Abstract
Tumor angiogenesis is a key factor in the progression of thymic epithelial tumors (TETs). Activin A, a member of the TGFβ family, and its antagonist Follistatin are involved in several human malignancies and angiogenesis. We investigated Activin A and Follistatin in serum and tumor tissue of patients with TETs in relation to microvessel density (MVD), WHO histology classification, tumor stage and outcome. Membranous Activin A expression was detected in all tumor tissues of TETs, while Follistatin staining was found in tumor nuclei and cytoplasm. Patients with TETs presented with significantly higher Activin A and Follistatin serum concentrations compared to healthy volunteers, respectively. Follistatin serum concentrations correlated significantly with tumor stage and decreased to physiologic values after complete tumor resection. Follistatin serum concentrations correlated further with MVD and were associated with significantly worse freedom from recurrence (FFR). Low numbers of immature tumor vessels represented even an independent worse prognostic factor for FFR at multivariable analysis. To conclude, the Activin A - Follistatin axis is involved in the pathogenesis of TETs. Further study of Follistatin and Activin A in TETs is warranted as the molecules may serve as targets to inhibit tumor angiogenesis and tumor progression.
Similar content being viewed by others
Introduction
Thymic epithelial tumors (TETs), comprising thymomas and thymic carcinomas (TCs) are extremely rare malignancies with an annual incidence of only 3.2 cases per million1. However, though rare, TETs are the most common malignancies in the anterior mediastinum in adults.
Clinical behavior and outcome differs significantly between thymomas and TCs. Thymomas, accounting for 72% to 89% of TETs, are characterized by a more benign tumor behavior compared to TCs and display a unique association with the paraneoplastic autoimmune disease Myasthenia Gravis (MG)2,3,4,5. Indeed, more than 30% of thymoma patients have thymoma associated MG (TAMG), while 10% to 20% of myasthenic patients have thymomas6. Conversely, TCs represent a minor group of TETs that are not associated with paraneoplastic MG, which show a more aggressive tumor behavior with worse outcome and more recurrences compared to thymomas7,8. The Masaoka-Koga staging system, which differentiates TETs according to level of invasiveness into four stages, ranging from non-invasive (stage I) to metastasized TETs (stage IV), is most commonly used for staging of TETs9. Recently a new TNM staging system for TETs was introduced10.
Surgical tumor resection represents the mainstay of therapy even in case of recurrent disease or in TETs with pleural metastases11. Recurrences can occur even decades after radical tumor resection, and therefore life-long oncologic follow up is recommended2,12,13. In our previous works we could demonstrate that different systemic inflammatory proteins, such as CRP or Fibrinogen and indices formed from inflammatory cell counts, such as neutrophil-to-lymphocyte ratio (NLR) were associated with higher tumor stage and worse prognosis3,14. However, despite improved insights also into genetic profiles of TETs, there exist no established markers for diagnosis or to predict tumor recurrence during oncologic follow-up of TETs15,16,17. Subsequently, a better insight into the pathogenesis of TETs and identification of new biomarkers are still needed.
Activin A, a member of the TGFβ superfamily of cytokines, binds to type II activin receptors on the cell surface, which leads to recruitment and phosphorylation of type I activin receptors that results in intracellular phosphorylation and activation of Smad proteins. The latter finally ends up in translocation of the activated Smad proteins into the nucleus and regulation of gene transcription18,19. Follistatin represents a natural antagonist of Activin A that prevents interaction of Activin A with type I and II activin receptors, resulting in endocytosis of the Activin A/Follistatin complex and lysosomal degradation19.
Within the past years, a deregulation or mutation of the activin signaling axis was found in numerous malignancies. Follistatin has also tumorigenic and proangiogenic functions, at least partially through antagonizing Activin A20,21.
Therefore, we hypothesized that Activin A and Follistatin may play a role in the pathophysiology of TETs. It was the primary aim of the study to evaluate whether serum Activin A and Follistatin serum concentrations correspond to histologic tumor subtype, stage and tumor behavior. In addition, we investigated whether TETs express Activin A or Follistatin, and whether there is an association between Activin A and Follistatin with tumor microvessel density (MVD) and outcome.
Results
Activin A and follistatin serum concentrations in thymic epithelial tumors
Activin A and Follistatin serum concentrations were significantly elevated in patients with TETs compared to healthy volunteers (p = 0.009 and p = 0.002; respectively). Patients with TCs and thymomas had significantly higher Activin A serum concentrations compared to volunteers (p = 0.004 and p = 0.001; Fig. 1A). Conversely, we detected highest Follistatin serum concentrations in patients with TCs, which were significantly higher compared to thymomas (p = 0.021) and volunteers (p = 0.002; Fig. 1B) (Table 1).
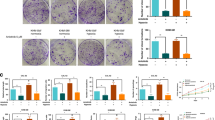
Activin A and Follistatin serum concentrations in patients with thymic epithelial tumors and Myasthenia Gravis Patients without TETs. Plots show preoperative Activin A and Follistatin serum concentrations in patients with thymic carcinomas (TCs), thymomas and healthy volunteers (A,B), and in patients with TETs according to Masaoka - Koga tumor stage (C,D). Further, the impact of tumor resection on postoperative Activin A and Follistatin serum levels is indicated (E,F). In addition, analysis of Activin A and Follistatin serum concentrations are illustrated in myasthenic patients (MG) compared to volunteers (G,H). Scatter dot plots are indicated as mean ± standard error of the mean.
Serum follistatin and Masaoka – Koga tumor stage
Preoperative Follistatin but not Activin A serum concentration was significantly different according to Masaoka - Koga tumor stage (p = 0.037 and p = 0.988; respectively; Fig. 1C,D). In particular, Follistatin serum concentrations were 806.5 ± 791.2 pg/mL and 713.4 ± 589.1 pg/mL in stage III and IV TETs, which was significantly different compared to 334.3 ± 286.1 pg/mL and 285.9 ± 246.0 pg/mL in stage I and II TETs (p = 0.005) (Table 1).
Decreased follistatin serum concentrations after complete tumor resection
Preoperative Follistatin serum concentrations decreased significantly after complete tumor resection (505.9 ± 564.9 pg/mL vs. 210.4 ± 280.4 pg/mL; p = 0.014; Fig. 1F). After radical tumor resection postoperative Follistatin serum concentrations reached physiologic serum levels, similar to those found in healthy volunteers (210.4 ± 280.4 pg/mL vs. 254.4 ± 164.5 pg/mL; p = 0.465). With regard to Activin A serum concentrations, tumor resection did not significantly influence Activin A serum levels (p = 0.773; Fig. 1E) (Table 1).
Impact of myasthenia gravis on Activin A and follistatin serum concentrations
Activin A and Follistatin serum concentrations did not significantly differ in patients with TAMG compared to TETs without MG (p = 0.751 and p = 0.211; respectively; Table 1). In addition, we analyzed serum samples of MG patients without thymic malignancy compared to sex- (p = 0.834) and age-matched (p = 0.079) healthy volunteers. Myasthenic patients showed also significantly elevated Activin A (p = 0.009; Fig. 1G) and Follistatin (p = 0.002; Fig. 1H) serum concentrations compared to controls (Table 1). Furthermore, we differentiated myasthenic patients based on their thymic pathology into patients with TETs, those with true thymic hyperplasia and those with follicular thymic hyperplasia. However, benign thymic pathology did not significantly impact Activin A and Follistatin serum concentration in patients with MG (p = 0.804 and p = 0.722; respectively).
Follistatin und Activin A expression in thymic epithelial tumors
We performed Follistatin and Activin A staining in 95 specimens of TETs (4 micronodular thymomas (MNT), 14 A, 14 AB, 10 B1, 21 B2, 17 B3 thymomas, 15 TCs; Fig. 2). Membranous Activin A expression was detected in all TETs without significant differences between thymomas and TCs or according to WHO subtype. Conversely, Follistatin staining was found in tumor nuclei and cytoplasm (p = 1.000). There was a trend towards absent nuclear Follistatin expression in TCs compared to thymomas. In particular, 75% of all TCs displayed absent Follistatin in nuclei, whereas absent nuclear Follistatin expression was found in 19% of all thymomas (p = 0.053). There were no semiquantitative differences in Follistatin staining intensity between thymomas and TCs.
Activin A and Follistatin expression in thymic malignancies. Strong cytoplasmic and membranous Activin A expression was found in all TETs. Representative Activin A staining is shown in a WHO type AB (A), B2 (C), and B3 (E) thymoma. Conversely, nuclear and cytoplasmic Follistatin staining was found in MNT (B), B2 thymoma (D), and TC (F) (magnification × 400; scale bar: 40 μm). WHO, World Health Organization; TC, thymic carcinoma; MNT, micronodular thymoma.
Follistatin serum levels correspond with microvessel density
We performed Spearman correlation analyses to evaluate whether serum Follistatin impacts MVD and vascular architecture in TETs (Fig. 3A–C). Follistatin serum levels showed a significant negative correlation with the number of immature (r: −0.443; p = 0.014) tumor vessels (Fig. 3D). In addition, there was also a significantly positive correlation between Follistatin and mature tumor vessels (r: 0.549; p = 0.002 Fig. 3E). As expected, immature tumor vessels correlated significantly with mature vessels (r: −0.440; p < 0.001) and total number of vessels (r: 0.932; p < 0.001), respectively.
Microvessel Density in thymic epithelial tumors. CD34 staining of a Masaoka - Koga stage I WHO type B2 thymoma (A), a stage II A thymoma (B) and a stage III A thymoma (C) are shown (200 × magnification; scale bar: 80 μm). Preoperative Follistatin serum concentrations showed a significant negative correlation (D) with the number of immature tumor vessels (r: −0.443; p = 0.014), and a significantly positive correlation (E) with the amount of mature tumor vessels (r: 0.549; p = 0.002). While the amount of immature tumor vessels decreased with higher tumor stage (F), the number of mature tumor vessels increased with level of invasiveness (G). Similarly, total numbers of tumor vessels decreased from stage I to stage IV tumors (H). High preoperative Follistatin serum levels were associated with significantly worse FFR (I).
Furthermore, we explored the possibility that other systemic inflammatory markers, such as Fibrinogen, CRP, Heat Shock Proteins or molecules of the RAGE axis, may also correlate with specific vascular architecture of TETs. However, beside serum Follistatin none of the abovementioned proteins showed significant correlation with mature, immature or total tumor vessels (Table 2). Nonetheless we found a significant correlation between Follistatin and CRP (r: 0.576; p = 0.006) and Follistatin with Fibrinogen (r: 0.512; p = 0.018).
Lowest number of immature, but highest number of mature vessels in thymic carcinomas
The mean number of immature and mature vessels was 79.5 ± 51.6 and 19.0 ± 16.8, respectively. TCs showed significantly higher numbers of mature (29.8 ± 17.1 vs. 17.2 ± 16.5), and lower numbers of immature tumor vessels (25.1 ± 10.8 vs. 90.0 ± 50.5) compared to thymomas (p = 0.010; p < 0.001). With regards to WHO thymoma subtypes, the highest number of immature vessels was found in MNT 121.1 ± 31.6 and WHO type AB thymomas 115.4 ± 25.8, respectively.
Moreover, TCs showed significantly lower immature to mature (1.11 ± 0.89 vs. 9.26 ± 10.6; p < 0.001) and immature to total vessel ratios (0.47 ± 0.16 vs. 0.81 ± 0.16; p < 0.001) compared to thymomas. Regarding to thymoma subtypes, WHO type B1 thymomas showed the highest immature to mature vessel ratios (15.2 ± 15.3), and AB thymomas the highest ratio of immature to total number of vessels of 0.89 ± 0.05, respectively. It is noteworthy that we detected the highest ratio of mature to total amount of tumor vessels in TCs, which was significantly higher compared to thymomas (0.53 ± 0.16 vs. 0.19 ± 0.16; p < 0.001) (Table 3).
Masaoka–Koga tumor stage and microvessel density
MVD and vascular architecture showed significant differences according to Masaoka – Koga tumor stage. The highest number of mature tumor vessels (38.7 ± 9.7) was detected in stage IV TETs (p < 0.001; Fig. 3G), while the number of immature vessels decreased gradually from stage I TETs (109.6 ± 46.4) to stage IV TETs (28.9 ± 3.9; p < 0.001; Fig. 3F). Similar, highest mature to total vessel ratio of 0.53 ± 0.05 and lowest immature to total vessel ratio of 0.47 ± 0.05 was found in metastasized stage IV TETs (Table 3).
Impact of microvessel density and serum parameters on outcome
Next, we performed Kaplan-Meier survival analysis to evaluate how Follistatin and Activin A serum concentrations and MVD impact freedom from recurrence (FFR) and cause specific survival (CSS). We calculated Youden-Indices to find optimal cut-off values for Activin A and Follistatin for predicting tumor recurrence. The highest Youden-Indices of 0.231 and 0.742 were found at cut-off values of 483.3 pg/mL and 776.5 pg/mL for Activin A and Follistatin, respectively. With respect to MVD, median number of mature (14.3), immature (71.3) and total tumor vessels (91.3) was used for dichotomizing patients into lower and higher subgroups, respectively. Low numbers of immature and total vessels were associated with significantly worse FFR (p = 0.001; p = 0.006) and CSS (p = 0.014; p = 0.017), respectively. High preoperative Follistatin serum concentrations were associated with significantly worse FFR (p < 0.001; Fig. 3I), but not CSS (p = 1.000), while Activin A serum levels did not impact outcome (Table 4).
Univariable and multivariable Cox-Regression analyses
Finally we performed univariable and multivariable Cox-regression analyses to assess the prognostic power of following clinical characteristics on FFR and CSS: histology (TC vs. thymoma), Masaoka-Koga tumor stage (III-IV vs. I-II), MG (neg. vs. pos.), sex (female vs. male), number of immature (low vs. high) and mature (high vs. low) tumor vessels and total number of vessels (low. vs. high).
At univariable analysis, presence of TC (HR 6.10; p = 0.002), tumor stage III-IV (HR 6.10; p = 0.003), low number of immature (HR 14.8; p = 0.010) and low number of total tumor vessels (HR 6.48; p = 0.016) represented significantly worse prognostic factors for FFR. Among these variables, only low number of immature tumor vessels stayed an independent worse prognostic factor at multivariable analysis. In particular, low number of immature tumor vessels showed a 35-fold higher risk for tumor recurrence compared to TETs with high number of immature tumor vessels (HR 35.3; p = 0.021). With respect to CSS, only presence of TC (HR 15.2; p = 0.002) was a significant worse prognostic factor at univariable testing (Table 5).
Discussion
Herein we investigated for the first time the role of Activin A and Follistatin serum concentrations and tumor expression in TETs. We found significantly elevated Activin A and Follistatin serum concentrations in patients with TETs compared to healthy volunteers. The findings were in line with the literature reporting of elevated Activin A serum levels in patients with malignant pleura mesothelioma22, lung adenocarcinoma23, breast cancer and prostate cancer24, hepatocellular carcinoma25, and endometrial and cervical carcinoma26. In our study, preoperative Activin A serum levels did not significantly correlate with Masaoka –Koga tumor stage, WHO classification or outcome, which was in contrast to literature, where increased Activin A serum levels were commonly associated with higher tumor stage and poor outcome.
Conversely to Activin A, elevated Follistatin serum concentrations were described only in few malignancies, including ovarian cancer27, lung adenocarcinoma28, hepatocellular carcinoma29, and prostate cancer30. In particular, high Follistatin serum levels were associated with shorter overall survival (OS) and poor prognosis in hepatocellular carcinoma29, emergence of bone metastases in prostate cancer30 or tumor proliferation of lung adenocarcinoma cells28. This is in agreement with our data, showing that highest Follistatin serum concentrations were found in patients with advanced stage TETs and TCs, and that high preoperative serum levels were associated with significantly worse FFR.
It is commonly assumed that the cancerogenic function of Follistatin depends primarily on its role as antagonist of Activin A21. Follistatin could prevent Activin A induced growth inhibition and apoptosis and therefore Follistatin fosters cell proliferation and tumor growth. Stove C. et al.31 found that in-vitro melanoma cells express activin receptors and that treatment with Activin A led to growth inhibition and apoptosis, which could be counteracted by addition of Follistatin31. In accordance to that, Chen F. et al.28 demonstrated that cultured lung adenocarcinoma cells secreted Follistatin, and that inhibition of Follistatin led to significantly augmented Activin A induced apoptosis28.
In addition, Follistatin also has effects on tumor-associated angiogenesis, which mostly depends on the inhibition of Activin A. Endothelial cells constitutively express activin receptors and VEGF, and binding of Activin A leads to inhibition of proliferation of cultured endothelial cells by downregulation of p21 and downregulation of VEGF expression19,32. In accordance to that, inhibition of p21 increased endothelial cell proliferation and resistance to Activin A mediated growth inhibition33. Moreover, Follistatin induces also angiogenesis by binding and activating Angiogenin, a potent activator of angiogenesis34.
Within our study, we could further link for the first time the pro-angiogenic function of serum Follistatin to MVD in TETs. Follistatin serum levels showed a significant negative correlation with absolute number of immature tumor vessels, and a significant positive correlation with mature tumor vessels. Herein, we defined maturity of tumor vessels as presence of vessel lumen that depends on the proliferation of endothelial cells. Therefore, the amount and characteristic pattern of tumor vessels in TETs most likely depend on proliferation of endothelial cells, which might be influenced by Follistatin.
Tumor angiogenesis represents one of the hallmarks of cancer and is essential for tumorigenesis, tumor growth and metastasis35. Already in 2002, Tomita et al. showed that mean MVD of TETs significantly correlated with tumor stage and invasiveness36. Reica et al. (2010) classified tumor vessels in thymomas as mature, intermediate and immature, and they further suggested that tumor invasiveness might correspond with tumor vessel subtype37. Recently, Pfister F. et al.38 could show that vascular architecture and expression profile of key angiogenic growth factors differ significantly in TETs. In particular, densest vascular networks of predominantly small vessels were found in A thymomas, while TCs showed the least dense vascular network with almost large capillaries and highest VEGF expression38.
Similar to that, patients with TCs showed the lowest MVD, but the highest amount of mature tumor vessels in our cohort. Furthermore, we could demonstrate that the number of immature vessels gradually decreased with level of invasiveness, while the amount of mature tumor vessels gradually increased with tumor stage. Indeed low numbers of immature tumor vessels represent a significantly worse prognostic factor for FFR with a 35-fold increased risk for recurrent disease. We managed to link the pro-angiogenic function of Follistatin with MVD and further to demonstrate that tumor vessel subtypes differ significantly according to WHO classification. Hence, manipulation of the Activin A and Follistatin system in TETs may represent a potential target to interfere with tumor angiogenesis, tumor growth and metastases in TETs.
According to data from the Unigene database, Follistatin is not expressed in normal (physiologic) thymus21. Herein, we could demonstrate that Follistatin and Activin A were generally expressed in TETs. We found constant membranous Activin A staining in all TETs, which was most likey caused by the usage of an anti-human Anti-Activin A receptor type IB antibody. Conversely, Follistatin as a soluble antagonist of Activin A, was detected in cytoplasm and nucleus of tumor cells. Soluble Follistatin molecules envelop Activin A and thus block the access of Activin A to type I and type II receptor binding sites39. Furthermore, Follistatin and Activin A binding complexes can interact with cell-surface proteoglycans followed by internalization and lysosomal degradation40, which could be an explanation of Follistatin expression in the cytoplasm of TETs. With respect to the nuclear function of Follistatin, Lin C. et al.41 (2016) could demonstrate that nuclear Follistatin prevented cultured human lung epithelial cells and mouse lunge tissue from reactive oxygen induced apoptosis and that down-regulation of Follistatin promoted apoptosis41. Similar, in HeLa cells, nuclear expression of Follistatin delayed glucose deprivation-induced apoptosis by attenuating RNA synthesis, which represents a key process of cellular energy homeostasis and cell survival42. Altogether we assume that nuclear expression of Follistatin in malignant thymic epithelial cells might cause cell-survival and inhibition of apoptosis, while the cytoplasmic Follistatin expression might result from degradation of Follistatin – Activin A complexes.
Follistatin serum concentrations decreased significantly after radical tumor resection, indicating that Follistatin serum levels are at least partially related to the presence of malignant thymic epithelial cells. Whether Follistatin is secreted directly by tumor cells, as demonstrated for human lung adenocarcinoma cells28, or indirectly by immune cells in response to tumor cells or as acute phase reaction43, remains elusive. Therefore, although our data are interesting, further studies are necessary to clarify the molecular and cellular effects of Activin A and Follistatin in TETs.
Our study has some limitations due to the mostly retrospective study design, the single center experience and the low number of patients, which limit our ability to draw conclusions. Moreover, although our data are interesting, we could not provide experimental data of Follistatin and Activin A in TET cell lines to further confirm our results. However, the strength of the study is that we could link the pro-angiogenic function of Follistatin to MVD in TETs, which corresponds to Masaoka-Koga tumor stage and outcome.
Taken together, we could demonstrate that preoperative Activin A and Follistatin serum concentrations were significantly elevated in patients with TETs and MG compared to controls. Follistatin serum levels were highest in patients with TCs and advanced tumor stage. Most importantly, Follistatin correlated significantly to MVD, which in turn was significantly different according to WHO subtype and Masaoka – Koga tumor stage. Number of immature tumor vessels represented a significant prognostic factor for FFR. Hence, manipulation of the Activin A and Follistatin system in TETs may represent a potential target to interfere with tumor angiogenesis and tumor progression in TETs.
Material and Methods
Study population
This study was conducted at the Department of Thoracic Surgery, Medical University of Vienna. From this cohort, patients with the diagnosis of a primary TET, who underwent surgical tumor resection, were included. Between 1999 and 2014, 98 patients with thymic malignancy were identified to meet criteria. Three patients with thymic neuroendocrine tumors (TNETs) were excluded.
Clinical and sociodemographic characteristics for each patient were obtained from medical hospital records, surgical and pathological reports, and imaging findings. Within our cohort, we had 56 female (58.9%) and 39 male (41.1%) patients with a mean age of 57.2 ± 15.9 years.
WHO classification was used for differentiating TETs into thymomas and TCs44. Accordingly, thymomas were classified as WHO type A, AB, B1, B2, B3 thymomas, and micronodular thymomas (MNT)44,45. We had 4 MNT (4.2%), 14 type A (14.7%), 14 AB (14.7%), 10 B1 (10.5%), 21 B2 (22.1%), 17 B3 thymomas (17.9%) and 15 TCs (15.8%; squamous cell carcinomas, SCCs), respectively. Within our cohort, we had 8 mixed type thymomas, including 7 B2/B3 and 1 B1/B2 thymomas. For the purpose of statistical analysis of this manuscript, WHO mixed type thymomas were classified according to the more malignant part (e.g. B2/B3 thymoma was classified as B3 thymoma).
Masaoka – Koga staging was as follows: stage I 23 (24.2%), stage II 45 (47.4%), stage III 13 (13.7%) and stage IV 14 cases (14.6%). Twenty - nine (30.5%) out of these 95 TETs were associated with TAMG.
Outcome analysis
The mean and median follow-up time was 95.5 and 72.4 months (range: 0.1–525.1 months), respectively. Within oncologic follow-up, periodic chest CT-scans were performed postoperatively every 3 to 6 months for the first three years followed by annual CT-scans. Thirteen (13.5%) patients experienced recurrent disease (local: n = 2; regional: n = 5; distant: n = 6) and six patients died from TETs (6.3%). According to the recommendations of the International Thymic Malignancy Interest Group (ITMIG), we used CSS and FFR as main oncologic outcome parameters within this study12. CSS was calculated in all patients and was defined as time from date of surgery to date of death from a TET, while unrelated deaths or unknown causes of death were censored. In contrast, FFR was only calculated in patients after radical tumor resection (R0) and was defined as time from date of surgery to date of recurrence.
Therapy
Among those 95 TETs who underwent surgical tumor resection, completeness of surgical resection could be achieved in 83 cases (87.4%), while R1 and R2 status were obtained in 9 (9.4%) and 3 (3.2%) of TETs, respectively. Forty-six patients (48.4%) underwent only surgical tumor resection, while the remaining 49 patients (51.6%) received multimodal therapy. In particular, 11 patients (11.6%) underwent neoadjuvant chemotherapy (ChT; n = 5) or concomitant radiochemotherapy (RChT; n = 6), while 42 patients (44.2%) received adjuvant therapy, including adjuvant ChT (n = 3), RT (n = 31) and RChT (n = 8), respectively.
Preparation of specimens and immunohistochemistry
In our clinical institute of pathology, tumor sections are processed from the capsule, every 1–2 cm of tumor tissue and from tumor regions, which appear different on gross inspection. However, our immunohistochemical analyses were done on selected slides from one block per patient that represented the main tumor component with a very homogenous histology.
Formaldehyde-fixed and paraffin embedded human specimens of all patients (n = 95) with TETs were available for immunohistochemical stainings. Staining was performed by using the automated Ventana Benchmark® platform (Ventana Medical Systems, Tucson, AZ, USA) according to standard protocol of the Clinical Institute of Pathology46. We used monoclonal rabbit anti-human Anti-Activin A Receptor Type IB antibody (ab204655, Abcam, Cambridge, UK) and monoclonal mouse anti-human Follistatin antibody IgG2a (MAB669, R&D Systems, Minneapolis, MN, USA) as primary antibodies to assess Activin A and Follistatin expression in TETs. In addition, we used monoclonal mouse anti-human CD34 antibody (LEICA, NovoCastra, Clone QBEnd/10, Nussloch, Germany) to assess microvascular density.
Evaluation of immunoreactivity in thymic epithelial tumors
We used a qualitative score to evaluate staining intensity of Activin A and Follistatin in tumor cells. Staining intensities were calculated as 0 (none), weak (1), moderate (2) or strong (3). Specimens were only counted as positive if more than 90% of tumor cells showed strong (3) Activin A or Follistatin expression.
Microvessel density
As already published and described by Weidner et al. 1992, we assessed microvessel density (MVD) using the “hotspot” method47. Briefly, slides were screened at low magnification to identify the areas with the greatest number of CD34 stained vessels (“hotspot”). MVD was determined by counting all CD34 positive vessels at 200x magnification (corresponding to 0.95 mm²) in 95 TETs. One specimen was removed due to lack of evaluable remaining thymic tissue. Three representative hotspots were calculated for each specimen.
Tumor vessels were further differentiated into immature and mature vessels according to the classification of Gee MG et al. based on the presence of perfused lumen and perivascular cells48. Accordingly, immature vessels are differentiated from intermediate and mature vessels by the presence or absence of vessel lumen, whereas intermediate and mature vessels are characterized by the presence or absence of perivascular cells. Herein, we used a simplified method and differentiated only between immature and mature vessels. Within the Results section, total number of immature, mature and all tumor vessels are indicated as well as following ratios: Ratio 1 (immature/mature), Ratio 2 (immature/total) and Ratio 3 (mature/total).
Enzyme-linked immunosorbent assays (ELISA)
Preoperative serum samples were available of 46 patients with TETs (17 TAMG, 29 non-TAMG) and 30 patients with MG for analysis of Activin A and Follistatin blood levels. Forty-nine sex and age-matched healthy volunteers were used as controls. Pre - and postoperative serum samples were available in a subset of 30 patients with TETs. Postoperative serum samples were collected in patients who underwent primary surgery 6 to 12 months ago, who did not receive adjuvant therapy within the last month, and who did not have signs of recurrence or a 2nd malignancy.
To assess Activin A and Follistatin serum concentrations, we used the commercial available human Activin A ELISA kit (R&D Systems, Minneapolis, MN, USA, DuoSet® human Activin A, DY338) and human Follistatin ELISA kit (R&D Systems, Minneapolis, MN, USA, DuoSet® human Follistatin, DY669). All tests were performed according to the Manufacture’s protocols. Samples were measured in duplicates and researches performing the assays were blinded to the groups associated with each sample.
Additionally to Activin A and Follistatin serum concentrations, Fibrinogen, CRP, heat shock protein 27 and 70, and molecules of the RAGE axis (sRAGE, esRAGE, HMGB1) as previously shown, were correlated with MVD3,14,46,49.
Statistical methods
Statistical analysis of data was performed using SPSS software (version 21; IBM SPSS Inc., IL, USA). The type of test used is indicated in the table and/or the result section. All data are reported as mean ± standard deviation (SD) within result section. Chi-square test was used to investigate the association between nominal variables. Unpaired Student’s t-test and one-way ANOVA were used to compare means of two or more than two independent groups with normal (Gaussian) distributions, respectively. Post-hoc Tukey’s - B and Bonferroni correction were used in case of multiple testing. Mann-Whitney-U test and Krusky-Wallis test were performed to analyze non-normal distributed variables with two or more than two groups, respectively. Paired Student’s t-test was performed for analyzing means of two dependent groups. Pearson correlation (r) was done to analyze linear relationships between two numerical measurements, while Spearman correlation analysis was applied for analysis of ranked variables. Kaplan-Meier analyses and Log-rank test were assessed for survival analysis. Uni - and multivariable Cox-regression analysis was used to evaluate the prognostic impact of different clinical variables on CSS and FFR. The receiver operating characteristic (ROC) curve and Youden-Index were calculated to find optimal cut-off values of 483.3 pg/mL for Activin A and 776.6 pg/mL for Follistatin for predicting tumor recurrence.
Ethics statement
Ethical approval was obtained from the Ethics Committee of the Medical University of Vienna (302/2011). All participating patients and control subjects gave their written informed consent, and all experiments were performed in accordance with the approved ethical guidelines.
References
de Jong, W. K. et al. Thymic epithelial tumours: a population-based study of the incidence, diagnostic procedures and therapy. Eur J Cancer. 44(1), 123–30 (2008).
Ruffini, E. et al. Tumours of the thymus: a cohort study of prognostic factors from the European Society of Thoracic Surgeons database. Eur J Cardiothorac Surg. 46(3), 361–8 (2014).
Janik, S. et al. Prognostic and diagnostic impact of fibrinogen, neutrophil-to-lymphocyte ratio, and platelet-to-lymphocyte ratio on thymic epithelial tumors outcome. Oncotarget. 9(31), 21861–21875 (2018).
Kondo, K. et al. WHO histologic classification is a prognostic indicator in thymoma. Ann Thorac Surg. 77(4), 1183–8 (2004).
Marx, A. et al. Thymoma related myasthenia gravis in humans and potential animal models. Exp Neurol. 270, 55–65 (2015).
Marx, A. et al. Thymoma and paraneoplastic myasthenia gravis. Autoimmunity. 43(5–6), 413–27 (2010).
Ruffini, E. et al. Thymic carcinoma: a cohort study of patients from the European society of thoracic surgeons database. J Thorac Oncol. 9(4), 541–8 (2014).
Ahmad, U. et al. Thymic carcinoma outcomes and prognosis: results of an international analysis. J Thorac Cardiovasc Surg. 149(1), 95–100 (2015).
Koga, K. et al. A review of 79 thymomas: modification of staging system and reappraisal of conventional division into invasive and non-invasive thymoma. Pathol Int. 44(5), 359–67 (1994).
Meurgey, A. et al. Assessment of the ITMIG Statement on the WHO Histological Classification and of the Eighth TNM Staging of Thymic Epithelial Tumors of a Series of 188 Thymic Epithelial Tumors. J Thorac Oncol. 12(10), 1571–1581 (2017).
Moser, B. et al. Surgical therapy of thymic tumours with pleural involvement: an ESTS Thymic Working Group Project. Eur J Cardiothorac Surg. 52(2), 346–355 (2017).
Huang, J. et al. Comparison of patterns of relapse in thymic carcinoma and thymoma. J Thorac Cardiovasc Surg. 138(1), 26–31 (2009).
Marulli, G. et al. Surgical treatment of recurrent thymoma: is it worthwhile?†. Eur J Cardiothorac Surg. 49(1), 327–32 (2016).
Janik, S. et al. Elevated CRP levels predict poor outcome and tumor recurrence in patients with thymic epithelial tumors: A pro- and retrospective analysis. Oncotarget. 8(29), 47090–47102 (2017).
Saito, M. et al. The genomic and epigenomic landscape in thymic carcinoma. Carcinogenesis. 38(11), 1084–1091 (2017).
Shitara, M. et al. Genetic profiling of thymic carcinoma using targeted next-generation sequencing. Lung Cancer. 86(2), 174–9 (2014).
Radovich, M. et al. The Integrated Genomic Landscape of Thymic Epithelial Tumors. Cancer Cell. 33(2), 244–258 (2018).
Attisano, L., Wrana, J. L., Montalvo, E. & Massagué, J. Activation of signalling by the activin receptor complex. Mol Cell Biol. 16(3), 1066–73 (1996).
Loomans, H. A. & Andl, C. D. Intertwining of Activin A and TGFβ Signaling: Dual Roles in Cancer Progression and Cancer Cell Invasion. Cancers (Basel). 7(1), 70–91 (2014).
Krneta, J. et al. Dissociation of angiogenesis and tumorigenesis in follistatin- and activin-expressing tumors. Cancer Res. 66(11), 5686–95 (2006).
Shi, L., Resaul, J., Owen, S., Ye, L. & Jiang, W. G. Clinical and Therapeutic Implications of Follistatin in Solid Tumours. Cancer Genomics Proteomics. 13(6), 425–435 (2016).
Hoda, M. A. et al. Circulating activin A is a novel prognostic biomarker in malignant pleural mesothelioma - A multi-institutional study. Eur J Cancer. 63, 64–73 (2016).
Hoda, M. A. et al. High circulating activin A level is associated with tumor progression and predicts poor prognosis in lung adenocarcinoma. Oncotarget. 7(12), 13388–99 (2016).
Leto, G. et al. Activin A circulating levels in patients with bone metastasis from breast or prostate cancer. Clin Exp Metastasis. 23(2), 117–22 (2006).
Pirisi, M. et al. Evaluation of circulating activin-A as a serum marker of hepatocellular carcinoma. Cancer Detect Prev. 24(2), 150–5 (2000).
Petraglia, F. et al. Expression and secretion of inhibin and activin in normal and neoplastic uterine tissues. High levels of serum activin A in women with endometrial and cervical carcinoma. J Clin Endocrinol Metab. 83(4), 1194–200 (1998).
Ren, P. et al. High serum levels of follistatin in patients with ovarian cancer. J Int Med Res. 40(3), 877–86 (2012).
Chen, F. et al. Follistatin is a novel biomarker for lung adenocarcinoma in humans. PLoS One. 9(10), e111398 (2014).
Tomoda, T. et al. Prognotic impact of serum follistatin in patients with hepatocellular carcinoma. J Gastroenterol Hepatol. 28(8), 1391–6 (2013).
Tumminello, F. M. et al. Serum follistatin in patients with prostate cancer metastatic to the bone. Clin Exp Metastasis. 27(8), 549–55 (2010).
Stove, C. et al. Melanoma cells secrete follistatin, an antagonist of activin-mediated growth inhibition. Oncogene. 23(31), 5330–9 (2004).
McCarthy, S. A. & Bicknell, R. Inhibition of vascular endothelial cell growth by activin-A. J Biol Chem. 268(31), 23066–71 (1993).
Kaneda, H. et al. Activin A inhibits vascular endothelial cell growth and suppresses tumour angiogenesis in gastric cancer. Br J Cancer. 105(8), 1210–7 (2011).
Gao, X., Hu, H., Zhu, J. & Xu, Z. Identification and characterization of follistatin as a novel angiogenin-binding protein. FEBS Lett. 581(28), 5505–10 (2007).
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell. 100(1), 57–70 (2000).
Tomita, M. et al. Correlation between tumor angiogenesis and invasiveness in thymic epithelial tumors. J Thorac Cardiovasc Surg. 124(3), 493–8 (2002).
Raica, M., Cimpean, A. M., Nico, B., Guidolin, D. & Ribatti, D. A comparative study of the spatial distribution of mast cells and microvessels in the foetal, adult human thymus and thymoma. Int J Exp Pathol. 91(1), 17–23 (2010).
Pfister, F. et al. Vascular architecture as a diagnostic marker for differentiation of World Health Organization thymoma subtypes and thymic carcinoma. Histopathology. 70(5), 693–703 (2017).
Thompson, T. B., Lerch, T. F., Cook, R. W., Woodruff, T. K. & Jardetzky, T. S. The structure of the follistatin:Activin complex reveals antagonism of both type I and type II receptor binding. Dev Cell. 9, 535–543 (2005).
Sugino, H., Sugino, K., Hashimoto, O., Shoji, H. & Nakamura, T. Follistatin and its role as an activin-binding protein. J Med Invest. 44, 1–14 (1997).
Lin, C. et al. Transcriptional activation of follistatin by Nrf2 protects pulmonary epithelial cells against silica nanoparticle-induced oxidative stress. Sci Rep. 6, 21133 (2016).
Gao, X. et al. Nucleolar follistatin promotes cancer cell survival under glucose-deprived conditions through inhibiting cellular rRNA synthesis. J Biol Chem. 285(47), 36857–64 (2010).
Phillips, D. J., Jones, K. L., Scheerlinck, J. Y., Hedger, M. P. & de Kretser, D. M. Evidence for activin A and follistatin involvement in the systemic inflammatory response. Mol Cell Endocrinol. 180(1-2), 155–62 (2001).
Travis, W. D., Brambilla, E., Muller-Hermelink, H. K. & Harris, C. C. (ed.). World Health Organization Classification of Tumours. Pathology and Genetics of Tumors of the Lung, Pleura, Thymus and Heart. (Lyon, IARC Press, 2004).
Suster, S. & Moran, C. A. Micronodular thymoma with lymphoid B-cell hyperplasia: clinicopathologic and immunohistochemical study of eighteen cases of a distinctive morphologic variant of thymic epithelial neoplasm. Am J Surg Pathol. 23(8), 955–62 (1999).
Moser, B. et al. Expression of RAGE and HMGB1 in thymic epithelial tumors, thymic hyperplasia and regular thymic morphology. PLoS One. 9(4), e94118 (2014).
Weidner, N. et al. Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma. J Natl Cancer Inst. 84(24), 1875–87 (1992).
Gee, M. S. et al. Tumor vessel development and maturation impose limits on the effectiveness of anti-vascular therapy. Am J Pathol. 162(1), 183–93 (2003).
Janik, S. et al. HSP27 and 70 expression in thymic epithelial tumors and benign thymic alterations: diagnostic, prognostic and physiologic implications. Sci Rep. 6, 24267 (2016).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: S.J., C.B., A.I.S., H.J.A. and B.M. Performed the experiments: S.J., C.B., P.H., T.R. and B.M. Analyzed the data: S.J., C.B., A.I.S, L.M., C.V. and B.M. Contributed reagents/materials/analysis tools: A.I.S., L.M., B.D., W.K., H.J.A. and B.M. Wrote the paper: S.J., C.V., L.M., H.J.A. and B.M.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Janik, S., Bekos, C., Hacker, P. et al. Follistatin impacts Tumor Angiogenesis and Outcome in Thymic Epithelial Tumors. Sci Rep 9, 17359 (2019). https://doi.org/10.1038/s41598-019-53671-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-53671-8
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.