Abstract
Tanshinones are important bioactive components in Salvia miltiorrhiza and mainly accumulate in the periderms of mature roots. Tanshinone biosynthesis is a complicated process, and little is known about the third stage of the pathway. To investigate potential genes that are responsible for tanshinone biosynthesis, we conducted transcriptome profiling analysis of two S. miltiorrhiza cultivars. Differential expression analysis provided 2,149 differentially expressed genes (DEGs) for further analysis. GO and KEGG analysis showed that the DEGs were mainly associated with the biosynthesis of secondary metabolites. Weighted gene coexpression network analysis (WGCNA) was further performed to identify a “cyan” module associated with tanshinone biosynthesis. In this module, 25 cytochromes P450 (CYPs), three 2-oxoglutarate-dependent dioxygenases (2OGDs), one short-chain alcohol dehydrogenases (SDRs) and eight transcription factors were found to be likely involved in tanshinone biosynthesis. Among these CYPs, 14 CYPs have been reported previously, and 11 CYPs were identified in this study. Expression analysis showed that four newly identified CYPs were upregulated upon application of MeJA, suggesting their possible roles in tanshinone biosynthesis. Overall, this study not only identified candidate genes involved in tanshinone biosynthesis but also provided a basis for characterization of genes involved in important active ingredients of other traditional Chinese medicinal plants.
Similar content being viewed by others
Introduction
Salvia miltiorrhiza (typically called danshen in Chinese) belongs to the Lamiaceae family and is a commonly used traditional Chinese medicine with great economic and medicinal value. The S. miltiorrhiza root has been widely used for the treatment of cardiovascular and cerebrovascular diseases1. Lipid-soluble tanshinones and water-soluble phenolic compounds are the two major groups of active components in S. miltiorrhiza2.
The red color of S. miltiorrhiza roots is largely ascribed to tanshinones and related quinones3. Tanshinones are abietane-type diterpenoids. They mainly accumulate in the periderms of mature roots4. To date, more than 40 tanshinones, including tanshinone I, tanshinone IIA, tanshinone IIB, cryptotanshinone, and dihydrotanshinone, have been isolated and structurally characterized2. Due to the medicinal and economic value of danshen, the production of tanshinone content can be effectively enhanced through genetic engineering or synthetic biology approaches. Therefore, elucidating the biosynthesis of tanshinones and identifying the key enzyme genes in the biosynthetic pathway are necessary for improving the production of tanshinones.
The putative biosynthetic pathway of tanshinones can be divided into three stages (Fig. 1). The first stage is the formation of terpenoid precursors. Isopentenyl diphosphate (IPP) and its isomer dimethylallyl diphosphate (DMAPP) are produced through the following two biosynthetic pathways: the mevalonate (MVA) pathway and the methylerythritol phosphate (MEP) pathway. The second stage is the construction of tanshinone skeletons. IPP and DMAPP are further catalyzed by geranylgeranyl diphosphate synthase (GGPPS) to form geranylgeranyl diphosphate (GGPP)5. The third stage is the formation of diverse tanshinones. Currently, little is known about the third stage of tanshinone biosynthesis. In the third stage of tanshinone biosynthesis, tanshinone skeletons produce various tanshinones by the catalysis of terpene synthases and by the modification of terpenoid-modifying enzymes2,6. Copalyl diphosphate synthase (CPS) and kaurene synthase-like cyclase (KSL) are two key terpenoid synthases involved in the biosynthesis of tanshinones. Studies have reported that SmCPS1 and SmCPS2 react with GGPP to form normal copalyl diphosphate (CPP) and that SmKSL1 further catalyzes the production of miltiradiene7,8. Twelve SmCPS and nine SmKSL homologs have been previously identified in S. miltiorrhiza4. Miltiradiene is the precursor of tanshinone and is further modified by cytochrome P450s (CYPs), dehydrogenases, demethylases, and other enzymes to produce structurally different compounds3. SmCYP76AH1 has been suggested to catalyze miltiradiene to produce ferruginol in vitro and in vivo9. RNA interference of SmCYP76AH1 in hairy roots led to significantly increased accumulation of miltiradiene, and the concentration of ferruginol decreased, revealing the key role in the biosynthesis of tanshinones10. Coexpression analysis showed that SmCYP76AH3 and SmCYP76AK1 are highly correlated with SmCYP76AH1. These two CYPs could function sequentially to form a bifurcating pathway for tanshinone biosynthesis, converting ferruginol into 11,20-dihydroxy ferruginol and 11,20-dihydroxy sugiol11. The postmodification of ferruginol to tanshinones remains obscure, and CYPs, dehydrogenases and other enzymes are speculated to play important roles in this process.
A schematic of the tanshinone biosynthetic pathway. AACT: acetyl-CoA C-acetyltransferase, HMGS: hydroxymethylglutaryl-CoA synthase, HMGR: 3-hydroxy-3-methylglutaryl-CoA reductase, MK: mevalonate kinase, PMK: 5-phosphomevalonate kinase, MDC: mevalonate pyrophosphate decarboxylase, DXS: 1-deoxy-D-xylulose-5-phosphate synthase, DXR: 1-deoxy-D-xylulose 5-phosphate reductoisomerase, MCT: 2-C-methyl-D-erythritol 4-phosphate cytidylyltransferase, CMK: 4-diphosphocytidyl-2-C-methyl-D-erythritol kinase, MDS: 2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase, HDS: 4-hydroxy-3-methylbut-2-enyl diphosphate synthase, HDR: 4-hydroxy-3-methylbut-2-enyl diphosphate reductase, IPP: isopentenyl pyrophosphate, DMAPP: dimethylallyl pyrophosphate, IDI: isopentenyl pyrophosphate isomerase, GGPP: geranylgeranyl diphosphate, GGPPS: geranylgeranyl diphosphate synthase, CPP: copalyl diphosphate, CPS: CPP synthase, and KSL: kaurene synthase-like cyclase.
CYPs are monooxygenases that are involved in numerous biosynthetic processes. Plant CYPs are the largest family of enzymes that contribute to the chemical diversity of secondary metabolites10,12,13. For example, the CYP71D subfamily is involved in the biosynthesis of terpenoids and flavonoids12. CYP72A1 is a secologanin synthase in the biosynthesis of terpene indole alkaloids in Catharanthus roseus14. The CYP76C subfamily is associated with monoterpenol metabolism in Arabidopsis thaliana15. In S. miltiorrhiza, CYPs in downstream tanshinone pathways have become a popular research field. SmCYP76AH1 is the first CYP that is responsible for the generation of oxygenated diterpenoid precursors in tanshinone biosynthesis9. It is always considered a marker gene for identifying possible genes involved in tanshinone biosynthesis postmodification. Gao et al. identified eight CYPs as targets for future investigation by transcriptomic analysis of S. miltiorrhiza hairy root culture16. Chen et al. identified 116 full-length and 135 partial-length CYPs in S. miltiorrhiza17. Coexpression and KEGG pathway analysis showed that three CYPs are potentially involved in terpenoid biosynthesis17. Xu et al. identified a total of 457 CYPs from the S. miltiorrhiza genome, and coexpression analysis suggested that 16 CYPs might be important in tanshinone biosynthesis4. Additionally, proteomic analysis also identified five CYPs as candidates that may be involved in tanshinone biosynthesis18. Other oxygenases and dehydrogenases may also be involved in tanshinone postmodification. The 2-oxoglutarate-dependent dioxygenase (2OGD) superfamily is a large gene family that is involved in the oxidic processes of natural products, such as terpenoid compounds. A total of 144 2OGDs have been identified in S. miltiorrhiza, and 16 2OGDs are more highly expressed in the periderm than in the rest of the root4. Recently, Sm2OGD5 was found to play a crucial role in the downstream biosynthesis of tanshinones19. Moreover, dehydrogenases were suspected to play roles in tanshinone biosynthesis. A total of 159 short-chain alcohol dehydrogenases (SDRs) have been identified in S. miltiorrhiza, and five of them may play a role in tanshinone biosynthesis4. These studies provided candidate genes that may be involved in tanshinone biosynthesis for further investigation.
“99–3” and “shh” are two S. miltiorrhiza cultivars that are bred by Prof. Xianen Li at our institute. The genome of “99–3” has been sequenced and annotated20. In the present study, we performed transcriptome profiling analysis of two S. miltiorrhiza cultivars to investigate potential genes that are responsible for the third step of the tanshinone biosynthetic pathway. We analyzed immature roots, mature roots and mature root periderms in “99–3” and “shh” by transcriptome sequencing with three biological repeats. Differential expression analysis and weighted gene coexpression network analysis (WGCNA) were carried out to identify putative genes that are involved in the biosynthetic pathway of tanshinones. This study provides important insight for further investigation of tanshinone biosynthesis in S. miltiorrhiza.
Results
Transcriptome sequencing and assembly
Tanshinones mainly accumulate in the periderms of mature roots4. Analysis showed that the content of tanshinones in immature roots is significantly less than that in mature roots (Fig. 2a).
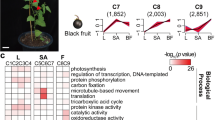
Tanshinone content of “99–3” and “shh” and expression analysis of differentially expressed genes (DEGs). (a) Tanshinone content in immature (99–3 W and shhW) and mature roots (99–3 R and shhR) of “99–3” and “shh”. Difference between mature roots and immature roots was analyzed by Student’s t-test. Significant difference (p < 0.001) was indicated by ***. (b) A venn diagram of DEGs in “99–3” and “shh”. (c) GO enrichment of DEGs.
To investigate the downstream biosynthetic pathway of tanshinone, transcriptome sequencing was performed using the roots of “99–3” and “shh”, which are two cultivars of S. miltiorrhiza. Three biological replicates were analyzed for immature root (named 99–3 W or shhW), mature root (named 99–3 R or shhR) and mature root periderms (named 99–3RE or shhRE) of the two cultivars, and 18 samples were sequenced by the Illumina HiSeqTM 2500 sequencing platform. A total of 908.98 million raw reads were generated from these samples. After removing the adapter reads, reads with greater than 10% of unknown nucleotides and low-quality reads, 887.06 million clean pair-end reads were generated for assembly. Each sample yielded more than 5.30 Gb clean reads. The Q20 and Q30 percentages were more than 98% and 95%, respectively. The average GC content of the transcriptome was 47.93% (Supplementary Table S1). Mapping the clean reads from the 18 samples against the S. miltiorrhiza reference genome (“99–3”)3 showed that 621.69 million reads (70.43%) were mapped in total and 590.81 million reads (66.95%) were mapped uniquely. A de novo assembly of filtered reads was assembled into 35,466 unique genes. Among them, 30,478 genes (85.94%) were identified in a previous study20, whereas the other 4,988 genes were novel.
The expression values of all these genes were determined by reads per kilo bases per million reads (RPKM). To verify the reproducibility of the sequencing data, Person’s correlation coefficients for three biological replicates were calculated by log10(RPKM + 1). The results showed that all biological replicate correlations were greater than 0.85 (Supplementary Fig. S1). A total of 27,440 genes with an RPKM ≥ 1 in at least one of these samples were used for further analysis (Supplementary Table S2).
Identification of differentially expressed genes
Since tanshinones mainly accumulate in the periderms of mature roots4, it is assumed that the expression levels of genes involved in tanshinone biosynthesis are significantly higher in mature roots than in immature roots. Two pairwise transcriptome comparisons were conducted to identify differentially expressed genes between mature roots (or periderms) and immature roots in both cultivars. Using a significance level of p < 0.05 and |log2(FoldChange)| > 1, we found 794 upregulated differentially expressed genes (DEGs) in 99–3 R (or 99–3RE) compared to 99–3 W and 2,275 upregulated DEGs in shhR (or shhRE) compared to shhW (Fig. 2b). Among them, 379 DEGs were redundant. Overall, a total of 2,690 DEGs with increased expression levels in mature roots compared with immature roots were identified. To gain more insight into the differential expression of these transcripts, hierarchical cluster and heat map analyses were performed based on the RPKM values. The results showed significant differential expression patterns (Supplementary Fig. S2). Except for 541 genes that were significantly downregulated in the periderms (99–3 RE and shhRE) than in roots (99–3 R and shhR), 2,149 DEGs were obtained. Among them, 1,104 genes were significantly up-regulated in the periderms (99–3RE and shhRE) compared with the roots (99–3R and shhR), suggesting they may have a periderm-specific profile. An additional 1,045 DEGs showed no significant difference between the periderms and roots, but they may contain genes with high expression levels in the periderms. Therefore, all 2,149 genes were further subjected to functional analysis.
To evaluate the major functional categories of DEGs, gene ontology (GO) enrichment was applied to identify the major functional categories represented by 2,149 DEGs. Among the biological process category, genes associated with metabolism, including metabolic process, organic substance metabolic process, cellular metabolic process, primary metabolic process, macromolecule metabolic process and single-organism metabolic process, were highly enriched (Fig. 2d). Additionally, DEGs were mapped to 118 KEGG pathways, and 20 significantly enriched pathways are shown in Table 1. The largest number of DEGs was annotated against biosynthesis of secondary metabolites and metabolic pathways. There are 22 genes associated with terpenoid backbone synthesis and 11 genes assigned to diterpenoid biosynthesis (Supplementary Table S3). The secondary metabolite biosynthesis pathway contains 17 functionally characterized tanshinone biosynthesis-related genes (Supplementary Table S3). These results suggest that the DEGs enriched in secondary metabolite biosynthesis may provide new information for analysis of tanshinone biosynthesis in S.miltiorrhiza.
Weighted gene coexpression network analysis
To further investigate genes that are related to the biosynthesis of tanshinones, highly coexpressed gene modules were inferred from all genes of 18 samples (three biological replicates) using WGCNA21. Highly interconnected genes clustered in the same modules were labeled by different colors. In each module, genes were coexpressed and functionally related. Therefore, we identified a total of 38 coexpression modules, and the 2,149 DEGs were clustered into 36 modules. (Supplementary Fig. S3). The majority of these DEGs were grouped into the “purple” and “cyan” modules that contained 24.71% (531) and 18.66% (401) of the total DEGs, respectively. The other 34 modules comprised 0.05–9.77% of the total DEGs (Table 2).
Further analysis showed that functionally characterized tanshinone biosynthesis related genes and their homologous genes4,6 were distributed in 18 modules (Table 2). The “cyan” module contains 15 previously reported genes that are involved in the tanshinone biosynthesis pathway (Table 2). This result indicated that genes in the “cyan” module were most likely associated with tanshinone biosynthesis. Further analysis was performed for the “cyan” module genes.
Potential genes related to tanshinone biosynthesis
As mentioned above, the “cyan” module most likely contains tanshinone biosynthesis-related candidate genes. The expression patterns showed that the majority of genes in this module had similar expression patterns and were specifically expressed in the mature root periderms (Fig. 3a). We visualized the coexpression networks of this module using Cytoscape22,23, and each node represents a gene and the connecting lines (edges) between genes represent coexpression correlations (Fig. 3b). Hub genes are genes with the most connections in a network. Thirty-three hub genes were selected from the “cyan” module (Supplementary Table S4). They included two key genes (SmMDS and SmGGPPS1) in the tanshinone biosynthesis pathway, further verifying our gene identification method. This result suggests that the other 31 hub genes might be associated with tanshinone biosynthesis.
Coexpression network analysis in the “cyan” module. (a) A heatmap of coexpressed genes in the “cyan” module. (b) Cytoscape representation of coexpressed genes with edge weights > 0.1 in the “cyan” module. The red, green, darkblue, lightblue, yellow and black nodes represent tanshinone biosynthesis-related genes, CYP genes, transcription factor genes, 2OGD, SDR and other genes, respectively. (c) Expression patterns of the tanshinone biosynthesis-related genes. (d) Expression patterns of CYPs in the “cyan” module. (e) Expression patterns of 2OGDs, SDR and transcription factor genes in the “cyan” module.
Since CYPs have been proven to play an important role in secondary metabolic biosynthesis, we focused on the 29 CYP genes in the “cyan” module (Table 3). They were classified into nine families and 14 subfamilies, and the CYP76, CYP71 and CYP72 families contained ten, eight and five members, respectively. The other members belonged to the CYP710, CYP714, CYP734, CYP81, CYP84 and CYP97 families. All these CYPs have high expression levels in the periderms, similar to the expression levels of key enzyme genes in tanshinone biosynthesis (Fig. 3d). To better understand the putative functions of CYPs, we constructed a phylogenetic tree with their amino acid sequences. The tree showed that all the 29 CYPs were distributed into three clades. The CYP76 family members were clustered into one clade. SmCYP84A60 and the CYP71 family members were grouped into another clade. The third clade contained CYP72 family members together with SmCYP81C16, SmCYP97B34, SmCYP710A59, SmCYP714G15 and SmCYP734A34 (Supplementary Fig. S4). The CYP76 family, which contains three key enzyme genes involved in the third stage of tanshinone biosynthesis9,11, was further analyzed. Both SmCYP76AH28 and SmCYP76AH29P belong to the CYP76AH subfamily. However, sequence analysis found that SmCYP76AH28 is a partial sequence of SmCYP76AH3 with only 4 bp nucleotide mutations (Supplementary Fig. S5 and Table S5). SmCYP76AH29P shares a 72.80% amino acid sequence identity with SmCYP76AH1 and an 88.80% identity with SmCYP76AH3, suggesting that it may have functions similar to SmCYP76AH1 and SmCYP76AH3 (Supplementary Fig. S5 and Table S5). SmCYP76AK3, SmCYP76AK4, SmCYP76AK5v1, SmCYP76AK5v2, SmCYP76AK9 and SmCYP76AK13 are members of the CYP76AK subfamily, which share 69.78%, 63.62%, 69.18%, 66.02%, 37.38% and 69.18% amino acid sequence identity with SmCYP76AK1, respectively (Supplementary Fig. S6 and Table S5). These CYP76AKs may also participate in tanshinone biosynthesis. Moreover, the CYP71 and CYP72 families were shown to be associated with terpene metabolite biosynthesis. It has been reported that the CYP71D subfamily is involved in the biosynthesis of terpenoids and flavonoids in Arabidopsis12. CYP72A1 is a secologanin synthase in the biosynthesis of terpene indole alkaloids in Catharanthus roseus14. Therefore, the eight CYP71s and five CYP72s may also warrant further investigation. GO and KEGG pathway analysis showed that 11 CYPs, including six members of the CYP71 family (SmCYP71BE31, SmCYP71BE51, SmCYP71BE59P, SmCYP71D373, SmCYP71D375 and SmCYP71D411), three members of the CYP76 family (SmCYP76A35, SmCYP76AK3 and SmCYP76B71), one member of the CYP710 family (SmCYP710A59) and one member of the CYP714 family (SmCYP714G15), are annotated against biosynthesis of secondary metabolites (Table 3 and Supplementary Table S3). Among them, SmCYP76A35 and SmCYP714G15 are also hub genes in the coexpression module (Supplementary Table S4), suggesting that they may play a central role in the module. GO and KEGG analysis also indicated that SmCYP734A34, SmCYP84A60 and SmCYP97B34 are associated with brassinosteroid biosynthesis, phenylpropanoid biosynthesis and carotenoid biosynthesis, respectively (Table 3). They were not considered to be involved in tanshinone biosynthesis. Overall, we identified 25 CYPs, of which 14 CYPs were previously reported4 and 11 CYPs were identified in this study, that may be involved in tanshinone biosynthesis.
Considering the highly oxidized nature of tanshinones, other oxygenases and dehydrogenases may also be involved in tanshinone biosynthesis. 2OGDs and SDRs were suspected to play roles in tanshinone production4. In the “cyan” module, we identified three 2OGDs (Sm2OGD-2, Sm2OGD-8 and Sm2OGD-15) and one dehydrogenase gene (SmSDR-3) (Fig. 3e). They were highly expressed in the periderm, which is consistent with previous reports4. In particular, 2OGD-8 and SDR-3 have been reported to exhibit a periderm-specific expression profile4. KEGG analysis indicated that 2OGD-15 was associated with diterpenoid biosynthesis (Supplementary Table S3). Thus, these genes were also considered to be candidates that may be related to tanshinone biosynthesis.
Some studies have shown that transcription factors could promote tanshinone accumulation24,25, but the regulatory mechanism remains unelucidated. In this study, eight transcription factors were identified and classified into four transcription factor families, including two WRKY transcription factors (SmWRKY9 and SmWRKY17)26, three MYB transcription factors (SmMYB27, SmMYB28 and SmMYB100)27, two putative NAC transcription factors and one putative DPB transcription factor. They were specifically expressed in the periderm (Fig. 3e), suggesting their putative roles in tanshinone biosynthesis.
Expression analysis of selected candidate genes in response to MeJA treatment
For the postmodification of tanshinone, CYPs, dehydrogenases and other enzymes are speculated to play important roles. As mentioned above, we identified 25 CYPs, three 2OGDs and one SDR that may be involved in this process. Among them, 14 CYPs, three 2OGDs and one SDR have reported in a previous study4. Therefore, the remaining 11 CYPs, including SmCYP710A59, SmCYP71BE59P, SmCYP72A436, SmCYP72A327, SmCYP72A450, SmCYP72A451, SmCYP76A35, SmCYP76AH29P, SmCYP76AK9, SmCYP76AK13 and SmCYP76B71, were further analyzed experimentally.
Methyl jasmonate (MeJA) is an effective elicitor that enhances the accumulation of tanshinone in S. miltiorrhiza28,29,30. We analyzed the expression of 11 CYP candidates in S. miltiorrhiza roots treated with 200 μM exogenous MeJA for 0, 12 and 24 h using qRT-PCR. The results showed that the expression levels of SmCYP76A35, SmCYP76AK13, SmCYP72A327 and SmCYP72A450 were all upregulated in roots treated with MeJA for 12 and 24 h (Fig. 4). Their expression profiles in response to MeJA were similar to those of SmCYP76AH1. No significant changes were observed in the expression level of SmCYP76AK9 after MeJA treatment. Additionally, the expression levels of SmCYP76B71, SmCYP710A59, SmCYP71BE59P and SmCYP72A451 were all decreased after MeJA treatment (Fig. 4). The expression of SmCYP72A436 and SmCYP76AH29P was not detected in the roots. All these results indicated that SmCYP76A35, SmCYP76AK13, SmCYP72A327 and SmCYP72A450 were upregulated by MeJA. These CYP450s can be selected for further investigation for their involvement in tanshinone biosynthesis.
Fold changes of CYPs in the roots of S. miltiorrhiza after MeJA treatment for 12 and 24 h. The expression levels were analyzed by qRT-PCR. CYP expression at 0 h were considered the controls. The values are representative of three biological replicates. Significant differences between MeJA treatment for 12 or 24 h and the control (0 h) were determined by Student’s t-test (*p < 0.05 and **p < 0.01).
Discussion
Tanshinones, which are the main bioactive components of S. miltiorrhiza, mainly accumulate in the periderms of mature roots4. Elucidating the biosynthesis of tanshinones and identifying the key enzyme genes in the biosynthetic pathway are necessary for improving the production of tanshinones.
WGCNA, which is one of the most reliable and effective methods for the analysis of gene functions21, using a large sample size ( ≥ 15) could comprehensively identify relationships between individual genes. This method has been successfully used in strawberry31, Prunus salicina32, rice33, lotus34, etc. In this study, we identified a “cyan” module that may be related to tanshinone biosynthesis by differential expression analysis and WGCNA. This module contained all the reported functionally characterized genes in the third stage of tanshinone biosynthesis. Among the 25 CYPs identified in the “cyan” module, 14 CYPs were consistent with those reported in a previous study4. All these results confirmed that our candidate genes were reliable.
The third stage of tanshinone biosynthesis is complex, and the postmodification from ferruginol to tanshinones remains obscure. CYPs, dehydrogenases, demethylases, and other enzymes are speculated to play roles in this process2. In this study, we also found that 25 CYPs, including 11 newly identified CYPs, might be involved in tanshinone biosynthesis by differential expression analysis and WGCNA (Fig. 3). The majority of the 25 candidate CYPs belong to the CYP76, CYP71 and CYP72 families (Table 3).
For the 14 previously reported CYPs4, our results also indicated that they were specifically expressed in the periderms, which was consistent with a previous study (Fig. 3). Eight CYPs, including SmCYP76AK3, SmCYP76AK4, SmCYP76AK5v1, SmCYP76AK5v2, SmCYP71D373, SmCYP71D375, SmCYP71D411 and SmCYP71AU66, were reported to be specifically expressed in the periderm4. Among these CYPs, GO and KEGG analysis showed that SmCYP71D373, SmCYP71D375, SmCYP71D411 and SmCYP76AK3 are associated with the biosynthesis of secondary metabolites (Table 3), implying that they are most likely to be involved in tanshinone biosynthesis. Proteomics analysis revealed five new CYPs as candidates that are involved in tanshinone biosynthesis18. In this study, sequencing analysis indicated that one of these CYPs is SmCYP76AK5v1 or SmCYP76AK5v2. Thus, SmCYP76AK5v1 and SmCYP76AK5v2 warrant further investigation. Moreover, SmCYP714G15 was identified as the hub gene in the tanshinone biosynthesis-related module and was annotated against the biosynthesis of secondary metabolites (Table 3, Supplementary Table S3 and S4), indicating that it may also be considered a candidate gene in tanshinone biosynthesis.
For the other 11 identified CYPs, SmCYP76A35, SmCYP76AK13, SmCYP72A327 and SmCYP72A450 were considered candidates that may be involved in tanshinone biosynthesis. SmCYP76A35 was the hub gene in the “cyan” module, which was associated with the biosynthesis of secondary metabolites (Supplementary Table S3, S4). The expression of SmCYP76A35 was enhanced by MeJA (Fig. 4), revealing its possible roles in the tanshinone biosynthesis pathway. The expression level of SmCYP76AK13 was also significantly increased after treatment with MeJA (Fig. 4). SmCYP76AK13, which shares 69.18% amino acid sequence identity with SmCYP76AK1, belongs to the CYP76AK subfamily (Supplementary Fig. S6 and Table S5), suggesting that it may have a similar function to SmCYP76AK1. Some CYP72A subfamily members were reported to be associated with the biosynthesis of secondary metabolites. CYP72A1 from Catharanthus roseus is a secologanin synthase in the biosynthesis of the seco-iridoid unit of terpene indole alkaloids14. CYP72A67 of Medicago truncatula35 and CYP72A154 of Glycyrrhiza uralensis36 have been reported to participate in oleanane triterpene scaffold oxidation. We also found that two CYP72A subfamily members, which include SmCYP72A327 and SmCYP72A450, were coexpressed with tanshinone biosynthesis-related genes and could be upregulated upon application of MeJA (Fig. 4), suggesting a possible role in tanshinone biosynthesis.
Two CYP76AH subfamily genes, which include SmCYP76AH28 and SmCYP76AH29P, were analyzed in this study. Sequence analysis showed that SmCYP76AH28 is a partial sequence of SmCYP76AH3 and that SmCYP76AH29P shares 88.80% identity with SmCYP76AH3 (Supplementary Fig. S5 and Table S5). Due to its highly similar sequence to SmCYP76AH3, the expression level of SmCYP76AH29P was analyzed using a specific region. However, the expression of SmCYP76AH29P in the roots was not detected, likely because the specific region of SmCYP76AH29P may not exist. This result suggested that SmCYP76AH28 and SmCYP76AH29P may be generated by incorrect sequence assembly and may be the same gene as SmCYP76AH3.
2OGDs and SDRs were suspected to play roles in tanshinone production4. A total of 144 2OGDs have been identified in S. miltiorrhiza, and 16 of these genes are more highly expressed in the periderm4. 2OGD5 was found to play a crucial role in the downstream biosynthesis of tanshinones19. Moreover, 159 SDRs were identified in S. miltiorrhiza, and five of them may be associated with tanshinone biosynthesis4. In this study, we found that three 2OGDs and one SDR may be involved in tanshinone biosynthesis. They were all highly expressed in the periderm (Fig. 3) and consistent with a previous study4. In particular, Sm2OGD-8 and SmSDR-3 were reported to exhibit a periderm-specific expression profile4. Sm2OGD-15 was assigned to diterpenoid biosynthesis by KEGG analysis (Supplementary Table S3). Therefore, Sm2OGD-8, Sm2OGD-15 and SmSDR-3 should be prioritized in further investigations of tanshinone biosynthesis.
Various transcription factors are involved in tanshinone biosynthesis by S. miltiorrhiza, but studies on transcription factors that regulate tanshinone biosynthesis are limited. The MYB gene family is the largest transcription factor family, and 110 SmMYB genes have been characterized in S. miltiorrhiza27. The overexpression of SmMYB36 in the hairy roots could promote tanshinone accumulation37. SmMYB9b could enhanced tanshinone concentration by stimulating the MEP pathway24. It was reported that 61 SmWRKYs were cloned and characterized from S. miltiorrhiza, and systematic analysis suggested that SmWRKY3 and SmWRKY9 are most likely activators in tanshinone biosynthesis25. Overexpression of SmWRKY2 in S. miltiorrhiza hairy roots significantly increases the accumulation of tanshinones38. A total of 127 bHLH transcription factor genes have been identified in S. miltiorrhiza, and 7 of them are potentially associated with the regulation of tanshinone biosynthesis25. SmbHLH10 could improve tanshinone production in S. miltiorrhiza hairy roots by activating the expression of the key enzyme genes involved in tanshinone biosynthesis39. Two AP2/ERF transcription factors, SmERF1L1 and SmERF128, have been reported to positively regulate tanshinone biosynthesis in S. miltiorrhiza40. Through DEG and WGCNA analysis, we also found that eight transcription factor genes might be involved in tanshinone biosynthesis (Fig. 3). They have a similar expression pattern and are coexpressed with tanshinone biosynthesis-related genes (Fig. 3). Among them, SmWRKY9, SmWRKY17, SmMYB27 and SmMYB100 were shown to be most highly expressed in roots26,27. The function of these transcription factors in tanshinone biosynthesis requires further confirmation.
In conclusion, we identified a “cyan” module that is associated with tanshinone biosynthesis by differential expression analysis and WGCNA. In this module, 25 CYPs, three 2OGDs, one SDR and eight transcription factors that they may be involved in tanshinone biosynthesis were identified. For the 25 CYPs, 14 CYPs have been reported previously4, whereas the other 11 CYPs were identified in this study. Expression analysis showed that four newly identified CYPs are upregulated upon the application of MeJA, suggesting their possible roles in tanshinone biosynthesis. Overall, the candidate genes involved in tanshinone biosynthesis that were identified in this study provide insight for future research. The function of these genes in the tanshinone biosynthesis pathway should be further investigated.
Materials and Methods
Plant materials
Two cultivars of S. miltiorrhiza, which included “99–3” and “shh”, were harvested from an experimental field in late August at the Institute of Medicinal Plant Development (IMPLAD). Root samples from “99–3” and “shh” were collected and named 99–3/shhW (immature roots), 99–3/shhR (mature roots) and 99–3/shhRE (mature root periderms). Immature roots are smaller roots without a tanshinone color, while mature roots are well-developed roots with a tanshinone color (Supplementary Fig. S7). Biological replicates were obtained from three independent plants.
Analysis of tanshinone content by HPLC-UV
Tanshinone content was analyzed as described in the Pharmacopoeia of the People’s Republic of China (2015)41. Three biological repeats were performed. Dried mature roots of “99–3” and “shh” were ground into a powder, and then each sample of weighed ground powder (0.3 g) was extracted with 50 ml of methanol. After 30 min of ultrasonic extraction, methanol was added to complement and maintain a constant weight. The sample was filtrated with 0.22 μm filter membrane, and then analyzed immediately using HPLC-UV. To determine tanshinone content, 10 μL of extraction product was injected into a Supelco C18 HPLC column (4.6 × 250 mm) and analyzed by HPLC-UV under the following conditions. Solvent system: methanol/H2O (75:25); flow rate: 0.8 mL/min; temperature: 25 °C; detection: absorbance measured at 270 nm. Tanshinone I, tanshinone IIA, cryptotanshinone and dihydrotanshinone standards were dissolved in methanol at a concentration of 0.04, 0.03, 0.02, and 0.02 mg.mL−1. Total content of tanshinone I, tanshinone IIA, cryptotanshinone and dihydrotanshinone in each sample was calculated.
RNA sequencing
For transcriptome sequencing, a total of 18 RNA samples were extracted by the Quick RNA isolation kit (Huayue Yang, Beijing, China) according to the manufacturer’s instructions. RNA degradation and contamination was monitored on 1% agarose gels. RNA purity was checked using the NanoPhotometer spectrophotometer (IMPLEN, CA, USA). RNA concentration was measured using the Qubit RNA Assay Kit Qubit 2.0 Flurometer (Life Technologies, CA, USA). RNA integrity was assessed using the RNA Nano 6000 Assay Kit and the Bioanalyzer 2100 system (Agilent Technologies, CA, USA). Sequencing libraries were generated using the NEBNext Ultra™ RNA Library Prep Kit for Illumina® (NEB, USA). The library preparations were sequenced on an Illumina Hiseq. 2500 platform, and 150 bp paired-end reads were generated.
RNA-seq data analysis
Clean data (clean reads) were obtained by removing reads containing adapters, reads containing ploy-N and low quality reads from raw data. Q20, Q30 and GC content of the clean data were calculated. All downstream analyses were based on high-quality clean data.
A reference genome and gene model annotation files were downloaded from http://www.ndctcm.org/shujukujieshao/2015-04-23/27.html20. The index of the reference genome was built using Bowtie v2.0.6 software, and paired-end clean reads were aligned to the reference genome using TopHat v2.0.9. HTSeq v0.6.1 was used to count the read numbers that were mapped to each gene. The RPKM of each gene was calculated based on the length of the gene and the read count mapped to this gene. The Cufflinks v2.1.1 reference annotation-based transcript (RABT) assembly method was used to construct and identify known and novel transcripts from the TopHat alignment results.
Differential expression analysis
Differential expression analysis was performed using the DESeq R package (1.10.1). The resulting p-values were adjusted using Benjamini and Hochberg’s approach for controlling the false discovery rate. Genes identified by DESeq with an adjusted p-value < 0.05 were considered differentially expressed. Venn diagram is conducted by the Venndiagram R package.
GO and KEGG enrichment analysis
Gene ontology (GO) enrichment analysis of differentially expressed genes was conducted by the GOseq R package, and the gene length bias was corrected. GO terms with a corrected p-value < 0.05 were considered significantly enriched by differential expression analysis. To identify the functions of DEGs, KEGG pathway enrichment analysis of the DEGs was performed using KOBAS software (http:// kobas.cbi.pku.edu.cn).
Gene coexpression network analysis
WGCNA can be used to identify modules of highly correlated genes and hub genes with important effects21. Gene expression was also evaluated with the WGCNA R package version 1.3421. We first combined the expression matrix of 27,440 genes as the input file for WGCNA analysis to identify modules of genes with strong co-expression. Next, WGCNA network construction and module detection were conducted using an unsigned type of topological overlap matrix (TOM). The soft power value is 18, minModuleSize is 30, and mergeCutHeight is 0.25. The most significantly correlated genes with a WGCNA edge weight > 0.1 were visualized using Cytoscape 3.5.1 software22,23. The phylogenetic tree was constructed using the Neighbor-Joining (NJ) method in MEGA6.
MeJA treatment and qRT-PCR analysis
Healthy S. miltiorrhiza plantlets (99–3 line) were subcultivated on Murashige and Skoog (MS) agar medium for 5 weeks under a 16/8 h light/dark photoperiod at 25 °C and then transferred to MS liquid medium for 2 days. Then, MeJA was added to the medium at a final concentration of 200 μM. Roots were collected at 0, 12 and 24 h after treatment. Three biological replicates were performed for each treatment.
Total RNA was extracted from the roots using an RNAprep Pure Plant Kit (Axygen, Beijing, China). Then, first-strand cDNA was synthesized with AMV RT (Invitrogen, CA, USA). qRT-PCR was performed using 100 ng cDNA, 10 μL of SYBR Premix Ex Taq (TaKaRa, Otsu, Japan) and 0.5 μM of each primer in a 20 μL volume. The transcription products were amplified using different primers (Supplementary Table S6). A standard 2-step protocol was followed: enzyme activation 10 min at 95 °C, followed by 40 cycles of 95 °C for 15 s, 60 °C for 45 s. The S. miltiorrhiza UBQ gene was used as a control for qRT-PCR reactions. The PCR experiment was repeated three times.
References
Yan, X. Liposoluble Chemical Constituents in Danshen in Dan Shen (Salvia miltiorrhiza) in Medicine 119−206 (Springer, 2015).
Ma, X. et al. The biosynthetic pathways of tanshinones and phenolic acids in Salvia miltiorrhiza. Molecules 20, 16235–16254 (2015).
Wang, J. & Wu, J. Tanshinone biosynthesis in Salvia miltiorrhiza and production in plant tissue cultures. Appl. Microbiol. Biotechnol. 88, 437–449 (2010).
Xu, Z. et al. Full-length transcriptome sequences and splice variants obtained by a combination of sequencing platforms applied to different root tissues of Salvia miltiorrhiza and tanshinone biosynthesis. Plant J. 82, 951–961 (2015).
Kai, G. et al. Metabolic engineering tanshinone biosynthetic pathway in Salvia miltiorrhiza hairy root cultures. Metab. Eng. 13, 319–327 (2011).
Ma, Y., Yuan, L., Wu, B., Li, X. & Chen, S. Genome-wide identification and characterization of novel genes involved in terpenoid biosynthesis in Salvia miltiorrhiza. J. Exp. Bot. 63, 2809–23 (2012).
Gao, W. et al. A functional genomics approach to tanshinone biosynthesis provides stereochemical insights. Org. Lett. 11, 5170–5173 (2009).
Cui, G. et al. Functional divergence of diterpene syntheses in the medicinal plant Salvia miltiorrhiza. Plant Physiol. 169, 1607–1618 (2015).
Guo, J. et al. CYP76AH1 catalyzes turnover of miltiradiene in tanshinones biosynthesis and enables heterologous production of ferruginol in yeasts. Proc. Natl. Acad. Sci. USA 110, 12108–12113 (2013).
Ma, Y. et al. RNA interference targeting CYP76AH1 in hairy roots of Salvia miltiorrhiza reveals its key role in the biosynthetic pathway of tanshinones. Biochem. Biophys. Res. Commun. 477, 155–160 (2016).
Guo, J. et al. Cytochrome P450 promiscuity leads to a bifurcating biosynthetic pathway for tanshinones. New Phytol. 210, 525–534 (2016).
Werck-Reichhart, D., Bak, S. & Paquette, S. Cytochromes P450. The Arabidopsis Book 1, e28 (2011).
Zhao, Y. J. et al. Research progress relating to the role of cytochrome P450 in the biosynthesis of terpenoids in medicinal plants. Appl. Microbiol. Biotechnol. 98, 2371–2383 (2014).
Irmler, S. et al. Indole alkaloid biosynthesis in Catharanthus roseus: new enzyme activities and identification of cytochrome P450 CYP72A1 as secologanin synthase. Plant J. 24, 797–804 (2000).
Hofer, R. et al. Dual Function of the cytochrome P450 CYP76 family from Arabidopsis thaliana in the metabolism of monoterpenols and phenylurea herbicides. Plant Physiol. 166, 1149–1161 (2014).
Gao, W. et al. Combining metabolomics and transcriptomics to characterize tanshinone biosynthesis in Salvia miltiorrhiza. BMC Genomics 15, 73 (2014).
Chen, H., Wu, B., Nelson, D. R., Wu, K. & Liu, C. Computational identification and systematic classification of novel cytochrome p450 genes in Salvia miltiorrhiza. PLoS ONE 9, e115149 (2014).
Contreras, A., Leroy, B., Mariage, P. & Wattiez, R. Proteomic analysis reveals novel insights into tanshinones biosynthesis in Salvia miltiorrhiza hairy roots. Sci Rep 9, 5768 (2019).
Xu, Z. & Song, J. The 2-oxoglutarate-dependent dioxygenase superfamily participates in tanshinone production in Salvia miltiorrhiza. J. Exp. Bot. 68, 2299–2308 (2017).
Xu, H. et al. Analysis of the genome sequence of the medicinal plant Salvia miltiorrhiza. Mol Plant 9, 949–952 (2016).
Langfelder, P. & Horvath, S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 9, 559 (2008).
Shannon, P. et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Research 13(11), 2498–504 (2003).
Demchak et al. Cytoscape: the network visualization tool for GenomeSpace workflows. F1000Res 3, 151 (2014).
Zhang, J. et al. Overexpression of SmMYB9b enhances tanshinone concentration in Salvia miltiorrhiza hairy roots. Plant Cell Rep. 36, 1297–1309 (2017).
Zhang, X. et al. Genome-wide characterisation and analysis of bHLH transcription factors related to tanshinone biosynthesis in Salvia miltiorrhiza. Sci Rep 5, 11244 (2015).
Li, C., Li, D., Shao, F. & Lu, S. Molecular cloning and expression analysis of WRKY transcription factor genes in Salvia miltiorrhiza. BMC Genomics 16 (2015).
Li, C. & Lu, S. Genome-wide characterization and comparative analysis of R2R3-MYB transcription factors shows the complexity of MYB-associated regulatory networks in Salvia miltiorrhiza. BMC Genomics 15, 277 (2014).
Shi, M. et al. Methyl jasmonate induction of tanshinone biosynthesis in Salvia miltiorrhiza hairy roots is mediated by JASMONATE ZIM-DOMAIN repressor proteins. Sci Rep 6, 20919 (2016).
Hao, X. et al. Effects of methyl jasmonate and salicylic acid on tanshinone production and biosynthetic gene expression in transgenic Salvia miltiorrhiza hairy roots. Biotechnol. Appl. Biochem. 62, 24–31 (2015).
Luo, H. et al. Transcriptional data mining of Salvia miltiorrhiza in response to methyl jasmonate to examine the mechanism of bioactive compound biosynthesis and regulation. Physiol Plant 152, 241–255 (2014).
Hollender, C. A. et al. Floral transcriptomes in woodland strawberry uncover developing receptacle and anther gene networks. Plant Physiol. 165, 1062–1075 (2014).
Farcuh, M. et al. Sugar metabolism reprogramming in a non-climacteric bud mutant of a climacteric plum fruit during development on the tree. J. Exp. Bot. 68, 5813–5828 (2017).
Qi, Z. et al. Meta-analysis and transcriptome profiling reveal hub genes for soybean seed storage composition during seed development. Plant Cell Environ. 41(9), 2109–2127 (2018).
Yang, M. et al. Digital gene expression analysis provides insight into the transcript profile of the genes involved in aporphine alkaloid biosynthesis in lotus (Nelumbo nucifera). Front Plant Sci 8, 80 (2017).
Biazzi, E. et al. CYP72A67 catalyzes a key oxidative step in Medicago truncatula hemolytic saponin biosynthesis. Mol Plant 8, 1493–1506 (2015).
Seki, H. et al. Triterpene functional genomics in licorice for identification of CYP72A154 involved in the biosynthesis of glycyrrhizin. Plant Cell 23, 4112–4123 (2011).
Ding, K. et al. SmMYB36, a Novel R2R3-MYB transcription factor, enhances tanshinone accumulation and decreases phenolic acid content in Salvia miltiorrhiza hairy roots. Sci Rep 7, 5104 (2017).
Deng, C. et al. Tanshinone production could be increased by the expression of SmWRKY2 in Salvia miltiorrhiza hairy roots. Plant Sci 284, 1–8 (2019).
Xing, B. et al. Overexpression of SmbHLH10 enhances tanshinones biosynthesis in Salvia miltiorrhiza hairy roots. Plant Sci 276, 229–238 (2018).
Zhang, Y., Ji, A., Xu, Z., Luo, H. & Song, J. The AP2/ERF transcription factor SmERF128 positively regulates diterpenoid biosynthesis in Salvia miltiorrhiza. Plant Mol. Biol. 100, 83–93 (2019).
Chinese Pharmacopoeia 2015 edition Volume 1. Pharmacopoeia Commission of People’s Republic of China. (Beijing: China Medical Science Press, 2015).
Acknowledgements
We thank Prof. Xian’en Li for providing S. miltiorrhiza plants. This work was supported by the National Natural Science Foundation of China (81773836, 81872964), the CAMS Innovation Fund for Medical Sciences (CIFMS) (2016-I2M-3–016), and the China Postdoctoral Science Foundation (2017M620685).
Author information
Authors and Affiliations
Contributions
S.L. and Y.C. designed the experiment; Y.C. and J.L. analyzed the data; M.W. and Y.C. performed the experiments; Y.C. wrote the paper; M.W. and S.L. reviewed the paper. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chang, Y., Wang, M., Li, J. et al. Transcriptomic analysis reveals potential genes involved in tanshinone biosynthesis in Salvia miltiorrhiza. Sci Rep 9, 14929 (2019). https://doi.org/10.1038/s41598-019-51535-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-51535-9
This article is cited by
-
Next-Generation Sequencing in Medicinal Plants: Recent Progress, Opportunities, and Challenges
Journal of Plant Growth Regulation (2024)
-
5-Azacytidine increases tanshinone production in Salvia miltiorrhiza hairy roots through epigenetic modulation
Scientific Reports (2022)
-
Comprehensive transcriptomic analysis in response to abscisic acid in Salvia miltiorrhiza
Plant Cell, Tissue and Organ Culture (PCTOC) (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.