Abstract
We recently developed a partial peptide of receptor activator of nuclear factor-кB ligand (RANKL) known as microglial healing peptide 1 (MHP1-AcN), that inhibits Toll-like receptor (TLR)-related inflammation through RANKL/RANK signaling in microglia and macrophages without promoting osteoclast activation. The abnormal activation of TLRs contributes to the initiation and maintenance of psoriasis, which is a chronic inflammatory skin disease that involves the aberrant expression of proinflammatory cytokines and the subsequent dermal γδ T cell and T helper 17 (Th17) cell responses. The inhibition of TLR-mediated inflammation provides an important strategy to treat psoriasis. Here, we examined the preventative effects of MHP1-AcN in a mouse model of imiquimod (a TLR 7/8 agonist)-induced psoriasis. Topical imiquimod application induced psoriasis-like skin lesions on the ear and dorsal skin. Systemic administration of MHP1-AcN by daily subcutaneous injection significantly prevented the development of skin lesions, including erythema, scaling and thickening. Mice treated with MHP1-AcN showed reduced levels of skin Il6 mRNA at 32 h and reduced levels of Il23 and Il17a mRNA at d9. Serum levels of IL-6 and IL-23 were reduced at 32 h, and IL-17A was reduced at d9. These results indicated that MHP1-AcN could decrease imiquimod-induced IL-6, IL-23 and IL-17A production. MHP1-AcN is potentially an alternative treatment for psoriasis.
Similar content being viewed by others
Introduction
Psoriasis is one of the most common immune-mediated disorders, and crosstalk between components of the innate and adaptive immune systems greatly contributes to its pathogenesis1. Imiquimod (IMQ), a Toll-like receptor (TLR) 7/8 agonist, exacerbates psoriasis in patients and causes psoriasis-like dermatitis in mice2. TLR activation triggers macrophages and dendritic cells to produce IL-23, IL-1β and other proinflammatory cytokines, including IL-6 and TNF-α3. IL-6 has been associated with macrophage activation, Th17 cell differentiation, and inhibition of regulatory T cell (Treg) activity during the development of psoriasis4,5. IL-23 is predominantly secreted by dendritic cells and macrophages to stimulate dermal γδ T cell activation and expansion and positively regulate differentiation or effector function of Th17 cells6,7. Dermal γδ T cells have been demonstrated to be the major IL-17 producers in mouse skin8. IL-17 acts on keratinocytes to induce hyperproliferation, contributing to the pathogenesis of psoriasis9,10. Additionally, antagonists of TLR7/8/9 have been demonstrated to inhibit Th1 and Th17 responses in a model of IL-23-induced psoriasis11. Thus, inhibition of TLR-mediated inflammation may provide an effective treatment strategy for psoriasis. Receptor activator of nuclear factor-кB ligand (RANKL) is one such candidate molecule since it can inhibit TLR-mediated inflammation in activated macrophages12 and microglia13. Additionally, a recent report showed that epidermal RANKL/RANK signaling changes dendritic cell function to maintain the number of peripheral Tregs and suppress allergic contact hypersensitivity responses and the development of systemic autoimmunity14. In addition, the developmental pathways of Th17 cells and Tregs are reciprocally regulated and influence the outcome of autoimmune and inflammatory diseases, including psoriasis. Thus, we speculated that RANKL/RANK signaling may improve psoriasis by inhibiting TLR-mediated inflammation and consequently decrease IL-17A production by dermal γδ T cells.
However, systemic administration of recombinant RANKL induces osteoporosis15, which is a problem for clinical applications. To solve this problem, we developed a novel peptide, microglia healing peptide-1 (MHP1), which is a partial RANKL peptide. This peptide was shown to inhibit TLR2-, 4-, and 7/8-related inflammation through the RANK signaling pathway without inducing osteoclast differentiation16,17. The peptide includes the DE loop and part of the EF loop, which is the binding site of RANKL for its receptor, RANK, but it does not include the AA’ and CD loops that are responsible for osteoclastogenesis. We further modified MHP1 with N-terminal acetylation and C-terminal amidation (MHP1-AcN), which increased its stability and strengthened its inhibitory effects on TLR-mediated inflammation in microglia/macrophages18. Systemic administration of MHP1 and MHP1-AcN has been shown to be effective for the treatment of ischemic stroke in mice16,18. Therefore, we hypothesized that this peptide may be a novel agent for the treatment of psoriasis.
Here, we examined the preventative effects of different doses of MHP1-AcN on psoriatic skin lesions and IL-17A production and determined the optimum dose in a mouse model of IMQ-induced psoriasis. The influence of MHP1-AcN on the IMQ-induced cytokines IL-6 and IL-23 in mouse skin and serum was assessed. Moreover, we examined the inhibitory effects of MHP1-AcN on IL-6 production in the R837 (TLR7 agonist)-stimulated macrophage cell line.
Results
Systemic administration of MHP1-AcN inhibited the development of IMQ-induced psoriasis-like skin lesions and IL-17A production
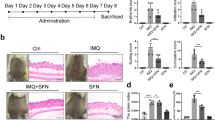
IMQ cream was applied to the shaved dorsal skin and right ears of BALB/c mice for 8 consecutive days. MHP1-AcN (0.1, 1, 10, 100 µg/mouse) or saline was systemically administered by daily subcutaneous injection at a distant site from IMQ application. Dorsal skin erythema and scales were measured on a four-point scale. Dorsal skin thickness was measured and calculated as the percentage change from baseline (Fig. 1A). Ear thickness was recorded on days 0–8 (Fig. 1B). Higher doses of MHP1-AcN resulted in reduced erythema and scale scores and less dorsal skin and ear thickening. However, a higher dose of MHP1-AcN (250 µg/mouse) did not further inhibit the development of IMQ-induced skin lesions (Supplementary Fig. 1). Because topical application of IMQ triggers TLR-mediated inflammation that activates dermal γδ T cells to produce IL-17A2, which plays a critical role in the pathogenesis of psoriasis, we next examined serum IL-17A levels in mice. Higher doses of MHP1-AcN showed a tendency to reduce serum IL-17A production without significant difference (Fig. 1C), whereas a much higher dose of MHP1-AcN (250 µg/mouse) did not further reduce IL-17A production (Supplementary Fig. 1). Therefore, we determined the optimum dose of MHP1-AcN (100 µg/mouse) for psoriasis treatment.
Systemic administration of MHP1-AcN inhibited the development of IMQ-induced skin lesions and IL-17A production. MHP1-AcN (0.1, 1, 10, or 100 µg/mouse) or saline was systemically administered by daily subcutaneous injection at a distant site from IMQ application. MHP1-AcN was injected immediately before IMQ application. (A) Dorsal skin erythema and scales were recorded and measured on a four-point scale (0 = none; 1 = slight; 2 = moderate; 3 = marked; and 4 = very marked). Dorsal skin thickness was measured using a digital caliper and calculated as the percentage change from baseline. (B) Right ear thickness from day 0–8. (C) Mice were sacrificed on d8, and serum IL-17A levels were measured by ELISA. N = 3 per normal group; N = 4 per saline-treated IMQ group; N = 4 per MHP1-AcN-treated IMQ group. All values are expressed as the mean with SEM.
MHP1-AcN inhibited IMQ-induced proinflammatory cytokine expression
We next examined IMQ-induced skin inflammation. MHP1-AcN (100 µg/mouse) or saline was systemically administered by daily subcutaneous injection at a distant site from IMQ application. As expected, MHP1-AcN inhibited the development of IMQ-induced skin erythema, scaling, and thickening (Fig. 2A). Hematoxylin and eosin (H&E)-stained IMQ-stimulated dorsal skin was characterized by increased epidermal thickening, parakeratosis, elongated rete ridges, and a mixed cellular infiltrate compared to normal skin (Fig. 2B). Thickening was calculated as the area of epidermis (µm2)/the length of the basement membrane (µm), and the inflammatory infiltrate was quantified as the number of infiltrated cells/(0.03 mm2). MHP1-AcN treatment decreased epidermal thickening and reduced inflammatory infiltrates compared to IMQ with saline-treated mice (Fig. 2B,C). These results suggest that MHP1-AcN inhibited IMQ-induced skin inflammation.
The optimum dose of MHP1-AcN inhibited the development of IMQ-induced skin lesions and inflammatory infiltrates in skin. MHP1-AcN (100 µg/mouse) or saline was systemically administered by daily subcutaneous injection at a distant site from IMQ application. (A) Dorsal skin erythema and scales were recorded and measured on a four-point scale (0 = none; 1 = slight; 2 = moderate; 3 = marked; and 4 = very marked). Dorsal skin thickness was measured using a digital caliper and calculated as the percentage change from baseline. (B) Representative H&E-stained section of dorsal skin. Scale bar: 100 μm. The white rectangular area in the left lower corner in each staining shows a magnified image of the corresponding area. (C) Thickness was calculated as the area of epidermis (µm2)/the length of the basement membrane (µm) in more than 10 fields. Inflammatory infiltrate in the dermis was quantified and shown as the number of infiltrated cells/(0.03 mm2). **P < 0.01 vs. the saline-treated IMQ group. N = 3 per normal group; N = 4 per saline-treated IMQ group; N = 4 per MHP1-AcN-treated IMQ group. All values are expressed as the mean with SEM.
Proinflammatory cytokines secreted by macrophages and dendritic cells contribute to the pathogenesis of psoriasis. Among these cytokines, IL-6 has been reported to be present at high levels in psoriatic skin and in the plasma of psoriasis patients, suggesting its critical role in psoriasis19. In addition to IL-6, a transient increase in IL-23 followed by IL-17A production was observed in the IMQ-induced psoriasis model2. Moreover, IMQ-induced dermatitis was almost completely blocked in IL-23- or IL-17RA-deficient mice2, proving the pivotal role of the IL-23/IL-17 axis in the pathogenesis of psoriasis9. In another IMQ-induced psoriasis study, IMQ-induced Il6 and Il23 mRNAs were highly expressed on day 2, whereas Il17a was highly expressed on day 420. Thus, we examined IL-6, IL-23, and IL-17A expression at 32 h (early stage) and d9 (late stage).
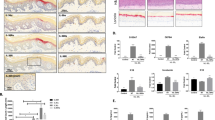
Systemic administration of MHP1-AcN inhibited IMQ-induced skin Il6 mRNA expression at 32 h, but the result at d9 was not significant. Interestingly, skin Il23 and Il17a mRNA expression was inhibited at d9, but not at 32 h (Fig. 3A). In serum, MHP1-AcN inhibited IMQ-induced IL-6 and IL-23 production at 32 h, whereas IL-17A was only inhibited at d9 (Fig. 3B). IL-6 or IL-23 was not detected at d9.
Systemic administration of MHP1-AcN inhibited IMQ-induced IL-6, IL-23 and IL-17A expression. MHP1-AcN (100 µg/mouse) or saline was systemically administered by daily subcutaneous injection at a distant site from IMQ application. Mice were sacrificed at 32 h or d9 in two independent experiments. Dorsal skin samples where IMQ was applied and serum samples were collected. (A) The mRNA expression of Il6, Il23, and Il17a at 32 h and d9 in dorsal skin was analyzed by real-time PCR. (B) Serum IL-6, IL-23, and IL-17A levels at 32 h and d9 were measured by ELISA. *P < 0.05, **P < 0.01 vs. the saline-treated IMQ group. 32 h: N = 3 per normal group; N = 4 per saline-treated IMQ group; N = 4 per MHP1-AcN-treated IMQ group, d9: N = 3 per normal group; N = 4 per saline-treated IMQ group; N = 4 per MHP1-AcN-treated IMQ group. All values are expressed as the mean with SEM.
MHP1-AcN inhibited R837 (TLR7 agonist)-induced IL-6 production in RAW 264.7 cells
We previously showed that MHP1 inhibited R848 (an analog TLR7/8 agonist)-induced IL-6 production in microglial cells16, and MHP1-AcN significantly inhibited lipopolysaccharide (LPS)-induced IL-6 at concentrations of 1 and 10 µg/mL18. RAW 264.7 cells were treated with 1 µg/mL of R837 (a TLR7 agonist) and MHP1-AcN (0.1, 1, 10 µg/mL) at the same time and incubated for 24 h, and supernatants were collected and assayed for IL-6 production. MHP1-AcN inhibited R837-induced IL-6 production in RAW 264.7 cells at a concentration of 10 µg/mL (Fig. 4).
MHP1-AcN inhibited R837-induced IL-6 production in RAW 264.7 cells. RAW 264.7 cells were stimulated with R837 (1 µg/mL) in the presence or absence of MHP1-AcN (0.1, 1, or 10 µg/mL) at the same time for 24 h. Cultured medium was collected and analyzed for IL-6 production by ELISA. *P < 0.05 vs. the group treated with R837 without MHP1-AcN. N = 4 per group. All values are expressed as the mean with SEM.
Discussion
In this study, we demonstrated that the novel, modified peptide MHP1-AcN, which was structurally designed from RANKL and modified with N-terminal acetylation and C-terminal amidation to improve its stability and effectiveness, significantly prevented the development of IMQ-induced psoriasis in mice.
Previous studies showed that MHP1-AcN is a partial agonist of RANK, and it can decrease the TLR2-, TLR4-, and TLR7/8-induced inflammatory cytokines in the microglial cell line MG6, as well as the TLR4-induced inflammatory cytokines in the macrophage cell line RAW264.716,17. In psoriasis, IL-6 and IL-23 are proinflammatory cytokines secreted by activated dendritic cells and macrophages in response to pathogen components or damage-associated molecular patterns (DAMPs) via TLRs and are able to induce dermal γδ T cell activation and expansion and Th17 cell differentiation, contributing to the initiation and maintenance of psoriasis4,8,21. In the present study, MHP1-AcN was shown to inhibit TLR7/8 agonist-induced IL-6 production in RAW 264.7 cells and in a mouse model of psoriasis at the early stage of disease. Compared to a recent study showing that an approximately 40% reduction in IL-6 in mice treated with cycloastragenol resulted in better clinical outcomes22, a 71.1% decrease in serum IL-6 by MHP1- AcN at the early stage might be enough to be associated with better clinical outcomes. There is evidence supporting the beneficial effects of IL-6 inhibition in the treatment of psoriasis. For example, psoriasis-like skin inflammation induced by intradermal injection of recombinant IL-23 is abrogated in IL-6 knockout mice23. However, the IL-6 blockade strategy shows few consistent beneficial effects when used to treat plaque-type psoriasis and psoriatic arthritis (PsA). For example, the humanized anti-IL-6 receptor monoclonal antibody, tocilizumab (Actemra), and the humanized anti-IL-6 monoclonal antibody, clazakizumab, do not improve psoriatic skin lesions24,25,26,27, and in fact, they even exacerbate the lesions in some patients26,28. A possible reason for these findings is that the therapeutic blockade of IL-6 may lead to overcompensation by other proinflammatory cytokines in the skin, which may abrogate the benefits of anti-IL-6 agents5,29. In contrast to antibodies against IL-6 or the IL-6 receptor, MHP1-AcN inhibited not only the expression of IL-6 but also IL-23, which emerged as an attractive therapeutic target for moderate-to severe psoriasis by using anti-IL-23 antibodies, such as guselkumab, tildrakizumab and risankizumab7. Although the direct action of MHP1-AcN on IL-23 and its effects in the IL-23-induced psoriasis model need further studies, one of the preventative effects of MHP1-AcN on psoriasis may be associated with the inhibition of TLR7/8-induced IL-6 and IL-23 expression.
Dermal γδ T cells are the main source of IL-17 upon IL-23 stimulation, which is predominantly secreted by skin dendritic cells and macrophages in the IMQ-induced psoriasis model8. Therefore, limited IL-17A production is one of the consequences of restricted macrophage activation by MHP1-AcN treatment in the present study. The action of MHP1-AcN in dendritic cells and other mechanisms regulating γδ T cell responses, such as the expression of CD69, BTLA, and PD-1, need further study.
Although IMQ-induced psoriasis recapitulates limited aspects of human psoriasis and is highly dependent on a prevalent population of dermal γδ T cells, which is a minor population in human skin30, the activation of TLR7/8 in human dendritic cells was reported to contribute to psoriasis31, and TLR7/8/9 antagonists reduced moderate-to-severe plaque psoriasis in a phase IIa trial32. Based on the aforementioned evidence, MHP1-AcN may also be effective for human psoriasis.
Considering that the plasma half-life of intravenously injected MHP1-AcN has been reported to be less than 10 min18, we speculate that the half-life of subcutaneously injected MHP1-AcN will also be short. However, the therapeutic effects of MHP1-AcN were demonstrated after a single subcutaneous injection each day. This is probably because some of the degradation products in the plasma include key sequences needed for anti-inflammatory effects16 and these key sequences may still be able to affect immune cells. A similar effect has been reported for glatiramer acetate, which is a copolymer of four amino acids with specific stoichiometry and ranging in length from 40 to 90 amino acids. Glatiramer acetate has shown efficacy in treating multiple sclerosis, despite its very short half-life33.
Psoriasis usually precedes PsA onset by an average of 10 years. Proinflammatory cytokines, such as IL-33, osteopontin (OPN), IL-17, and TNF-α, are involved in both psoriasis and PsA pathogenesis as well as in bone homeostasis. These cytokines induce the release of a wide range of pro-osteoclastogenic factors in the skin, such as RANKL. Furthermore, RANKL serum levels and osteoclast number and activity are influenced by the severity of skin lesions in psoriatic patients with and without arthritis34. We previously reported that MHP1 could inhibit RANKL-induced osteoclast differentiation17. MHP1-AcN treatment may prove advantageous for psoriatic arthritis, which needs further study.
Taken together, we showed that the systemic administration of the novel, modified partial agonist of RANKL, MHP1, with N-terminal acetylation and C-terminal amidation (MHP1-AcN), could inhibit the development of IMQ-induced psoriatic skin lesions and IL-6, IL-23, and IL-17A production by inhibiting TLR-mediated inflammation. Although the exact molecular mechanisms and appropriate long-term risk-benefit effects need further study, the inhibitory effects of MHP1-AcN on IMQ-induced psoriasis and RANKL-induced osteoclast activation make it a promising agent for psoriatic diseases.
Methods
Peptide design and synthesis
Synthetic MHP1-AcN (Ac-LMVYVVKTSIKIPSSHNLMKGGSTKNWSGN-NH2) was purchased from ILS, Inc. (Ibaragi, Tsukuba, Japan) and dissolved in double-distilled H2O to make a 2 mg/mL solution and stored at 4 °C until use.
IMQ-induced psoriasis model in mice
All experiments were approved by the Institutional Animal Care and Use Committee of Osaka University (27-020-032) and conducted in accordance with the Osaka University Guidelines, which are based on the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals, and with the ARRIVE guidelines. All procedures were performed under isoflurane anesthesia, and all efforts were made to minimize suffering. Nine-week-old male BALB/c mice, that weighed 22–23 g, were purchased from CLEA Japan, Inc. (Tokyo, Japan). Each mouse received a topical dose of 62.5 mg of commercially available 5% IMQ cream (Aldara; 3 M Pharmaceuticals; Maplewood, MN, USA) on the shaved back and the right ear for 8 or 9 consecutive days. Because our previous study showed the effectiveness of intravenous injection of MHP1-AcN at 75–300 µg/mouse in a stroke model18 and others showed the effectiveness and safety of subcutaneous injection of 3435 or 2536 amino acids of synthetic peptides at 0.3, 1, 3, and 10 µg/mouse35 or 150 µg/mouse36, MHP1-AcN groups were subcutaneously injected (distant site from IMQ application) with 0.1, 1, 10, 100 µg or 250 µg of MHP1-AcN daily for 8 or 9 days immediately before IMQ application. Considering that the mice weighed 22–23 g, we believe that the influence of body weight was small. As a control for MHP1-AcN treatment, 0.45% saline was injected in a similar manner. The scores of erythema and scaling on the dorsal skin at the site of IMQ application were measured on a four-point scale (0 = none; 1 = slight; 2 = moderate; 3 = marked; and 4 = very marked). The relative increase in dorsal skin thickness was measured using a digital caliper and calculated as the percentage change from the baseline thickness. The thickness of the ear was also measured using a digital caliper. Although each animal experiment was performed once, the effectiveness of MHP1-AcN (100 µg/mouse) was examined three times (Figs 1, 2 and Supplemental Fig. 1). Sixty-eight mice were examined in this study.
Cell culture
RAW 264.7 cells were obtained from the RIKEN Gene Bank (Tsukuba, Japan) and maintained in 5% CO2 at 37 °C in Dulbecco’s modified Eagle medium (DMEM; Nacalai Tesque, Kyoto, Japan) supplemented with 10% fetal bovine serum (FBS; Thermo Fisher Scientific, Waltham, MA, USA). Cells (4 × 105 cells) were plated in 96-well plastic culture dishes. After overnight culture, the medium was replaced with DMEM supplemented with 4% FBS. R837 (Imiquimod; InvivoGen, San Diego, CA, USA) and MHP1-AcN were added at the same time to the medium, which was then harvested 24 h after stimulation. The final concentration of R837 was 1 µg/mL, following product information. The concentrations of MHP1-AcN were 0.1, 1, and 10 μg/mL because MHP1-AcN at 1 and 10 μg/mL exerted a significant inhibitory effect on LPS-induced inflammation in microglial cells, as previously reported18.
Enzyme-linked immunosorbent assay (ELISA)
The concentrations of IL-6, IL-23, and IL-17A were measured using the following commercially available ELISA kits: IL-6, Mouse IL-6 Quantikine ELISA Kit (R&D Systems, Minneapolis, MN, USA); IL-23, Mouse IL-23 Quantikine ELISA Kit (R&D systems); and IL-17A, Mouse IL-17A Quantikine ELISA Kit (R&D Systems). The serum concentration of each cytokine was determined in duplicate for each mouse sample. The IL-6 concentration in the macrophage culture medium was also analyzed in duplicate for each experimental condition.
Real-time reverse transcription polymerase chain reaction (qRT-PCR)
Dorsal skins were collected at 32 h and d9 after IMQ application. mRNA was isolated using an RNeasy Fibrous Tissue Mini Kit (QIAGEN, Germantown, MD, USA) according to the manufacturer’s instructions. cDNA was synthesized using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions. The oligonucleotide primers were purchased according to the following identifications: Il6, Mm00446190; Il23, Mm00518984; Il17a, Mm00439618; and Gapdh, Mm99999915 (Applied Biosystems). The 5′ nuclease assay PCRs were performed in a MicroAmp Optical 384-well reaction plate using an ABI PRISM 7900 Sequence Detection System (Applied Biosystems). The expression levels of the target genes were quantified with triplicate repeated wells by comparing the fluorescence generated by each example with that of a serially diluted standard and were then normalized to the level of Gapdh expression in each individual sample. We selected the reference gene according to previous studies2,20.
Histological analysis
Mice were perfused with 4% paraformaldehyde (PFA), and the dorsal skin was fixed in 10% neutral-buffered formalin and then processed and embedded in paraffin blocks. Samples were cut into 4-μm-thick sections using a rotary microtome and stained with H&E (Muto Pure Chemicals Co., Ltd.; Tokyo; Japan). Stained sections were observed using a digital microscope (FSX-100; Olympus, Tokyo, Japan). Acanthosis was calculated as the area of epidermis (µm2)/the length of the basement membrane (µm) using ImageJ (National Institutes of Health, Bethesda, MD, USA). Inflammatory infiltrate was quantified and shown as the number of infiltrated cells/(0.03 mm2).
Statistical analysis
All values are expressed as the mean ± standard error of the mean (SEM). Multiple comparisons were evaluated by ANOVA, followed by Dunnett’s multiple comparison test. Two groups were compared using an unpaired t test. Differences were considered significant at p < 0.05. Statistical analyses were performed using the software Prism 6.0 (GraphPad, Inc.; San Diego; CA; USA).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Lowes, M. A., Bowcock, A. M. & Krueger, J. G. Pathogenesis and therapy of psoriasis. Nature 445, 866–873 (2007).
van der Fits, L. et al. Imiquimod-Induced Psoriasis-Like Skin Inflammation in Mice Is Mediated via the IL-23/IL-17 Axis. J. Immunol. 182, 5836–5845 (2009).
Hari, A., Flach, T. L., Shi, Y. & Mydlarski, P. R. Toll-like receptors: role in dermatological disease. Mediators Inflamm. 2010, 437246 (2010).
Cai, Y., Fleming, C. & Yan, J. New insights of T cells in the pathogenesis of psoriasis. Cell. Mol. Immunol. 9, 302–309 (2012).
Blauvelt, A. IL-6 Differs from TNF-α: Unpredicted Clinical Effects Caused by IL-6 Blockade in Psoriasis. J. Invest. Dermatol. 137, 541–542 (2017).
Tesmer, L. A., Lundy, S. K., Sarkar, S. & Fox, D. A. Th17 cells in human disease. Immunol. Rev. 223, 87–113 (2008).
Fotiadou, C., Lazaridou, E., Sotiriou, E. & Ioannides, D. Targeting IL-23 in psoriasis: current perspectives. Psoriasis (Auckland, N.Z.) 8, 1–5 (2018).
Cai, Y. et al. Pivotal Role of Dermal IL-17-Producing γδ T Cells in Skin Inflammation. Immunity 35, 596–610 (2011).
Schön, M. P. & Erpenbeck, L. The Interleukin-23/Interleukin-17 Axis Links Adaptive and Innate Immunity in Psoriasis. Front. Immunol. 9, 1323 (2018).
Nestle, F. O., Kaplan, D. H. & Barker, J. Mechanisms of disease psoriasis. N. Engl. J. Med. 30, 773–773 (2009).
Jiang, W. et al. A Toll-Like Receptor 7, 8, and 9 Antagonist Inhibits Th1 and Th17 Responses and Inflammasome Activation in a Model of IL-23-Induced Psoriasis. J. Invest. Dermatol. 133, 1777–1784 (2013).
Maruyama, K. et al. Receptor activator of NF-kappa B ligand and osteoprotegerin regulate proinflammatory cytokine production in mice. J. Immunol. 177, 3799–805 (2006).
Shimamura, M. et al. OPG/RANKL/RANK axis is a critical inflammatory signaling system in ischemic brain in mice. Proc. Natl. Acad. Sci. 111,8191–8196 (2014).
Loser, K. et al. Epidermal RANKL controls regulatory T-cell numbers via activation of dendritic cells. Nat. Med. 12, 1372–1379 (2006).
Tomimori, Y. et al. Evaluation of Pharmaceuticals With a Novel 50-Hour Animal Model of Bone Loss*. J. Bone Miner. Res. 24, 1194–1205 (2009).
Shimamura, M. et al. Therapeutic Effects of Systemic Administration of the Novel RANKL-Modified Peptide, MHP1, for Ischemic Stroke in Mice. Biomed Res. Int. 2018, 1–9 (2018).
Kurinami, H. et al. A Novel Therapeutic Peptide as a Partial Agonist of RANKL in Ischemic Stroke. Sci. Rep. 6, 1–11 (2016).
Shimamura, M. et al. Development of a novel RANKL-based peptide, microglial healing peptide1-AcN (MHP1-AcN), for treatment of ischemic stroke. Sci. Rep. 8, 1–10 (2018).
Grossman, R. M. et al. Interleukin 6 is expressed in high levels in psoriatic skin and stimulates proliferation of cultured human keratinocytes. Proc. Natl. Acad. Sci. USA 86, 6367–6371 (1989).
Funakoshi, A. et al. Cholecystokinin Downregulates Psoriatic Inflammation by Its Possible Self-Regulatory Effect on Epidermal Keratinocytes. J. Immunol. 202, 2609–2615 (2019).
Saggini, A., Chimenti, S. & Chiricozzi, A. IL-6 as a Druggable Target in Psoriasis: Focus on Pustular Variants. J. Immunol. Res. 2014, 1–10 (2014).
Deng, G. et al. Inhibition of NLRP3 inflammasome-mediated pyroptosis in macrophage by cycloastragenol contributes to amelioration of imiquimod-induced psoriasis-like skin inflammation in mice. Int. Immunopharmacol. 74, 105682 (2019).
Lindroos, J. et al. IL-23-Mediated Epidermal Hyperplasia Is Dependent on IL-6. J. Invest. Dermatol. 131, 1110–1118 (2011).
Grasland, A., Mahé, E., Raynaud, E. & Mahé, I. Psoriasis onset with tocilizumab. Jt. Bone Spine 80, 541–542 (2013).
Laurent, S., Le Parc, J.-M., Clérici, T., Bréban, M. & Mahé, E. Onset of psoriasis following treatment with tocilizumab. Br. J. Dermatol. 163, 1364–1365 (2010).
Wendling, D., Letho-Gyselinck, H., Guillot, X. & Prati, C. Psoriasis onset with tocilizumab treatment for rheumatoid arthritis. J. Rheumatol. 39, 657 (2012).
Mease, P. J. et al. The Efficacy and Safety of Clazakizumab, an Anti-Interleukin-6 Monoclonal Antibody, in a Phase IIb Study of Adults With Active Psoriatic Arthritis. Arthritis Rheumatol. 68, 2163–2173 (2016).
Palmou-Fontana, N., Sánchez Gaviño, J. A., McGonagle, D., García-Martinez, E. & Iñiguez de Onzoño Martín, L. Tocilizumab-Induced Psoriasiform Rash in Rheumatoid Arthritis. Dermatology 228, 311–313 (2014).
Fritz, Y. et al. Induction of Alternative Proinflammatory Cytokines Accounts for Sustained Psoriasiform Skin Inflammation in IL-17C+IL-6KO Mice. J. Invest. Dermatol. 137, 696–705 (2017).
Hawkes, J. E., Gudjonsson, J. E. & Ward, N. L. The Snowballing Literature on Imiquimod-Induced Skin Inflammation in Mice: A Critical Appraisal. J. Invest. Dermatol. 137, 546–549 (2017).
Ganguly, D. et al. Self-RNA–antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J. Exp. Med. 206, 1983–1994 (2009).
Balak, D. M. W. et al. IMO-8400, a toll-like receptor 7, 8, and 9 antagonist, demonstrates clinical activity in a phase 2a, randomized, placebo-controlled trial in patients with moderate-to-severe plaque psoriasis. Clin. Immunol. 174, 63–72 (2017).
Skotnicki, J. S. & Huryn, D. M. Treatment of Transplantation Rejection and Multiple Sclerosis. Compr. Med. Chem. II 917–934 (2007).
Raimondo, A. et al. Psoriatic cutaneous inflammation promotes human monocyte differentiation into active osteoclasts, facilitating bone damage. Eur. J. Immunol. 47, 1062–1074 (2017).
Yates, A. J. et al. Effects of a synthetic peptide of a parathyroid hormone-related protein on calcium homeostasis, renal tubular calcium reabsorption, and bone metabolism in vivo and in vitro in rodents. J. Clin. Invest. 81, 932–8 (1988).
Kolonin, M. G., Saha, P. K., Chan, L., Pasqualini, R. & Arap, W. Reversal of obesity by targeted ablation of adipose tissue. Nat. Med. 10, 625–632 (2004).
Acknowledgements
This work was supported by the Program for Creating Start-ups from Advanced Research and Technology (START Program), the Translational Research Program, and the Strategic Promotion for Practical Application of Innovative Medical Technology (TR-SPRINT) from the Japan Agency for Medical Research and Development, AMED (to M.S.).
Author information
Authors and Affiliations
Contributions
M. Shimamura and H. Nakagami designed the experiments; M. Shimamura, N. Ju, H. Hayashi and Y. Ikeda performed the experiments; M. Shimamura, N. Ju, A. Nakamura and S. Yoshida analyzed the data; N. Ju, M. Shimamura, H. Rakugi and R. Morishita wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
A patent application has been filed for the MHPs (Application No. 2015-102502 and PCT/JP2016/064446; M.S., H.N. and R.M.). The Department of Clinical Gene Therapy is financially supported by Novartis, AnGes, Shionogi, Boeringher, Fancl, Saisei Mirai Clinics, Rohto, and Funpep. The Department of Health Development and Medicine is financially supported by AnGes, Funpep, and DAICEL. R.M. is a founder and shareholder of AnGes and a former board member. R.M. is also a shareholder of Funpep. M.S. and H.N. are shareholders of MH Peptide. This company has a contract for priority rights of negotiation regarding the MHP-1 patent with Osaka University. There are no conflicts of interest between any authors and MH Peptide, as adjudicated by the Conflict of Interest Committee at Osaka University. The company was not involved in the “design and conduct of the study,” “collection, management, analysis, and interpretation of data,” “preparation, review, or approval of the manuscript,” or the “decision to submit the manuscript for publication”.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ju, N., Shimamura, M., Hayashi, H. et al. Preventative effects of the partial RANKL peptide MHP1-AcN in a mouse model of imiquimod-induced psoriasis. Sci Rep 9, 15434 (2019). https://doi.org/10.1038/s41598-019-51681-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-51681-0
This article is cited by
-
Prevention of bleomycin-induced pulmonary fibrosis by a RANKL peptide in mice
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.