Abstract
Vitellogenin is the main yolk precursor protein in insect oocytes. It is synthesized in the fat body and released into the hemolymph. To reach the oocyte surface, vitellogenin must cross a single layer of follicular epithelium cells. The transport of vitellogenin across the follicular epithelium has been suggested to occur through the enlarged intercellular spaces (patency) by a paracellular route or by endocytosis by follicular cells and release onto oocyte surface in a transcelluar route. In this study, we investigated whether vitellogenin transport in the meroistic telotrophic ovary of Podisus nigrispinus (Hemiptera) occurs via a paracellular or transcellular route. Light and transmission electron microscopies showed that short cell–cell contacts with well-developed occluding septate junctions were present in follicular cells with patency. Immunofluorescence microscopy revealed the presence of vitellogenin receptors in the plasma membrane and of vitellogenin in the cytoplasm of follicular cells. Data suggest that cell–cell contacts serve as a barrier to large vitellogenin molecules and that this protein is transported via a transcellular route of receptor-mediated endocytosis.
Similar content being viewed by others
Introduction
The female insect reproductive tract is formed by a pair of ovaries connected to the common oviduct by a pair of lateral oviducts. Each ovary has many functional units, the ovarioles, in which oocytes grow surrounded by a follicular epithelium1.
In Heteroptera (Hemiptera), which includes bugs, ovaries are of the meroistic telotrophic type2,3,4 and each ovariole is divided into terminal filament, tropharium, vitellarium, and pedicel3,5. The distal portion of the ovariole is composed of the terminal filament, formed by connective tissue6; a tropharium with oogonia (which differentiate into oocytes) and nurse cells, which produce cytoplasm compounds that are transferred to oocytes during development5,6,7.
Oocyte maturation and vitellogenesis occur in the vitellarium2,8. Vitellogenesis is crucial for insect reproduction and comprises the synthesis of vitellogenin and yolk accumulation in the ooplasm9. Synthesis of the glycolipophosphoprotein vitellogenin occurs in the fat body under juvenile hormone control2,10. Vitellogenin is released into the hemolymph, taken up by growing oocytes, and stored as vitellin, the major yolk protein11,12.
Vitellogenin uptake into oocytes occurs via endocytosis, mediated by membrane vitellogenin receptors (VgRs) belonging to the low-density lipoprotein receptor (LDLR) superfamily9,11,13,14,15. LDLRs shares some structural features such as ligand-binding domain (LBD), epidermal growth factor precursor (EGFP) homology domain, O-linked sugar domain, a single transmembrane domain and a cytoplasmic domain14,15,16. Insects have two LBDs with different numbers of class A cysteine-rich ligand binding repeats (LBRs)16,17,18,19,20. These VgRs have been reported to combine with some different ligands in vertebrates21, and their mRNA are also detected in the hypopharingeal glands22 and fat body16,23 in insects, suggesting that VgR may recognize other ligands, but in insect ovary vitellogenin is the unique ligand of VgR endocytosed by oocytes11,13,14,15,24. However, to reach the oocyte surface, vitellogenin must be transported through the follicular epithelium25, which surrounds the oocyte2,6 and regulates the flow of vitellogenin to the oocyte5,7.
A model proposed over fifty years ago to explain the vitellogenin transport through the follicular epithelium remains almost intact. Extracellular canals formed by enlargement of the intercellular spaces between the follicular cells (patency) facilitate diffusion of soluble proteins from the hemolymph until the oocyte surface via a paracellular route. Transmission electron microscopy and immunohistochemical studies in Hyalophora cecropia (Lepidoptera)26,27,28 and Bemisia tabaci (Hemiptera)29, dynamics of the vital dyes trypan blue in Aedes aegypti (Diptera)30 and Evan’s blue in Rhodnius prolixus (Hemiptera)31 suggest the occurrence of paracellular transport of proteins trough the ovarian follicular epithelium. Conversely, in social Hymenoptera with meroistic polytrophic ovaries, ultrastructural studies indicate patency in the follicular epithelium25, but immunocytochemistry of vitellogenin and VgR show that both are localized in the plasma membrane and cytoplasm of follicular cells, suggesting a transcellular route in which vitellogenin initially diffuses through intercellular spaces to the follicular cells surface, bind to VgR, following clathrin-mediated endocytosis and transport to another plasma membrane domain (transcytosis) closely associated with oocyte surface32. This recent finding stimulated us to verify whether in other insects with meroistic telotrophic ovaries, patency is already an evidence of paracellular route for the vitellogenin transport to the oocyte surface for further endocytosis.
Podisus nigrispinus (Hemiptera: Pentatomidae) is an important generalist predator used in biological control of agricultural insect pests33. The development34, histology, cytology35,36,37, predator–prey interaction38, and biochemical processes39 of this predator have been studied. However, to the best of our knowledge, data on the morphology and reproductive physiology of P. nigrispinus females are scarce4,40 and the route for vitellogenin transport from the hemolymph to the oocyte surface has yet to be determined.
The objective of this study was to investigate whether vitellogenin follows a paracellular or transcellular route through the follicular epithelium in P. nigrispinus and thus contribute to the comprehension of this insect’s reproduction cycle.
Results
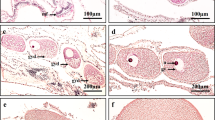
Podisus nigrispinus oocytes in the vitellarium were enveloped by a single layer of binucleate follicular cells (Fig. 1A). Previtellogenic oocytes were characterized by their small size and homogeneous cytoplasm, lined by columnar follicular cells with narrow intercellular spaces (Fig. 1A).
Light micrographs of the ovariole of Podisus nigrispinus. (A) Previtellogenic oocytes (PV) with homogeneous ooplasm and nucleus (n) lined by follicular epithelium (FE) and peritoneal sheath (PS). (B) Vitellogenic oocyte (VO) with ooplasm showing many yolk granules. Note polar follicular epithelium (PF) with columnar and juxtaposed cells and lateral follicular epithelium (LF) with enlarged intercellular spaces. (C) Lateral follicular epithelium of vitellogenic oocyte (VO) showing binucleated (N) cells with enlarged intercellular spaces (IS) and small points of cell-cell contact (arrowheads). (D) Late vitellogenic oocyte (OO) showing chorion (Ch), perioocytic space (PO) and follicular cells (FC) with narrowed intercellular spaces (arrows). NC – nutritive cord.
During vitellogenesis, oocytes increased in size and had many yolk granules and lipid droplets in the cytoplasm (Fig. 1B). Follicular cells at the anterior and posterior poles of the ovarian follicles were columnar and juxtaposed (Fig. 1B), whereas those in lateral regions were cubic and separated by enlarged intercellular spaces (Fig. 1B,C) but always showed small points of contact (Fig. 1C).
In advanced vitellogenesis, the perioocytic space (between oocyte surface and follicular cells) increased in size, eggshell (chorion) synthesis occurred, and intercellular spaces of follicular cells narrowed (Fig. 1D).
The ultrastructure of follicular cells during vitellogenesis in Hemiptera was previously described2. Therefore, we used transmission electron microscopy to evaluate intercellular contacts between follicular cells in previtellogenic and vitellogenic follicles. In the former, follicular cells showed narrow intercellular spaces along almost all their extension (Fig. 2A) as well as some septate junctions (Fig. 2B). In the vitellogenic follicle, there were enlarged intercellular spaces, which were interrupted by short contacts between adjacent follicular cells (Fig. 3A). At these cell–cell contact points, the intercellular space was narrow and plasma membranes showed septate junctions along almost the entire length (Fig. 3B,C).
Transmission electron micrographs of the follicular epithelium of the previtellogenic follicles in the ovarioles of Podisus nigrispinus. (A) Three adjacent follicular cells (FC1, FC2 and FC3) showing narrowed intercellular spaces (arrows). N – nucleus, Nu – nucleolus. (B) Detail of two adjacent follicular cells (FC1 and FC2) showing plasma membrane (arrowheads) and narrowed intercellular spaces (IS). M – mitochondria.
Transmission electron micrographs of the vitellogenic follicles in the ovarioles of Podisus nigrispinus. (A) Adjancent follicular cells (FC) showing enlarged intercellular space (IS) and a small point of cell-cell contract (arrowheads). (B) Region of follicular cell-cell contact showing narrowed intercellular space with many occluding junctions (arrows). (C) Detail of the occluding junction with parallel rows characterizing septate junction. N – nucleus, Nu – nucleolus.
To investigate whether vitellogenin is transported to the perioocytic space via a paracellular or transcellular route, we analyzed previtellogenic and vitellogenic follicles using immunofluorescence microscopy for detection of VgR. Western blotting showed that the antibody used recognized VgR, confirming the presence of this protein in the plasma membrane of P. nigrispinus oocytic follicles (Fig. 4). Immunofluorescence microscopy revealed the presence of VgR (Fig. 5) on the surface of follicular cells in both previtellogenic (Fig. 5A) and vitellogenic follicles, with a high fluorescent signal in the latter (Fig. 5B). Since we found high amount of VgR in the vitellogenic follicle, these were evaluated for the uptake of the vitellogenin, showing presence of this protein into the follicular cells (Fig. 6).
Immunofluorescence staining of follicles of ovarioles of Podisus nigrispinus. (A) Occurrence of vitellogenin receptor (green) in follicular cells of the previtellogenic follicle. (B) Vitellogenin receptor (green) in follicular cells of the vitellogenic follicle. (C) Negative control by omission of incubation with vitellogenin receptor antibody. Nuclei (N) of follicular cells in red.
Immunofluorescence staining of vitellogenic follicles of ovarioles of Podisus nigrispinus. (A) Occurrence of vitellogenin (green) in follicular cells of the vitellogenic follicle. (B) Detail of vitellogenin (green) in the follicular cell. (C) Negative control by omission of incubation with vitellogenin antibody.Nuclei (N) of follicular cells in red.
Discussion
Our findings show that the follicular epithelium in vitellogenic follicles of P. nigrispinus is differentiated into columnar and juxtaposed cells at the anterior and posterior poles, whereas follicular cells in lateral regions are cubic with dilated intercellular spaces. A similar morphology was observed in R. prolixus (Hemiptera) at the onset of vitellogenesis, when cells of the follicular epithelium at the anterior and posterior poles are morphologically distinct from cells in the lateral portion41. The intercellular spaces between follicular cells of the epithelium lateral to the oocyte enlarge progressively with oocyte growth, whereas polar follicular cells continue to possess narrow intercellular spaces and might act as a barrier for the passage of yolk proteins42,43, but the physiological significance of this difference remains unknown.
Enlargement of intercellular spaces in the follicular epithelium (patency) during vitellogenesis, as observed in the present study in P. nigrispinus, has been reported in H. cecropia26,27, R. prolixus28,41 and B. tabaci29. Those studies show accumulation of hemolymph proteins in the intercellular spaces of follicular cells, which has been interpreted as an evidence that these proteins, including vitellogenin, are transported to the oocyte surface via a paracellular route. However, in bees, ants, and wasps, although patency occurs, vitellogenin is transported to the oocyte surface by receptor-mediated endocytosis, that is, via a transcellular route25,32.
Light and transmission electron microscopies showed that patency occurs in the follicular epithelium of P. nigrispinus vitellogenic follicles but also revealed that follicular cells display septate junctions in small cell–cell contact regions. Septate junctions play an important role in maintaining the mechanical and physiological integrity of the epithelium41,43,44. In addition, septate junctions are important permeability barriers that control the transit of molecules through invertebrate epithelia45,46,47,48. Some chemical species such as lanthanum ions (La3+), which have high charge density and occur in a hydrated state, can cross septate junctions43,49,50; however, the passage of vitellogenin, which is a large protein with a molecular mass of 200–700 kDa14,51, seems unlikely, as septate junctions in different epithelia of insects are effective barriers to some ions and molecules larger than 7 kDa49,52,53,54.
Together, ultrastructural and immunofluorescence data show that intercellular spaces are blocked by septate junctions and that the plasma membrane of follicular cells contains VgR and vitellogenin, suggesting a transcellular route of vitellogenin transport to the perioocytic space, as has been demonstrated in Hymenoptera32. However, the question remains as to why patency occurs in follicular cells of insects during vitellogenesis. A possible explanation would be that the almost complete separation of adjacent follicular cells allows the exposure of a larger area of the plasma membrane to the hemolymph and thus exposes many VgR molecules for vitellogenin uptake, resulting in a high transport rate of reserve proteins to the oocyte.
The present study provides insight into the transcellular route of vitellogenin transport through the follicular epithelium in meroistic telotrophic ovaries, which seems to be a conserved route in insects, as it also occurs in insects with meroistic polytrophic ovaries. Further investigation is needed to elucidate the routes of vitellogenin transport in insects with panoistic ovaries.
Methods
Insects
Mated P. nigrispinus females were obtained from the Biological Control Laboratory of the Federal University of Viçosa (UFV), Viçosa, Minas Gerais, Brazil.
Light microscopy
Insects were cryoanesthetized at −4 °C for 90 s, and their ovaries were dissected in 125 mM NaCl. Five pairs of ovaries were transferred to 4% paraformaldehyde in 0.1 M phosphate buffered saline (PBS), pH 7.2. Then, samples were washed in the same buffer, dehydrated in a graded ethanol series (70, 80, 90, and 95%), and embedded in historesin (Leica). Sections of 2 μm, obtained using a rotary microtome with a glass knife, were stained with hematoxylin and eosin and analyzed under a light microscope.
Transmission electron microscopy
Five females were cryoanesthetized at −4 °C for 90 s and dissected. The ovaries were transferred to 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer, pH 7.2, for 12 h. After being washed in the same buffer, samples were post-fixed in 1% osmium tetroxide for 2 h, dehydrated in a graded ethanol series (70, 80, 90, 95, and 98%), and embedded in LR White resin. Ultrathin sections, obtained using an ultramicrotome equipped with a glass knife, were stained with 2% aqueous uranyl acetate and lead citrate and analyzed using a transmission electron microscope (Zeiss LIBRA 120).
Western blot analysis
Podisus nigrispinus females were dissected, and the ovaries were homogenized in 50 mM Tris-HCl buffer, pH 7.5, containing 10% protease inhibitor cocktail (P2714-1BTL, Sigma–Aldrich) using a cordless motor. Procedures were performed in triplicate. Then, samples were centrifuged at 10,000 × g for 15 min, and the supernatant containing soluble proteins was collected. The pellet was resuspended in Tris-HCl buffer, pH 7.5, 1% Triton X-100, and 10% protease inhibitor, homogenized, placed in an ultrasonic bath for extraction of membrane proteins, and centrifuged at 10,000 × g for 15 min. Supernatants containing soluble membrane proteins were submitted to SDS-PAGE55. The gel was incubated in transfer buffer [0.58% (w/v) Tris-HCl, 0.28% (v/v) glycine, 20% (v/v) methanol, and ultrapure water] and transferred to a nitrocellulose membrane by electroblotting at 190 A and 4 °C for 3 h (Mini Trans-Blot Cell®, Bio-Rad). Subsequently, the nitrocellulose membrane was incubated in Tris-buffered saline (TBS) (25 mM Tris and 150 mM NaCl, pH 7.5) with 1% Tween 20 (TBST) and 5% skimmed milk powder for 16 h. The nitrocellulose membrane was rapidly washed with TBST, washed twice with TBST for 15 min, and incubated for 2 h with primary antibodies against vitellogenin receptor (anti-VgR)32 (1:500). After washing with TBST, the nitrocellulose membrane was incubated with peroxidase-conjugated anti-mouse IgG secondary antibody (1:100) for 2 h. Then, samples were revealed with a diaminobenzidine solution [0.1% (w/v) DAB, 50 mM Tris-HCl, and H2O2].
Immunofluorescence microscopy
After dissection, the ovarioles had the peritoneal sheath removed with tweezers and were immersed in Zamboni fixative [4% (w/v) paraformaldehyde and 0.4% (w/v) picric acid in 0.1 M PBS, pH 7.2] for 2 h. Samples were transferred to PBS with 0.05% Triton X-100 (PBST) for 2 h and incubated in anti-VgR primary antibody32 (1:100 dilution in PBST) for 16 h. Another set of ovarioles were incubated in anti-vitellogenin primary antibody10 (1:100 dilution in PBST) for 16 h. Then, samples were incubated in a 1:400 dilution of FITC-conjugated anti-mouse IgG in PBST for 2 h in the dark. Samples were transferred to 50 μL of TO-PRO-3 iodide (Thermo Fisher Scientific) in PBS (1:1000), incubated for 30 min in the dark, mounted on slides with 50% sucrose., and analyzed on a Zeiss 510 Meta confocal fluorescence microscope.
Negative controls were subjected to the same procedures, except that the primary antibodies were replaced with normal mouse (for VgR) or rabbit (for vitellogenin) serum.
Ethical statement
This article does not contain any studies with animals and humans participants performed by any of the authors.
Data availability
All data and protocols used in this study are available at Universidade Federal de Viçosa.
References
Chapman, R. F. The Insects. Structure and Function (Elsevier, 2013).
Büning, J. The Insect Ovary – Ultrastructure, Previtellogenic Growth and Evolution (Chapman & Hall, 1994).
Simiczyjew, B., Ogorzalek, A. & Stys, P. Heteroptera ovaries: variations on the theme. Folia Histochem. Cytobiol. 36, 147–156 (1998).
Lemos, W. P., Ramaho, F. S., Serrão, J. E. & Zanuncio, J. C. Morphology of female reproductive tract of the predator Podisus nigrispinus (Dallas) (Heteroptera, pentatomidae) fed on different diets. Braz. Arch. Biol. Tech. 48, 129–138 (2005).
Huebner, E. The Ultrastructure and Development of the Telotrophic Ovary in Insect Ultrastructure (eds King, R.C. & Akai, H.) 3–48 (Plenum Press, 1982).
Snodgrass, R. E. Principles of Insect Morphology (McGraw-Hill, 1935).
Lutz, D. A. & Huebner, E. Development of nurse cell-oocyte interactions in the insect telotrophic ovary (Rhodnius prolixus). Tiss. Cell 13, 321–335 (1981).
Souza, E. A., Neves, C. A., Campos, L. A. O., Zanuncio, J. C. & Serrão, J. E. Effect of mating delay on the ovary of Melipona quadrifasciata anthidioides (Hymenoptera: Apidae) queens. Micron 38, 471–477 (2007).
Valle, D. Vitellogenesis in insects and other groups: a review. Mem. Inst. Oswaldo Cruz 88, 1–26 (1993).
Azevedo, D. O., de Paula, S. O., Zancunico, J. C., Martinez, L. C. & Serrão, J. E. Juvenile hormone downregulates vitellogenin production in Ectatomma tuberculatum (Hymenoptera: Formicidae) sterile workers. J. Exp. Biol. 219, 103–108 (2016).
Raikhel, A. S. & Dhadialla, T. S. Accumulation of yolk proteins in insect oocytes. Annu. Rev. Ent. 37, 217–251 (1992).
Tufail, M. & Takeda, M. Molecular characteristics of insect vitellogenins. J. Ins. Physiol. 54, 1447–1458 (2008).
Abu-Hakima, R. & Davey, K. G. The action of juvenile hormone on the follicle cells of Rhodnius prolixus: the importance of volume changes. J. Exp. Biol. 69, 33–44 (1977).
Sappington, T. W. & Raikhel, A. S. Molecular characteristics of insect vitellogenins and vitellogenin receptors. Ins. Biochem. Molec. Biol. 28, 277–300 (1998).
Tufail, M. & Takeda, M. Insect vitellogenin/lipophorin receptors: Molecular structures, role in oogenesis, and regulatory mechanisms. J. Ins. Physiol. 55, 88–104 (2009).
Liu, L. et al. LBD1 of vitellogenin receptor specifically binds to the female-specific storage protein SP1 via LBR1 and LBR3. PLoS One 11, e0162317, https://doi.org/10.1371/journal.pone.0162317 (2016).
Lu, K. et al. Molecular characterization and RNA interference analysis of vitellogenin receptor from Nilaparvata lugens (Stal). J. Ins. Physiol. 73, 20–29 (2015).
Shu, Y. H. et al. The first vitellogenin receptor from a lepidopteran insect: molecular characterization, expression patterns and RNA interference analysis. Ins. Molec. Biol. 20, 61–73 (2011).
Lin, Y. et al. Vitellogenin receptor mutation leads to the oogenesis mutant phenotype “scant vitellin” of the silkworm, Bombyx mori. J. Biol. Chem. 288, 13345–13355 (2013).
Upadhyay, S. K., Singg, H., Dixit, S., Mendu, V. & Verma, C. Moleular characterization of vitellogenin and vitellogenin recptor of Bemisia tabaci. PLoS One 11, 20155306, https://doi.org/10.1371/journal.pone.0155306 (2016).
Hiesberger, T. et al. The chicken oocyte receptor for yolk precursor as a model for studying the action of receptor-associated protein and lactoferrin. J. Biol. Chem. 270, 18219–18226 (1995).
Amdam, G. V., Norberg, K., Hagen, A. & Omholt, S. W. Social explotation of vitellogenin. Proc. Natl. Acad. Sci. USA 100, 1799–1802 (2003).
Lin, W. J., Chien, C. Y., Tsai, C. L. & Chen, M. E. A nonovary-specific vitellogenin receptor from the Oriental fruit fly, Bactrocera dorsalis (Hendel). Arch. Insect Biochem. Physiol. 90, 169–180 (2015).
Han, C. et al. Vitellogenin receptor selectively endocytoses female-specific and highly-expressed hemolymph proteins in the silkworm. Bomby mori. Biochem. Cell Biol. 95, 510–516 (2017).
Ronnau, M. et al. Changes in the follicular cells architecture during vitellogenin transport in the ovary of social Hymenoptera. Protoplasma 253, 815–820 (2016).
Telfer, W. H. The route of entry and localization of blood protein in the oocytes of saturniid moths. J. Bioph. Biochem. Cytol. 9, 747–753 (1961).
Stay, B. Protein uptake in the oocytes of the cecropia moth. J. Cell Biol. 26, 49–62 (1965).
Anderson, L. M. & Telfer, W. H. Extracellular concentrating of proteins in the cecropia moth follicle. J. Cell. Physiol. 76, 37–53 (1970).
Guo, J. Y., Wan, F. H. & Ye, G. Y. Oogenesis in the Bemisia tabaci MEAM1 species comples. Micron 83, 1–10 (2016).
Yonge, C. & Hagedorn, H. H. Dynamics of vitellogenin uptake in Aedes aegypti as demonstrated by trypan blue. J. Ins. Physiol. 23, 1199–1203 (1977).
Davey, K. G. Hormonal control of vitelogenin uptake in Rhodnius prolixus. Stal. Am. Zool. 21, 701–705 (1981).
Dohanik, V. T., Gonçalves, W. G., Oliveira, L. L., Zanuncio, J. C. & Serrão, J. E. Vitellogenin transcytosis in folicular cells of the honeybee Apis mellifera and the wasp Polistes simillimus. Protoplasma 255, 1703–1712 (2018).
Zanuncio, J. C., Alves, J. B., Sartório, R. C. & Garcia, J. F. Hemipterous predators of eucalypt defoliator caterpillars. For. Eco. Manag. 65, 65–73 (1994).
Lacerda, M. C. et al. Development and reproduction of Podisus distinctus (Heteroptera: Pentatomidae) fed on larva of Bombyx mori (Lepidoptera: Bombycidae). Braz. J. Biol. 64, 237–242 (2004).
Martinez, L. C. et al. Stink bug predator kills prey with salivary non-proteinaceous compounds. Ins. Biochem. Molec. Biol. 68, 71–78 (2016).
Martinez, L. C., Plata-Rueda, A., Zanuncio, J. C., Tavares, W. S. & Serrão, J. E. Comparative morphology of the odoriferous system in three predatory stink bugs (Heterotpera: Asopinae). Protoplasma 254, 1965–1972 (2017).
Martinez, L. C., Fialho, M. C. Q., Zanuncio, J. C. & Serrão, J. E. Ultrastructure and cytochemistry of salivary glands of the predator Podisus nigrispinus (Hemiptera: Pentatomidae). Protoplasma 251, 535–543 (2014).
Ferreira, J. A. M., Zanuncio, J. C., Torres, J. B. & Molina-Rugama, A. J. Predatory behaviour of Podisus nigrispinus (Heteroptera: Pentatomidae) on different densities of Anticarsia gemmatalis (Lepidoptera: Noctuidae) larvae. Biocontrol Sci. Tech. 18, 711–719 (2008).
Fialho, M. C. Q. et al. Prey digestion in the midgut of the predatory bug Podisus nigrispinus (Hemiptera: Pentatomidae). J. Ins. Physiol. 58, 850–856 (2012).
Rodrigues, A. R. S., Serrão, J. E., Teixeira, V. W., Torres, J. B. & Teixiera, A. A. Spermatogenesis, changes in reproductive structures, and time constraint associated with insemination in Podisus nigrispinus. J. Ins. Physiol. 54, 1543–1551 (2008).
Huebner, E. & Anderson, E. A cytological study of the ovary of Rhodnius prolixus. I Ontogeny of the follicular epithelium. J. Morph. 136, 459–493 (1972).
Huebner, E. & Injeyan, H. S. Patency of the follicular epithelium in Rhodnius prolixus: A re-examination of the hormone response and technique refinement. Can. Zool. 58, 1617–1625 (1980).
Telfer, W. H., Huebner, E. & Smith, D. S. The cell biology of vitellogenic follicles in Hyalophora and Rhodnius in Insect Ultrastructure (eds King, R.C. & Akai, H.) 118–149 (Plenum Press, 1982).
Huebner, E. & Injeyan, H. Follicular modulation during oocyte development in an insect: Formation and modification of septate and gap junctions. Develop. Biol. 83, 101–113 (1981).
Flower, N. E. Septate and gap junctions between the epithelial cells of an invertebrate, the mollusc Cominella maculosa. J. Ultrastruc. Res. 37, 259–268 (1971).
Baskin, D. G. The fine structure of Polychaete septate junctions. Cell Tiss. Res. 174, 55–68 (1976).
Jonusaite, S., Donini, A. & Kelly, S. P. Occluding junctions of invertebrate epithelia. J. Comp. Physiol. 186B, 17–43 (2016).
Serrão, J. E., Marques-Silva, S. & Martins, G. F. The rectum of Oxaea flavescens (Andrenidae) has a specialized structure among bees. Micron 35, 245–253 (2004).
Skaer, H. L., Maddrell, S. H. P. & Harrison, J. B. The permeability properties of septate junctions in Malpighian tubules of Rhodnius. J. Cell Sci. 88, 251–265 (1987).
Cruz-Landim, C., Roat, T. C. & Berger, B. Fat body, hemolymph and ovary routes for delivery of substances to ovary in Melipona quadrifasciata anthidioides: Differences among castes through the use of electron-opaque tracers. Microscopy 62, 457–466 (2013).
Azevedo, D. O., Zanuncio, J. C., Delabie, J. H. C. & Serrão, J. E. Temporal variation of vitellogenin synthesis in Ectatomma tuberculatum (Formicidae: Ectatomminae) workers. J. Ins. Physiol. 57, 972–977 (2011).
O’Donnell, M. J., Maddrell, S. H. & Gardiner, B. O. Passage of solutes through walls of Malpighian tubules of Rhodnius by paracellular and transcellular routes. Am. J. Physiol. 246, 759–769 (1984).
Zhu, W. et al. Proteolitic breakdown of the Neb-trypsin modulating oostatic fator (Neb-TMOF) in the haemolymph of different insects and its gut epithelial transport. J. Ins. Physiol. 47, 1235–1242 (2001).
Jonusaite, S., Kelly, S. P. & Donini, A. Identification of the septate junction protein gliotactin in the mosquito Aedes aegypti: evidence for a role in increased paracellular permeability in larvae. J. Exp. Biol. 220, 2354–2363 (2017).
Laemmli, U. K. Cleavage of structural proteins during assembly of head of bacteriophage-T4. Nature 227, 680–685 (1970).
Acknowledgements
This research was supported by Brazilian research agencies CNPq, CAPES, FAPEMIG, FINEP and Programa Cooperativo sobre Proteção Florestal/PROTEF do Instituto de Pesquisas e Estudos Florestais/IPEF. We thanks to the Center of Microscopy and Microanalysis at Federal University of Viçosa for technical assistance.
Author information
Authors and Affiliations
Contributions
M.Q.A. and J.E.S. conceived and designed the experiments; M.Q.A., V.T.D. and L.L.d.O. performed the experiments; M.Q.A., V.T.D. and J.E.S. analyzed the data; M.Q.A., J.C.Z. and J.E.S. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Assis, M.Q., Dohanik, V.T., Oliveira, L.L.d. et al. Evidence for a transcellular route for vitellogenin transport in the telotrophic ovary of Podisus nigrispinus (Hemiptera: Pentatomidae). Sci Rep 9, 16441 (2019). https://doi.org/10.1038/s41598-019-52789-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-52789-z
This article is cited by
-
Bicaudal C is required for the function of the follicular epithelium during oogenesis in Rhodnius prolixus
Development Genes and Evolution (2021)
-
Morphology of the reproductive tract of females of leaf beetle Chrysomela populi (Chrysomelidae: Coleoptera)
Biologia (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.