Abstract
The elements constituting a layered double hydroxides material provide many alternatives for its optimization. Ten different layered double hydroxides materials with various combinations of Ni, Cu, Zn, Al, Cr, and Fe elements were studied as sorbent materials for phosphate ion. The type of element used in the layered double hydroxides affected the uptake capacity of phosphate. The influence of a specific element alone was not the primary role of enhancing the sorption performance of phosphate ion on the LDHs material. However, using specific two or three elements together is the key to achieve the best result due to synergistic effects. BET surface area of the sorbent showed no correlation with phosphate uptake. From the examined materials, Four layered double hydroxides of Cu-Zn-Cr, Zn-Cr, Ni-Al, and Cu-Ni-Cr showed high phosphate sorption capability. Sorption equilibrium isotherm, reaction kinetics, and desorption of phosphate from the sorbent materials were also investigated.
Similar content being viewed by others
Introduction
Pollution of water resources hinders access to clean water to meet the needs of the continuously growing world population. Phosphate resulting from agricultural runoffs, domestic sewage, and through the sewage generated from mining activities, is a major pollutant of water bodies. Phosphate creates significant problems in aquatic environments; because as a fertilizer, it causes the growth of algae and microorganisms known as eutrophication, leading to the depletion of dissolved oxygen and causing deterioration in the aquatic environment and water quality. Several studies have investigated methods to remove phosphorus from aqueous solutions; these include enhanced biological phosphorus adsorption, chemical precipitation, crystallization, ion exchange, and adsorption1,2. Among these methods, adsorption is an effective and promising technology regarding cost and ease of operation3,4.
Layered double hydroxides (LDHs) are a class of anionic clays that show high anion exchange capacity, making it a promising adsorbent material in many industrial applications. LDHs have been widely used as an adsorbent, ion exchange, or a catalyst material to remove organic and inorganic impurities from polluted water5,6,7,8. The structure of LDHs contains positively charged layers due to the presence of two metal hydroxides in different oxidation states, which retained its charge-balance by the presence of anions in the interlayer region9. Different studies that used LDHs for phosphate removal and recovery showed promising results10,11. However, most of these studies focused on using LDHs that consist of Mg and Al elements in their structure without investigating the possibilities of using different elements11,12,13,14,15,16. Other studies replaced Mg and Al elements with Zn and Fe17,18,19,20,21. LDHs can provide more options for elements that can be used for their synthesis9, which opens the door for tuning this material for phosphate removal and recovery from aqueous solution. Different variables can be utilized in the synthesis of LDHs to produce materials specifically designed for the required application. Adjusting LDHs is a critical factor for the preparation of high-performance sorption materials9,22. The refinement can be accomplished by altering of the metal cations, changing the proportions between divalent and trivalent ions, accommodating an appropriate anion into the interlayer space, controlling thermal activation, and stimulants by doping with different elements. A screening study for phosphate sorption using thirteen different LDHs and related structure shows a Zn-Fe-Zr combination with high potential for phosphate uptakes23.
Many natural copper deposits contain some amount of phosphate ion in its structure, like Turquoise, Coeruleolactite, Wooldridgeite, Cloncurryite, and Hentschelite mineral. Although copper may harm the environment, the tendency of copper elements to attract phosphate ions make it a potential ingredient candidate for the preparation of LDHs, whereas no previous study examines this possibility.
While LDHs naturally exist as mineral deposits, they are feasible and economically produced. Two different metal hydroxides with different oxidation state forming the layers of LDHs material produces a positive charge between these layers24. Balancing negatively charged anions resides the interlayer space to sustain its integrity. The general formula of the LDHs is expressed as [M1−x(II) Mx(III) (OH)2] Ax/nn− mH2O. Where An− is the anion occupying the interlayers space with water molecules, M(II) and M(III) is the divalent and trivalent metal ion, respectively. The primary mechanism that allows the sorption of anions by LDHs is the anion exchange with the interlayers LDHs’ anion24,25,26.
The objective of this study was to screen a different combination of divalent and trivalent ions incorporated in the LDHs structure for the best sorption removal of phosphate. Divalent ions consisting of Ni, Cu, Zn, and trivalent ions consisting of Al, Cr, and Fe were used to prepare the LDHs material, which gave a different combination of LDHs materials. The effects of some factors, such as time, initial phosphate concentration, and pH, were investigated. The composition of the LDHs sorbents was confirmed using inductively coupled plasma optical emission spectrometer, and the morphological structure was examined with X-ray diffraction.
Experimental
Preparation of sorbent materials
Different preparation routes for LDHs have been described in the literature; the most straightforward and widely used methods are coprecipitation25,27. In this method, solutions of divalent and trivalent elements containing the anion that is to be incorporated into the LDHs are used as precursors. The synthesis is performed under a condition of supersaturation, to ensure simultaneous precipitation of the two cations. Metals nitrates were used for making the first metal solutions of M(II) and M(III). Caustic soda was used as a base solution and was related to the metal solution according to the following factor, [NaOH] = 1.6 [M(II) + M(III)], with the interlayer anion of carbonate, [CO32−] = 2.0 [M(III)]. The metals solution and the base solution were mixed concurrently for 2 min; then the formed mixture was aged at 85 °C for 24 h. The precipitates were washed with distilled water to remove the remaining salts and then dried at 100 °C for 24 h28. The final solids were ground and passed through 0.25 mm sieve, then stored for later use. Obtaining pure LDHs was indicated by limiting the ratio M(II)/M(III) between 2 and 424,25. The exchange capacity of the LDHs could be increased by increasing the amount of the trivalent cations in the LDHs, which consequently increase the net positive charge in the interlayer region24. In this study, the ratio M(II)/M(III) was chosen to equal two to increase the amount of the trivalent cations and hence the anion exchange capacity.
Characterization of prepared sorbents
A sample of prepared material was digested by nitric acid, using a 1:1 weight ratio of nitric acid. The solution was heated for two hours at 90 °C to dissolve the metals. The concentration of metals was measured using an inductively coupled plasma optical emission spectrometer (ICP-OES, Varian 730-ES). An X-ray diffraction patterns were obtained using an X-ray diffractometer (Bruker-D8 Discover) equipped with Cu Kα radiation (λ = 1.5406 Å). Data were recorded over a 2θ range of 5°–70° with a step size of 0.02°. The test was run on a standard glass slide for the background correction. The specific surface area for the prepared samples was measured using a Micrometrics Tristar II surface area analyzer, by N2 adsorption, whereas degassing of the samples were conducted at 80 °C for each 0.3 g of the LDHs material. The concentration of phosphate ions in the solution phase was determined using the standard procedure of vanadomolybdo-phosphoric acid colorimetric method29 and a portable Spectrophotometer (HACH DR/3000).
Preparation of aqueous phosphate solution
A stock solution of phosphate (P), containing 1000 mg P/L, was prepared by dissolving potassium dihydrogen orthophosphate (KH2PO4) powder in distilled water. Working solutions of phosphate with different concentrations were prepared by diluting the stock solution with distilled water. The working solutions were used for batch sorption test with a pH of about 6.0 except for sorption test of pH-effects.
Procedural specification for batch sorption experiment
Firstly, for screening the best LDHs material for phosphate uptakes, a sample size of 200 mg of LDHs material was used. Then, 100 mL of phosphate solution containing 20 mg P/L was added to an Erlenmeyer flask and mixed with the LDHs. The experiments were performed at pH = 6 to approximate the real condition of pH neutrality. The flask was covered and placed on a shaker at 150 rpm for 24 h at room temperature of 23 °C, to ensure the equilibrium of phosphate concentration between the liquid phase and the solid phase. After the 24 h period, the suspension was filtered through 1.6-μm borosilicate glass microfiber filters and then analyzed for phosphate concentration. Secondly, for sorption isotherm, sorption kinetics, pH effects, and phosphate desorption performance, the detail of experimentation characteristics are shown in Table 1.
Measurement of phosphate uptake
Mass balances, as expressed in Eq. (1), were used to calculate the mass of phosphate adsorbed per unit mass of the LDHs material after equilibrium (Qe, mg P/g). V is the volume of solution (L); Ci and Ce are the initial and equilibrium concentration of phosphate in solution after adsorption process (mg/L), respectively; and m is the LDHs mass (g).
The affinity of dissolved phosphate to the LDHs material was evaluated using Freundlich and Langmuir isotherm models described in Eqs (2) and (3), respectively. Kf and KL represent the Freundlich isotherm capacity parameter ((mg/g)(L/mg)1/n) and the Langmuir bonding term related to interaction energies (L/mg), respectively; 1/n is the Freundlich isotherm intensity parameter (unitless); qmax denotes the Langmuir maximum capacity (mg/g).
Pseudo-first-order and pseudo-second-order kinetic models30 as expressed in Eqs (4) and (5), respectively, were used to estimate the rate of adsorption of phosphate on the LDHs material. Qt is the mass of phosphate adsorbed per unit mass of LDHs at time t (mg/g); k1 and k2 are the first-order and second-order reaction rate constants (1/h and g/mg/h), respectively.
Effects of pH and desorption study
The effect of pH on phosphate uptake was investigated at different pH values using HCl or NaOH solution, which reflect a range of acidic and basic solutions; Table 1 describes the conditions of the experiment. Desorption study was conducted for the exhausted LDHs material, whereas the LDHs was subjected to sorption with an initial phosphate concentration of 20 mg P/L using 400 mg of sorbent, more detail in Table 1. Two desorption solutions were used, a solution of nitric acid with pH = 4, and a solution of sodium hydroxide, as alkaline, with pH = 12.
Result and Discussion
Characteristics of prepared sorbents
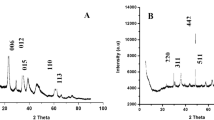
Combining the divalent ions of Ni, Cu, Zn with the trivalent ions of Al, Cr, Fe results in nine LDHs materials. However, based on Reichle31 study, Cu-LDHs require an additional divalent ion along with Cu to obtain the desired structure of LDHs. Accordingly, twelve different sorbent materials were formed (see Supplementary Fig. S1). However, after characterizing the synthesized material by XRD, Zn-Fe, and Cu-Zn-Fe compounds failed to acquire the morphology structure of the LDHs material. Figure 1 shows the XRD for the other ten LDHs materials. The type of synthesized LDHs along with their ICP-OES chemical composition and other characteristics are presented in Table 2. A good match between the chemical composition of the nominal and as-synthesized LDHs material was achieved. High variation of BET surface area for the prepared LDHs material had been observed, which could influence the sorption capacity of the LDHs; Cu-Zn-Cr compound showed the highest surface area that can be elucidated due to the formation of the smallest nanoparticle sized compared to other LDHs material. The attribute of the smallest particle size for Cu-Zn-Cr LDHs could be explained by the synergetic effect of these metals when used as precursors in the formation of the LDHs utilizing the coprecipitation method, more investigation is required.
Screening of phosphate uptake by different adsorbents
The results of phosphate removal by the different LDHs indicate the influence of the type of metal ions incorporated into the structure of the sorbent. Cu-Zn-Cr, Zn-Cr, Ni-Al, and Cu-Ni-Cr LDHs material showed higher sorption uptake of phosphate, Fig. 2. The chemical formula for these compounds can be written based on the general formula of LDHs described previously along with as-synthesized material shown in Table 2, i.e., \({{\rm{Cu}}}_{0.33}{{\rm{Zn}}}_{0.32}{{\rm{Cr}}}_{0.35}{({\rm{OH}})}_{2}{({{\rm{CO}}}_{3}^{2-})}_{0.17}\cdot {{\rm{mH}}}_{2}{\rm{O}}\). The nature of LDHs that consists of a layered structure of positive charge facilitate the attraction of the phosphate ion. Whereas there is always a competing anion for the interlayer site, the type of metals and their proportion in the LDHs structure play a key role in attracting the phosphate ion. Furthermore, the synergistic effect of the elements may influence the improvement of phosphate ion sorption. These synergistic effects could be observed when comparing the three LDHs of Ni-Al, Zn-Al, and Zn-Cr. While Zn-Al achieved less sorption of phosphate, Ni-Al and Zn-Cr achieved high sorption. The results in Table 2 and Fig. 2 reveal that the BET surface area had less influence than the type and composition of the sorbent, which implies a chemisorption mechanism had taken place in the sorption process of phosphate to the LDHs material.
LDHs metal ions showed a different affinity to adsorb phosphate. For the divalent ions, the affinity to phosphate has the following order Zn > Cu > Ni; and for the trivalent ions, the affinity order is Cr > Fe > Al as demonstrated in Fig. 3. The aluminum roles is mainly a structural aspect that guarantees the formation of the layered structure for LDHs material as observed by a previous study28. Of these four enhanced LDHs compounds, Cu-Zn-Cr LDHs was selected for more kinetics and sorption isotherm studies, as it achieves the higher phosphate uptakes.
Phosphate sorption isotherm
The results of phosphate sorption isotherm for Cu-Zn-Cr LDHs are shown in Fig. 4, where experimental data was compared to Freundlich and Langmuir isotherm, Eqs (2) and (3) respectively. The experimental data reveal that for an initial concentration of phosphate in solution (Ci) up to 15–20 mg/L, the sorbent was able to uptake all phosphate from solution, whereas the phosphates concentration after equilibrium was below 0.1 mg/L. Then, gradually with initial phosphate increased, the equilibrium concentration of phosphate (Ce) demonstrated a linearity relation with the quantity of sorbed phosphate in the LDHs material (Qe). As shown in Fig. 4, at higher values of the equilibrium concentration of phosphate, the Freundlich model provided a better fit to the experimental data; whereas, the Langmuir model was better at lower values of initial concentrations.
Phosphate sorption kinetics
The dependence of the adsorption rate on the concentration of the adsorbate is of particular interest. The phosphate sorption by the Cu-Zn-Cr LDHs as a function of time is shown in Fig. 5. The sorption rate was relatively faster in the first 20 min, and then gradually increased until it reached an almost stable value after 100–120 min due to saturation of adsorbent. As demonstrated from the figure, the pseudo-second-order reaction fitted well the experimental data compared to pseudo-first-order; with the reaction rate constant k2 = 0.53 g/mg/h and k1 = 4.13/h, respectively. This result indicates the inclination of the reaction towards chemisorption instead of physisorption reaction reflecting the influence of the type of element selected on the performance of the sorbent material.
The superiority Cu-Zn-Cr over the other LDHs compounds can be readily correlated to HSAB principle32. According to the HSAB concept, Cr3+ has high hardness acidity (high positive charge and low polarization) compared to Al3+ and Fe3+, which is more suited to adsorb hard bases like phosphate. The presence of Cr3+ in the LDHs compound shows an advantage over the other ions, excluding Ni-Cr, whereas the effect of the BET surface area lessens its sorption capacity.
Effect of pH on phosphate uptake
The Cu-Zn-Cr LDHs material was investigated for its performance to uptake phosphate for different pH values of the solution. As expected from a previous study33, the lower the pH value of the solution, the more capacity of LDHs sorbent material to uptake phosphate. The sorption capacity versus pH values of the solution is shown in Fig. 6, the phosphate uptake at pH = 5 is 50% greater than at pH = 11. This advantages of low pH solution were explained well by Chitrakar et al.34, as the OH− ion increases the surface charge will be more negative which will interfere and decrease the anion exchange capacity of LDHs material.
Desorption study
The exhausted Cu-Zn-Cr LDHs sorption material was tested for desorption of phosphate ion, whereas acidic and alkaline solutions were used. The alkaline solution desorbed 83% of the phosphate from the LDHs material, whereas the acidic solution desorbed only 39%. This result could be related to the incorporation of the hydroxides ions in the structure of the freshly prepared LDHs that gave the bases characteristic of this material35. The concentration of the Cu, Zn, and Cr metal ions were analyzed for the exhaust alkaline solution used for desorption study, whereas it was found 28.4 ± 2.5, 2.4 ± 0.2, 0.9 ± 0.1 mg/L, respectively. The copper ion seems to be likely to desorb with using an alkaline solution like NaOH, which contaminates the water and degrade the sorption material. However, as many methods could be used to remove copper ions contamination from water, the requirement for further treatment could render the recovery of phosphate ion economically infeasible. In this sense, the use of Zn-Cr LDHs material will be more justifiable where it shows excellent performance for phosphate sorption with stable Zn and Cr ions.
Conclusion
Incorporating different type of ions in the LDHs structure leads to a versatile sorbent material with better characteristics that target specific compounds. Phosphate sorption on LDHs of a different composition was studied in a batch system. Divalent ions of Ni, Cu, Zn and trivalent ions of Al, Cr, and Fe were considered as potential candidates to synthesize LDHs for better sorption and uptake of phosphate. Using Cu, Zn, and Cr in the LDHs structure showed the best performance of sorption of phosphate from its aqueous solution. The synergy between these three ions improved the sorption of phosphate and thus its separation from water bodies. However, due to contamination of the recovery solution, that used to regenerate the sorption material with copper, the copper ion is proposed to be eliminated, and the adopting of LDHs material consist of Zn-Cr will be preferable.
Freundlich isotherm showed the best fit of the affinity of dissolved phosphate to Cu-Zn-Cr LDHs, and a pseudo-second-order reaction rate expression best described the experimental data. Chemisorption process was concluded as the type of bonding between the phosphate and the LDHs material, whereas the BET area of the sorbent has less influence on phosphate uptake.
References
Ramasahayam, S. K., Guzman, L., Gunawan, G. & Viswanathan, T. A Comprehensive Review of Phosphorus Removal Technologies and Processes. J. Macromol. Sci. Part A 51, 538–545 (2014).
Everaert, M. et al. Phosphate-Exchanged Mg–Al Layered Double Hydroxides: A New Slow Release Phosphate Fertilizer. ACS Sustain. Chem. Eng. 4, 4280–4287 (2016).
De Gisi, S., Lofrano, G., Grassi, M. & Notarnicola, M. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sustain. Mater. Technol. 9, 10–40 (2016).
Ince, M. & Kaplan İnce, O. An Overview of Adsorption Technique for Heavy Metal Removal from Water/Wastewater: A Critical Review. Int. J. Pure Appl. Sci. 3, 10–19 (2017).
Koilraj, P. & Srinivasan, K. High Sorptive Removal of Borate from Aqueous Solution Using Calcined ZnAl Layered Double Hydroxides. Ind. Eng. Chem. Res. 50, 6943–6951 (2011).
Koilraj, P. & Kannan, S. Phosphate uptake behavior of ZnAlZr ternary layered double hydroxides through surface precipitation. J. Colloid Interface Sci. 341, 289–297 (2010).
Guo, Q. et al. Enhanced Removal of Arsenic from Water by Synthetic Nanocrystalline Iowaite. Sci. Rep. 7, 17546 (2017).
Cai, J., Zhang, Y., Qian, Y., Shan, C. & Pan, B. Enhanced Defluoridation Using Novel Millisphere Nanocomposite of La-Doped Li-Al Layered Double Hydroxides Supported by Polymeric Anion Exchanger. Sci. Rep. 8, 11741 (2018).
Debecker, D. P., Gaigneaux, E. M. & Busca, G. Exploring, Tuning, and Exploiting the Basicity of Hydrotalcites for Applications in Heterogeneous. Catalysis. Chem. - A Eur. J. 15, 3920–3935 (2009).
Das, J., Patra, B. S., Baliarsingh, N. & Parida, K. M. Adsorption of phosphate by layered double hydroxides in aqueous solutions. Appl. Clay Sci. 32, 252–260 (2006).
Li, R. et al. Enhancing phosphate adsorption by Mg/Al layered double hydroxide functionalized biochar with different Mg/Al ratios. Sci. Total Environ. 559, 121–129 (2016).
Bernardo, M. P., Moreira, F. K. V., Colnago, L. A. & Ribeiro, C. Physico-chemical assessment of [Mg-Al-PO4]-LDHs obtained by structural reconstruction in high concentration of phosphate. Colloids Surfaces A Physicochem. Eng. Asp. 497, 53–62 (2016).
Everaert, M. et al. Phosphate-Exchanged Mg-Al Layered Double Hydroxides: A New Slow Release Phosphate Fertilizer. ACS Sustain. Chem. Eng. 4, 4280–4287 (2016).
Khitous, M., Salem, Z. & Halliche, D. Removal of phosphate from industrial wastewater using uncalcined MgAl-NO 3 layered double hydroxide: batch study and modeling. Desalin. Water Treat. 57, 15920–15931 (2016).
Cheng, X., Wang, Y., Sun, Z., Sun, D. & Wang, A. Pathways of phosphate uptake from aqueous solution by ZnAl layered double hydroxides. Water Sci. Technol. 67 (2013).
Novillo, C. et al. Evaluation of phosphate removal capacity of Mg/Al layered double hydroxides from aqueous solutions. Fuel 138, 72–79 (2014).
Yang, K. et al. Adsorptive removal of phosphate by Mg-Al and Zn-Al layered double hydroxides: Kinetics, isotherms and mechanisms. Sep. Purif. Technol. 124, 36–42 (2014).
Cheng, X., Huang, X., Wang, X. & Sun, D. Influence of calcination on the adsorptive removal of phosphate by Zn-Al layered double hydroxides from excess sludge liquor. J. Hazard. Mater. 177, 516–523 (2010).
Koilraj, P. & Sasaki, K. Fe3O4/MgAl-NO3 layered double hydroxide as a magnetically separable sorbent for the remediation of aqueous phosphate. J. Environ. Chem. Eng. 4, 984–991 (2016).
Chen, X., Cheng, X., Sun, D., Ma, W. & Wang, X. Adsorptive removal of phosphate from secondary effluents in WWTPs by ZnAl layered double hydroxides granules. Desalin. Water Treat. 54, 1216–1225 (2015).
Sun, X. et al. Adsorption of phosphate using calcined Mg3–Fe layered double hydroxides in a fixed-bed column study. J. Ind. Eng. Chem. 20, 3623–3630 (2014).
Li, C., Wei, M., Evans, D. G. & Duan, X. Recent advances for layered double hydroxides (LDHs) materials as catalysts applied in green aqueous media. Catal. Today 247, 163–169 (2015).
Drenkova-Tuhtan, A. et al. Influence of cation building blocks of metal hydroxide precipitates on their adsorption and desorption capacity for phosphate in wastewater—A screening study. Colloids Surfaces A Physicochem. Eng. Asp. 488, 145–153 (2016).
Goh, K.-H., Lim, T.-T. & Dong, Z. Application of layered double hydroxides for removal of oxyanions: A review. Water Res. 42, 1343–1368 (2008).
Cavani, F., Trifirò, F. & Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 11, 173–301 (1991).
Mandel, K. et al. Layered double hydroxide ion exchangers on superparamagnetic microparticles for recovery of phosphate from waste water. J. Mater. Chem. A 1, 1840–1848 (2013).
Bagbi, Y., Sarswat, A., Mohan, D., Pandey, A. & Solanki, P. R. Lead and Chromium Adsorption from Water using L-Cysteine Functionalized Magnetite (Fe3O4) Nanoparticles. Sci. Rep. 7, 7672 (2017).
Zahid, W. M., Othman, M. A. & Abasaeed, A. E. Enhanced sulfur removal by a tuned composite structure of Cu, Zn, Fe, and Al elements. J. Hazard. Mater. 331, 273–279 (2017).
APHA. Standard Methods for the Examination of Water and Wastewater. (American Public Health Association, 2005).
Yuh-Shan, H. Citation review of Lagergren kinetic rate equation on adsorption reactions. Scientometrics 59, 171–177 (2004).
Reichle, W. T. Synthesis of anionic clay minerals (mixed metal hydroxides, hydrotalcite). Solid State Ionics 22, 135–141 (1986).
Chattaraj, P. K., Lee, H. & Parr, R. G. HSAB principle. J. Am. Chem. Soc. 113, 1855–1856 (1991).
Chitrakar, R. et al. Uptake properties of phosphate on a novel Zr-modified MgFe-LDH(CO3). J. Colloid Interface Sci. 349, 314–320 (2010).
Chitrakar, R. et al. Adsorption of phosphate from seawater on calcined MgMn-layered double hydroxides. J. Colloid Interface Sci. 290, 45–51 (2005).
Chibwe, M., Valim, J. B. & Jones, W. The Synthesis, Characteristics and Applications of Layered Double Hydroxides. In Multifunctional Mesoporous Inorganic Solids 191–206, https://doi.org/10.1007/978-94-015-8139-4_16 (Springer Netherlands, 1993).
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through the Research Project No. R17-03-70.
Author information
Authors and Affiliations
Contributions
S.A. designed and performed the experimental work, contributed to sample preparation and analysis of phosphate ion in the water. M.O. conceived and presented the idea, contributed to the characterization of the adsorbent material. All authors wrote the paper, discussed the results and contributed to the final manuscript.
Corresponding author
Ethics declarations
Competing interests
Both authors, S.A. and M.O., declare no competing interests as defined by Nature Research, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Almojil, S.F., Othman, M.A. Screening Different Divalent and Trivalent Metals Containing Binary and Ternary Layered Double Hydroxides for Optimum Phosphate Uptake. Sci Rep 9, 15511 (2019). https://doi.org/10.1038/s41598-019-52031-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-52031-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.