Abstract
Respiratory sensations such as breathlessness are prevalent in many diseases and are amplified by increased levels of anxiety. Cortical activation in response to inspiratory occlusions in high- and low-anxious individuals was found different in previous studies using the respiratory-related evoked potential method. However, specific brain areas showed different activation patterns remained unknown in these studies. Therefore, the purpose of this study was to compare cortical and subcortical neural substrates of respiratory sensation in response to inspiratory mechanical occlusion stimuli between high- and low-anxious individuals using functional magnetic resonance imaging (fMRI). In addition, associations between brain activation patterns and levels of anxiety, and breathlessness were examined. Thirty-four (17 high- and 17 low-anxious) healthy non-smoking adults with normal lung function completed questionnaires on anxiety (State Trait Anxiety Inventory - State), and participated in a transient inspiratory occlusion fMRI experiment. The participants breathed with a customized face-mask while respiration was repeatedly interrupted by a transient inspiratory occlusion of 150-msec, delivered every 2 to 4 breaths. Breathlessness was assessed by self-report. At least 32 occluded breaths were collected for data analysis. The results showed that compared to the low-anxious group, the high-anxious individuals demonstrated significantly greater neural activations in the hippocampus, insula, and middle cingulate gyrus in response to inspiratory occlusions. Moreover, a significant relationship was found between anxiety levels and activations of the right inferior parietal gyrus, and the right precuneus. Additionally, breathlessness levels were significantly associated with activations of the bilateral thalamus, bilateral insula and bilateral cingulate gyrus. The above evidences support stronger recruitment of emotion-related cortical and subcortical brain areas in higher anxious individuals, and thus these areas play an important role in respiratory mechanosensation mediated by anxiety.
Similar content being viewed by others
Introduction
Respiratory sensations such as breathlessness are aversive and prevalent symptoms in many cardiorespiratory diseases. Brain mechanisms underlying respiratory mechanosensation remain poorly understood, thus, limiting our understanding of mechanisms that contribute to the experience of breathlessness. In the past two decades, respiratory-related evoked potential (RREP) studies have been conducted, providing excellent temporal resolution for investigating the neural processing of respiratory sensations such as respiratory occlusions by electroencephalography1,2,3,4,5,6. Short- and long- latency RREP peaks are indicative of the exogenous and endogenous nature of respiratory sensory processing of mechanical stimuli7,8,9. Source analyses and animal studies suggested that respiratory occlusions elicited activations over several brain areas including the somatosensory cortex (SI), somatosensory association cortex (SII), prefrontal, premotor, and supplementary motor cortices as well as over parietal areas, thalamus, and brainstems8,10,11,12,13. However, the limited spatial resolution of the RREP technique prevents robust interferences about the specific localisations of these activations within the brain. Therefore, recent studies have been using the high spatial resolution of the functional magnetic resonance imaging (fMRI) technique to investigate cortical and subcortical areas related to respiratory mechanosensation14,15,16,17,18,19,20,21,22,23. Most studies have utilized resistive loads to measure brain substrates related to dyspnea14,15,17,19,22,24,25, whereas a few other studies have used inhalation of elevated CO2 levels26,27 or threshold loads, respectively23. These studies found that not only sensorimotor areas such as the sensorimotor cortex, supplementary motor area, SII and thalamus, but also emotion-related areas such as insular cortex, amygdala, anterior cingulate cortex (ACC), hippocampus, and periaqueductal gray (PAG) were activated with inspiratory resistive loads. Partly, these activations in emotion-related areas such as insula, ACC, hippocampus and amygdala were associated with elevated levels of negative affect such as anxiety or increased unpleasantness of breathlessness14,15,19,21,25,28,29,30,31. In addition, a few researchers have used transient inspiratory occlusion in a single breath to measure brain activation associated with breathlessness16,18. For example, Jack et al. (2010) suggested that transient inspiratory occlusions (TIO) elicited activation in sensorimotor, pre-motor, and insular cortices in patients with idiopathic hyperventilation16. In our recent study, we found that TIO elicited significant brain activations in the thalamus, caudate, premotor area, and SII18.
Whether these brain activation patterns in response to transient inspiratory occlusions would differ between low- and high- anxious individuals remained unclear from these studies. However, various behavioural studies have provided evidence that anxiety is associated with elevated levels of breathlessness29,32,33,34,35,36. For example, greater anxiety was found associated with elevated levels of dyspnea during ergometer exercise32 and during activities of daily life in patients with COPD37. Marines-Price and the colleagues (2019) found that levels of dyspnea on exertion is associated with subjective ratings of anxiety and negative emotions in healthy obese women36. Moreover, RREP studies demonstrated that in an aversive environment, anxious individuals directed more neural processing capacities to respiratory sensory stimuli compared to a neutral environment38. Similarly, high-anxious individuals were also found to show decreased central neural inhibitory function in respiratory sensation39,40,41,42. However, the specific cortical and subcortical brain areas underlying these different activation patterns to TIO in individuals with different levels of anxiety were not identified.
Therefore, the purpose of this fMRI study was to identify differences between high- and low-anxious individuals in their cortical and subcortical brain activation patterns associated with respiratory mechanosensation as elicited by inspiratory occlusions. Across all individuals, we expected to observe neural activation in the somatosensory cortex, middle frontal cortex, the inferior parietal cortex, lentiform nucleus, caudate, and thalamus, as in our earlier study using TIO18. More specifically, we further hypothesized that high compared to low anxious individuals would demonstrate different levels of brain activations in emotion-related brain areas including the insula, hippocampus, and cingulate cortex. In additional explorative analyses we examined the associations between brain activation patterns and individual levels of anxiety and breathlessness.
Methods
Participants
Healthy nonsmoking adults with self-reported absence of cardio-respiratory and/or neurological diseases were pre-screened to ensure there were no metal implants, pacemakers or braces, and claustrophobia. Women with an age over 50 years were excluded to avoid potential confounding effects of menopause-related symptoms. A total of 34 participants (20 females, mean age = 23 ± 3.4 years) signed the informed consent and participated in the study. This study was reviewed and approved by the Institutional Review Board of the Chang Gung Medical Foundation (106-0880D). All methods in this study were performed in accordance with the relevant guidelines and regulations.
Respiratory apparatus
The general setting of the apparatus was described previously18,43,44,45. MRI experiments were conducted by a 3T MRI scanner (Prisma, Siemens, Erlangen, Germany) with 20-ch head coil at National Taiwan University. Briefly, participants were laying in supine position in the MRI scanner. They were breathing through a two-way non-rebreathing valve with a facemask (Hans Rudolph Inc., Kansas City, USA). The participant’s mouth pressure was monitored by a pressure tubing at the center of the non-rebreathing valve connected to a differential pressure transducer of a pneumotachograph amplifier (1110 series, Hans Rudolph Inc., Kansas City, MO, USA) and a PowerLab signal recording unit (ADInstruments Inc., Bella Vista, NSW, Australia). The two-way non-rebreathing valve was connected to a customized occlusion valve (Hans Rudolph Inc., Kansas City, USA), which can be controlled manually. To be compatible with the MRI requirement, the customized occlusion valve was placed 3 meters away from the MRI scanner (Hans Rudolph Inc., Kansas City, USA). The occlusion valve was manually controlled by the experimenter via a customized trigger outside of the scanning room. For the stimulus events, the experimenter manually triggered a Transistor-Transistor Logic (TTL) pulse to activate the solenoid, which allows the pressure air tank to close the occlusion valve.
Experiment protocol
After signing the consent form, participants performed a spirometric pulmonary function test to ensure that their baseline lung function (forced expiratory volume in 1 second, FEV1) was at least 70% of the normative values46. Then, the participants filled out the demographic information sheet and the State-Trait Anxiety Inventory (STAI) questionnaire. The STAI has established reliability and validity and is commonly used as a tool for measuring subjective ratings on state and trait levels of anxiety47. We used the state-version containing 20 items with the summary scores ranging from 20 (not anxious at all) to 80 (extremely anxious). Upon completion, participants were assisted to position the facemask and earplugs while laying supine with the head immobilized in the scanner head coil. The participants were instructed to breathe as normal as possible throughout the experiment. Occasional inspiratory occlusions of 150-ms were delivered randomly every 2 to 4 breaths at the onset of inspiration. The scan time was 12 minutes, which allowed at least 32 occluded breaths to be collected for data analysis. After the experiment, participants were given verbal instruction “please rate the level of breathless you experienced during this session that just ended” and rated their feeling of breathlessness during scanning using a Visual Analog Scale (0 = not at all breathless, and 100 = maximal level of breathlessness).
Image acquisition
A T1-weighted image was acquired using a three-dimensional gradient-echo sequence (MP-RAGE) with an isotropic resolution of 1 mm. The fMRI experiment was performed using a 3-T whole-body scanner (Siemens MRI Scanner MAGNETOM Prisma, Erlangen, Germany) with a 20-channel head array coil. The blood oxygenation level-dependent (BOLD) response in fMRI signals was taken as the surrogate of local neuronal activation following each occlusion event. 32 continuous axial slices (thickness = 4 mm) were acquired by using a gradient echo, echo-planar T2*-sensitive imaging sequence (TR = 2 s, TE = 32 ms; flip angle = 90 degrees; iPAT factor = 2, matrix = 64 × 64; field of view = 220 × 220 mm2).
Data analysis
The fMRI image processing and statistical analysis was performed with SPM8 (http://www.fil.ion.ucl.ac.uk/spm/, Department of Imaging Neuroscience, University College London, UK) and analysis of functional neuroimages (AFNI)48. All images were realigned to the first image, spatially normalized into standard anatomical space based on the MNI template and smoothed with an isotropic Gaussian kernel of 6-mm full-width at half-maximum. At the individual level, every short inspiratory occlusion was regarded as a stimulus event that induced corresponding hemodynamic responses, whereas the flat signal fluctuations during normal breaths were taken as the baseline. The statistical analysis was performed with the general linear model with time and dispersion derivatives, and the six motion parameters were included as nuisance regressors. After the model estimation, the generated beta map for every individual reflected the magnitude of the BOLD responses induced by transient inspiratory occlusions. At the group level, the activation map for all participants was determined using the voxel-wise one-sample t-tests with the significance level of p < 0.05 corrected for family-wise error (FWE) rate. Moreover, one-sample t-tests were used for the whole group of 34 participants to examine the group averaged beta values within selected regions of interest (ROIs) across the cortical and subcortical areas, where spherical ROIs were centered at the peak of activated brain areas with a radius of 5 mm.
Participants were then divided into a high anxious group (N = 17) and low anxious group (N = 17) based on a median split of the group’s STAI-S score (median = 36.5). Independent two-sample t-tests were used to examine the differences in brain activations between groups (high-anxious vs. low-anxious). To further investigate the group difference in brain activations, we reported the fMRI results at a corrected significance level of p < 0.05 using AFNI 3dClustSim correction concerning the autocorrelation function (uncorrected threshold of p < 10−4 and cluster size of 39 voxels). In addition, the ROI-based beta contrasts were used to examine the differences in BOLD activity between low- and high-anxious individuals.
Finally, associations of brain activation during inspiratory occlusions with individual levels of anxiety (STAI-S) and breathlessness (VAS) across the full group (N = 34) were examined by off-line correlation analyses (Pearson’s r) using SPSS software (SPSS Inc., Chicago, IL) using a significant level of p < 0.05.
Results
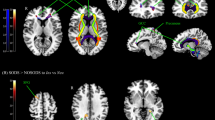
Table 1 presents baseline characteristics with mean (SD) for all participants. Briefly, 34 participants with 20 females and 14 males were tested. There was no significant difference between the high- and low-anxious groups in terms of age and FEV1 in % of predicted value. There were more females in the high-anxious group compared to the low-anxious group (13 and 7 females, respectively). As expected, the high-anxious group had higher average scores for the STAI, compared to the low-anxious group (44.76 ± 6.13 vs. 28.65 ± 4.9, p < 0.05). Figure 1 shows the activation map of the effect of inspiratory occlusions for the full group of 34 individuals. Significant activations were observed in the thalamus, caudate, putamen, precuneus, supramarginal gyrus, cingulate cortex, temporal lobe, SII, frontal cortex, and inferior parietal cortex using a threshold of p < 0.05 (FWE corrected).
Averaged brain activation maps for the full group of participants (N = 34) during inspiratory occlusion compared to baseline conditions. Significant activations were observed in the thalamus, caudate, putamen, precuneus, supramarginal gyrus, cingulate cortex, temporal lobe, SII, frontal cortex, and inferior parietal cortex using a threshold of p < 0.05 (FWE corrected).
The subgroup analysis compared brain activations during inspiratory occlusions between the high- (N = 17) and low-anxious (N = 17) groups. The SPM analysis revealed no significant differences in activation under the FWE correction criteria between groups. However, the voxel-wise one sample t-tests revealed significantly activated areas within each group. Table 2 and Fig. 2 represent the regions significantly activated in the low-anxious group, whereas Table 3 and Fig. 3 represent the regions significantly activatied in the high-anxious group (corrected p < 0.05). Our results showed that low anxious individuals demonstrated higher levels of brain activation in the supramarginal gyrus compared to the higher anxious individuals. In contrast, the high-anxious group showed strong activations in the middle cingulate cortex, insular cortex, and hippocampus, which were not observed in the low-anxious group.
Explorative correlational analyses showed that the STAI-S scores were negatively correlated with activation (beta values) of the right inferior parietal cortex (r = −0.39, p = 0.02), and the right precuneus (r = −0.36, p = 0.04) (see Fig. 4). In addition, levels of breathlessness were positively correlated with activation (beta values) of the right thalamus (r = 0.49, p = 0.003), the left thalamus (r = 0.45, p = 0.008), the right insula (r = 0.43, p = 0.01), the left insula (r = 0.48, p = 0.004), the right cingulate cortex (r = 0.50, p = 0.002), and the left cingulate cortex (r = 0.58, p < 0.001) (see Fig. 5).
Scatter plots showing the correlations between the participants’ subjective ratings on breathlessness and the brain activation levels (expressed as the beta values); Correlations between breathlessness level and activation in bilateral thalamus (a), bilateral insula (b), and bilateral cingulate cortex (c).
Discussion
The present study examined the brain activation patterns in response to transient inspiratory occlusions. We further analyzed the difference in the activated brain areas between a group of high- and low-anxious individuals and explored associations between levels of anxiety and breathlessness with brain activations. Our results demonstrated that cortical and subcortical regions including thalamus, inferior parietal lobe, frontal lobe, temporal lobe, and SII were activated by respiratory mechanical stimulation, consistent with our recent study18. Our results further revealed that low anxious individuals showed a significant level of brain activation, especially at supramarginal gyrus. The high-anxious group showed a strong activation level in the middle cingulate cortex, insular cortex, and hippocampus, which were not observed in the low-anxious group. Moreover, significant relationships were found between anxiety levels and activations of the inferior parietal gyrus and the precuneus as well as between breathlessness levels and activations of the thalamus, insula, superior frontal gyrus, and cingulate gyrus.
The present findings are consistent with earlier studies examining brain activations in response to respiratory stimulation and breathlessness14,15,18,19,20,21,23,27. For example, Evans et al.27 used hypercapnic breathing together with reduced tidal volume as manipulated by mechanical ventilation as experimental stimuli to test related brain substrates. Comparable to the present study, they found that thalamus, caudate, sensorimotor, parietal and prefrontal cortices, next to other areas, were related to dyspnea perception. McKay et al.20 investigated neural correlates of voluntary breathing and found that cortical and subcortical areas such as sensory motor areas, thalamus, and caudate were activated next to cerebellum and medulla. Raux et al.23 discovered that single threshold loaded breathing increased brain activations areas including premotor cortices, right parietal cortex, bilateral thalamus, and bilateral insula, whereas it decreased BOLD activities in other areas including the anterior cingulate cortices, and temporal cortices. Our earlier study in testing brain activations with similar mechanical stimuli also showed that thalamus, supramarginal gyrus, middle frontal cortex, and caudate were activated (Chan et al., 2018). Together, these studies suggest common activations in the thalamus, supramarginal gyrus, middle frontal gyrus, premotor cortices, parietal lobe, bilateral insula, and caudate during different forms of respiratory stimulation including inspiratory occlusions and loaded breathing.
Moreover, during the respiratory occlusion events, the low-anxious group showed a trend of more extensive hemodynamic response in the inferior parietal cortex (IPC), especially the supramarginal gyrus, and in the middle frontal gyrus, than the high-anxious group did. This trend suggests that the low-anxious group might have possessed more resources in subsequent neural processes after initial sensing of respiratory occlusions than the high-anxious group. This notion is paralleled by the findings in some previous reports in the field38,42,45,49. For example, Chan et al. (2014) found that patients with generalized anxiety disorder exhibited lower amplitudes of the RREP P3 peak, suggesting they used less neural resources to process attention-related tasks in respiratory sensation45. With high-density EEG methodology, von Leupoldt et al. (2011) further suggested that that the sources of P2 and P3 peaks of the RREP were likely diversified in the association cortices38. Miller & Cohen (2001) mentioned that the frontal area retains emotion-related memories from the limbic system50. Taken together, the above evidence indicates that long-latency RREP peaks were probably generated in both the secondary somatosensory cortices and association motor cortices, and higher-order cognitive processing of respiratory sensation is closely related to the individuals’ anxiety status38,43,45.
Our results also demonstrated that the high-anxious group had a trend of increased neural activation in some ROIs including the insula, middle cingulate cortex, and hippocampus during respiratory occlusion events, which were not observed in the lower anxious group. These findings are consistent with some previous studies examining the effects of anxiety and negative affect, or diseased states14,15,19,21,25,29,30,31. For example, Stoeckel et al. (2016) used resistive loading to elicit dyspnea sensation in healthy subjects. They found that the participants’ showed increased level of neural activations in the limbic system, where activation in the insula and anterior cingulate cortex (ACC) during anticipation were positively associated with anticipatory fear. This evidence together with another study of Stoeckel et al.’s (2018) indirectly supported our notion of state anxiety interacting with neural processing of respiratory mechanosensation. Moreover, in the study of Carlson et al.51 using an auditory anticipation task, the right insular was found predictive of the subjects’ anticipatory anxiety for aversive and neutral stimuli, whereas the amygdala was predictive of anxiety for aversive stimuli only. In our study, the transient inspiratory occlusions given intermittently during the inspiratory phase were not perceived as aversive stimuli by most subjects, which may be the reason that activation of the amygdala did not emerge to be significant in the current study51. In our study, the hippocampus was significantly activated in the higher anxious group. This is comparable with some previous studies where patients with anxiety were found to more easily overgeneralized for dangerous stimuli in different contexts52,53. It can be speculated that, in the higher-anxious group, our transient, non-threat occlusion stimuli still somehow mimic dyspneic experiences and triggered hippocampal activation, although this concept needs to be further investigated. Lastly, Esser et al.17 reported that emotion-related areas such as the hippocampus were more activated in patients with moderate-to-severe COPD compared to healthy controls during the anticipation of increasing dyspnea, which was related to increased anxiety levels in the patient group. The above evidence underlines the prominent role of the limbic system structures for mediating the effects of anxiety and negative affect on respiratory sensation.
The high- and low- anxious groups were divided based on the median of the participants’ anxiety score measured by the STAI. There was no significant difference between the two sub-groups on their reported breathlessness score measured by the VAS. The reason for this is that the VAS scale measures the subjects’ feeling of breathlessness, where the subjects may have mainly focused on rating the intensity of the breathlessness, rather than the aversive feeling, resulted from transient inspiratory occlusions. According to the literature, it is the affective aspect of the dyspnea that is directly related to the individuals’ emotional status54. Therefore, the difference in the reported breathlessness levels did not directly reflect the subjects’ anxiety levels in our study. Another possibility for the inconsistency between the anxiety levels and the dyspnea rating is that the study participants are all healthy adults with only high- and low- levels of anxiety states, rather than comparisons between healthy adults and patients with diagnosis, and therefore no differences were found statistically. Previous literature has demonstrated that negative affective states enhance patients’ dyspnea perception55. It will be interesting for future studies to test the brain activation patterns in response to respiratory occlusions in patients with the fMRI.
Our correlational analyses between the behavioural data and the brain activation levels showed several significant relationships. Although our participants did not rate the level of breathlessness intensity and level of breathlessness unpleasantness separately, our results showed that the higher the subjective ratings of overall breathlessness, the higher the activations in the thalamus, insula, and cingulate cortex. The relationship between the insula and the unpleasantness dimension of breathlessness was highlighted in some earlier studies14,56. In addition, our results demonstrated that the higher the anxiety of the individuals, the lower the activation of the right inferior parietal lobe and the right precuneus. This finding about the somatosensory association cortex again supports the previous findings of RREP studies where higher anxious individuals exhibited lower levels of neural activations in the cognitive processing of respiratory sensation38,45. In addition, Farb and colleagues (2013) also suggested that the precuneus is a part of the medial parietal network associated with respiratory interoceptive awareness57. In line with these previous reports, our results suggest that the precuneus might mediate respiratory interoception as a function of individuals’ emotional status.
In summary, the present study examined cortical and subcortical brain substrates in response to transient inspiratory occlusions in relation with the effect of individuals’ anxiety state. Our results suggest that, in response to transient inspiratory occlusions, higher anxious individuals have significant neural activations in the insular cortex, middle cingulate cortex, and hippocampus, which was not observed in lower anxious individuals. Also, individuals’ anxiety levels were closely associated with the neural activation levels in specific brain areas including the inferior parietal gyrus and precuneus, suggesting important roles of these areas in mediating cerebral respiratory sensation elicited by inspiratory occlusions.
References
Davenport, P. W., Friedman, W. A., Thompson, F. J. & Franzen, O. Respiratory-related cortical potentials evoked by inspiratory occlusion in humans. J Appl Physiol 60, 1843–1848 (1986).
Chan, P. Y. & Davenport, P. W. Respiratory related evoked potential measures of cerebral cortical respiratory information processing. Biol Psychol 84, 4–12 (2010).
von Leupoldt, A., Chan, P. Y., Esser, R. W. & Davenport, P. W. Emotions and neural processing of respiratory sensations investigated with respiratory-related evoked potentials. Psychosom Med 75, 244–252, https://doi.org/10.1097/PSY.0b013e31828251cf (2013).
Fauroux, B. et al. Impaired cortical processing of inspiratory loads in children with chronic respiratory defects. Respir Res 8, 61, https://doi.org/10.1186/1465-9921-8-61 (2007).
Ruehland, W. R. et al. Sensory detection of threshold intensity resistive loads in severe obstructive sleep apnoea. Respir Physiol Neurobiol 236, 29–41, https://doi.org/10.1016/j.resp.2016.10.014 (2017).
Harver, A., Squires, N. K., Bloch-Salisbury, E. & Katkin, E. S. Event-related potentials to airway occlusion in young and old subjects. Psychophysiology 32, 121–129 (1995).
Webster, K. E. & Colrain, I. M. The respiratory-related evoked potential: effects of attention and occlusion duration. Psychophysiology 37, 310–318 (2000).
Davenport, P. W., Colrain, I. M. & Hill, P. M. Scalp topography of the short-latency components of the respiratory-related evoked potential in children. J Appl Physiol 80, 1785–1791 (1996).
Davenport, P. W., Chan, P. Y., Zhang, W. & Chou, Y. L. Detection threshold for inspiratory resistive loads and respiratory-related evoked potentials. J Appl Physiol 102, 276–285 (2007).
Logie, S. T., Colrain, I. M. & Webster, K. E. Source dipole analysis of the early components of the RREP. Brain Topogr 11, 153–164 (1998).
von Leupoldt, A., Keil, A. & Davenport, P. W. Respiratory-related evoked potential measurements using high-density electroencephalography. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology 122, 815–818, https://doi.org/10.1016/j.clinph.2010.10.031 (2011).
Davenport, P. W., Reep, R. L. & Thompson, F. J. Phrenic nerve afferent activation of neurons in the cat SI cerebral cortex. J Physiol 588, 873–886, https://doi.org/10.1113/jphysiol.2009.181735 (2010).
Yates, J. S., Davenport, P. W. & Reep, R. L. Thalamocortical projections activated by phrenic nerve afferents in the cat. Neurosci Lett 180, 114–118 (1994).
von Leupoldt, A. et al. The unpleasantness of perceived dyspnea is processed in the anterior insula and amygdala. Am J Respir Crit Care Med 177, 1026–1032 (2008).
von Leupoldt, A. et al. Down-regulation of insular cortex responses to dyspnea and pain in asthma. Am J Respir Crit Care Med 180, 232–238, https://doi.org/10.1164/rccm.200902-0300OC (2009).
Jack, S., Kemp, G. J., Bimson, W. E., Calverley, P. M. & Corfield, D. R. Patterns of brain activity in response to respiratory stimulation in patients with idiopathic hyperventilation (IHV). Adv Exp Med Biol 669, 341–345, https://doi.org/10.1007/978-1-4419-5692-7_70 (2010).
Esser, R. W. et al. Brain Activation during Perception and Anticipation of Dyspnea in Chronic Obstructive Pulmonary Disease. Front Physiol 8, 617, https://doi.org/10.3389/fphys.2017.00617 (2017).
Chan, P. Y. et al. Cortical and Subcortical Neural Correlates for Respiratory Sensation in Response to Transient Inspiratory Occlusions in Humans. Front Physiol 9, 1804, https://doi.org/10.3389/fphys.2018.01804 (2018).
Hayen, A. et al. Opioid suppression of conditioned anticipatory brain responses to breathlessness. NeuroImage 150, 383–394, https://doi.org/10.1016/j.neuroimage.2017.01.005 (2017).
McKay, L. C., Evans, K. C., Frackowiak, R. S. & Corfield, D. R. Neural correlates of voluntary breathing in humans. J Appl Physiol (1985) 95, 1170–1178, https://doi.org/10.1152/japplphysiol.00641.2002 (2003).
Paulus, M. P. et al. Subjecting elite athletes to inspiratory breathing load reveals behavioral and neural signatures of optimal performers in extreme environments. PLoS One 7, e29394, https://doi.org/10.1371/journal.pone.0029394 (2012).
Yu, L. et al. Functional connectivity and information flow of the respiratory neural network in chronic obstructive pulmonary disease. Human brain mapping 37, 2736–2754, https://doi.org/10.1002/hbm.23205 (2016).
Raux, M. et al. Functional magnetic resonance imaging suggests automatization of the cortical response to inspiratory threshold loading in humans. Respir Physiol Neurobiol 189, 571–580, https://doi.org/10.1016/j.resp.2013.08.005 (2013).
Peiffer, C., Poline, J. B., Thivard, L., Aubier, M. & Samson, Y. Neural substrates for the perception of acutely induced dyspnea. Am J Respir Crit Care Med 163, 951–957 (2001).
Peiffer, C., Costes, N., Herve, P. & Garcia-Larrea, L. Relief of dyspnea involves a characteristic brain activation and a specific quality of sensation. Am J Respir Crit Care Med 177, 440–449, https://doi.org/10.1164/rccm.200612-1774OC (2008).
Binks, A. P., Evans, K. C., Reed, J. D., Moosavi, S. H. & Banzett, R. B. The time-course of cortico-limbic neural responses to air hunger. Respir Physiol Neurobiol 204, 78–85, https://doi.org/10.1016/j.resp.2014.09.005 (2014).
Evans, K. C. et al. BOLD fMRI identifies limbic, paralimbic, and cerebellar activation during air hunger. Journal of neurophysiology 88, 1500–1511 (2002).
Faull, O. K. & Pattinson, K. T. The cortical connectivity of the periaqueductal gray and the conditioned response to the threat of breathlessness. Elife 6, https://doi.org/10.7554/eLife.21749 (2017).
Stoeckel, M. C., Esser, R. W., Gamer, M., Buchel, C. & von Leupoldt, A. Dyspnea catastrophizing and neural activations during the anticipation and perception of dyspnea. Psychophysiology 55, https://doi.org/10.1111/psyp.13004 (2018).
Stoeckel, M. C., Esser, R. W., Gamer, M., Buchel, C. & von Leupoldt, A. Brain mechanisms of short-term habituation and sensitization toward dyspnea. Frontiers in psychology 6, 748, https://doi.org/10.3389/fpsyg.2015.00748 (2015).
Stoeckel, M. C., Esser, R. W., Gamer, M., Buchel, C. & von Leupoldt, A. Brain Responses during the Anticipation of Dyspnea. Neural Plast 2016, 6434987, https://doi.org/10.1155/2016/6434987 (2016).
Janssens, T. et al. Dyspnea perception in COPD: association between anxiety, dyspnea-related fear, and dyspnea in a pulmonary rehabilitation program. Chest 140, 618–625, https://doi.org/10.1378/chest.10-3257 (2011).
Brenes, G. A. Anxiety and chronic obstructive pulmonary disease: prevalence, impact, and treatment. Psychosom Med 65, 963–970 (2003).
Livermore, N., Sharpe, L. & McKenzie, D. Panic attacks and panic disorder in chronic obstructive pulmonary disease: a cognitive behavioral perspective. Respir Med 104, 1246–1253, https://doi.org/10.1016/j.rmed.2010.04.011 (2010).
von Leupoldt, A. & Janssens, T. Could targeting disease specific fear and anxiety improve COPD outcomes? Expert Rev Respir Med 10, 835–837, https://doi.org/10.1080/17476348.2016.1198697 (2016).
Marines-Price, R., Bernhardt, V., Bhammar, D. M. & Babb, T. G. Dyspnea on exertion provokes unpleasantness and negative emotions in women with obesity. Respir Physiol Neurobiol 260, 131–136, https://doi.org/10.1016/j.resp.2018.11.008 (2019).
Reijnders, T. et al. The impact of disease-specific fears on outcome measures of pulmonary rehabilitation in patients with COPD. Respir Med 146, 87–95, https://doi.org/10.1016/j.rmed.2018.12.004 (2019).
von Leupoldt, A., Chan, P. Y., Bradley, M. M., Lang, P. J. & Davenport, P. W. The impact of anxiety on the neural processing of respiratory sensations. NeuroImage 55, 247–252, https://doi.org/10.1016/j.neuroimage.2010.11.050 (2011).
Chan, P. Y., von Leupoldt, A., Bradley, M. M., Lang, P. J. & Davenport, P. W. The effect of anxiety on respiratory sensory gating measured by respiratory-related evoked potentials. Biol Psychol 91, 185–189, https://doi.org/10.1016/j.biopsycho.2012.07.001 (2012).
Chan, P. Y., Cheng, C. H., Hsu, S. C., Liu, C. Y. & Von Leupoldt, A. Respiratory Sensory Gating measured by Respiratory-Related Evoked Potentials in Generalized Anxiety Disorder. Frontiers in psychology (2015).
Herzog, M. et al. Reduced neural gating of respiratory sensations is associated with increased dyspnoea perception. Eur Respir J 52, https://doi.org/10.1183/13993003.00559-2018 (2018).
Chan, P. Y. & Davenport, P. W. The role of nicotine on respiratory sensory gating measured by respiratory-related evoked potentials. J Appl Physiol (1985) 108, 662–669, https://doi.org/10.1152/japplphysiol.00798.2009 (2010).
Chan, P. Y., Cheng, C. H., Jhu, Y. J., Chen, C. L. & von Leupoldt, A. Being Anxious, Thinking Positively: The Effect of Emotional Context on Respiratory Sensory Gating. Front Physiol 7, 19, https://doi.org/10.3389/fphys.2016.00019 (2016).
Chan, P. Y., Cheng, C. H. & von Leupoldt, A. The Effect of Development in Respiratory Sensory Gating Measured by Electrocortical Activations. Neural Plast 2015, 389142, https://doi.org/10.1155/2015/389142 (2015).
Chan, P. Y., von Leupoldt, A., Liu, C. Y. & Hsu, S. C. Respiratory perception measured by cortical neural activations in individuals with generalized anxiety disorder. Respir Physiol Neurobiol 204, 36–40, https://doi.org/10.1016/j.resp.2014.09.009 (2014).
Miller, M. R. et al. Standardisation of spirometry. Eur Respir J 26, 319–338, https://doi.org/10.1183/09031936.05.00034805 (2005).
Spielberger, C. D. & Vagg, P. R. Psychometric properties of the STAI: a reply to Ramanaiah, Franzen, and Schill. J Pers Assess 48, 95–97, https://doi.org/10.1207/s15327752jpa4801_16 (1984).
Cox, R. W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29, 162–173 (1996).
von Leupoldt, A. et al. Dyspnea and pain share emotion-related brain network. NeuroImage 48, 200–206, https://doi.org/10.1016/j.neuroimage.2009.06.015 (2009).
Miller, E. K. & Cohen, J. D. An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24, 167–202, https://doi.org/10.1146/annurev.neuro.24.1.167 (2001).
Carlson, J. M., Greenberg, T., Rubin, D. & Mujica-Parodi, L. R. Feeling anxious: anticipatory amygdalo-insular response predicts the feeling of anxious anticipation. Social cognitive and affective neuroscience 6, 74–81, https://doi.org/10.1093/scan/nsq017 (2011).
Lissek, S. Toward an account of clinical anxiety predicated on basic, neurally mapped mechanisms of Pavlovian fear-learning: the case for conditioned overgeneralization. Depression and anxiety 29, 257–263, https://doi.org/10.1002/da.21922 (2012).
Morey, R. A. et al. Fear learning circuitry is biased toward generalization of fear associations in posttraumatic stress disorder. Transl Psychiatry 5, e700, https://doi.org/10.1038/tp.2015.196 (2015).
von Leupoldt, A., Mertz, C., Kegat, S., Burmester, S. & Dahme, B. The impact of emotions on the sensory and affective dimension of perceived dyspnea. Psychophysiology 43, 382–386 (2006).
von Leupoldt, A., Taube, K., Henkhus, M., Dahme, B. & Magnussen, H. The impact of affective states on the perception of dyspnea in patients with chronic obstructive pulmonary disease. Biol Psychol 84, 129–134, https://doi.org/10.1016/j.biopsycho.2009.07.010 (2010).
Stewart, J. L. et al. You are the danger: attenuated insula response in methamphetamine users during aversive interoceptive decision-making. Drug Alcohol Depend 142, 110–119, https://doi.org/10.1016/j.drugalcdep.2014.06.003 (2014).
Farb, N. A., Segal, Z. V. & Anderson, A. K. Attentional modulation of primary interoceptive and exteroceptive cortices. Cereb Cortex 23, 114–126, https://doi.org/10.1093/cercor/bhr385 (2013).
Acknowledgements
The authors wish to thank the Imaging Center for Integrated Body, Mind and Culture Research of the National Taiwan University for technical and facility (fMRI) supports. This study was supported by the MOST- 103-2420-H-182-003-MY2 and MOST-105-2420-H-182-002-MY3 research grants from the Ministry of Science & Technology as well as the BMRPB96 grant from the Chang Gung Memorial Hospital in Taiwan. A.v.L. was supported by grants from the Research Fund KU Leuven, Belgium (STRT/13/002), the Research Foundation – Flanders, Belgium (G0A3718N), by an infrastructure grant from the Herculesstichting, Belgium (AKUL/13/07) and by the ‘Asthenes’ long-term structural funding Methusalem grant (METH/15/011) by the Flemish Government, Belgium.
Author information
Authors and Affiliations
Contributions
P.Y.C. conceived and designed the experiments. Y.T.W., A.L.H. and C.W.L. performed the experiments. Y.T.W., A.L.H., C.W.L. and C.W.W. analyzed the data. P.Y.C., C.W.W., A.v.L. and S.C.H. interpreted the data. P.Y.C. wrote the manuscript. All authors reviewed and revised the manuscript critically.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chan, PY.S., Wu, YT., Hsu, AL. et al. The effect of anxiety on brain activation patterns in response to inspiratory occlusions: an fMRI study. Sci Rep 9, 15045 (2019). https://doi.org/10.1038/s41598-019-51396-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-51396-2
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.