Abstract
The positive effects of arbuscular mycorrhizal fungi (AMF) have been demonstrated for plant biomass, and zinc (Zn) and phosphorus (P) uptake, under soil nutrient deficiency. Additionally, a number of Zn and P transporter genes are affected by mycorrhizal colonisation or implicated in the mycorrhizal pathway of uptake. However, a comprehensive study of plant physiology and gene expression simultaneously, remains to be undertaken. Medicago truncatula was grown at different soil P and Zn availabilities, with or without inoculation of Rhizophagus irregularis. Measures of biomass, shoot elemental concentrations, mycorrhizal colonisation, and expression of Zn transporter (ZIP) and phosphate transporter (PT) genes in the roots, were taken. Mycorrhizal plants had a greater tolerance of both P and Zn soil deficiency; there was also evidence of AMF protecting plants against excessive Zn accumulation at high soil Zn. The expression of all PT genes was interactive with both P availability and mycorrhizal colonisation. MtZIP5 expression was induced both by AMF and soil Zn deficiency, while MtZIP2 was down-regulated in mycorrhizal plants, and up-regulated with increasing soil Zn concentration. These findings provide the first comprehensive physiological and molecular picture of plant-mycorrhizal fungal symbiosis with regard to soil P and Zn availability. Mycorrhizal fungi conferred tolerance to soil Zn and P deficiency and this could be linked to the induction of the ZIP transporter gene MtZIP5, and the PT gene MtPT4.
Similar content being viewed by others
Introduction
Zinc (Zn) is important both for agricultural production and for human development. A deficiency of Zn can seriously affect plant and human development because Zn is a regulatory co-factor and structural constituent in proteins and enzymes involved in many biochemical pathways1,2. Soil Zn deficiency affects millions of hectares of cropland worldwide, and is particularly prevalent in developing countries, and in the cereal-growing regions of Australia2,3. In addition, approximately one third of the global population suffers from an inadequate dietary intake of Zn4,5,6. Therefore, increasing the level of Zn uptake by crops - known as biofortification - is a subject of considerable international interest7,8,9.
Phosphorus (P) is one of the most important macronutrients for plant growth. Soil P deficiency leads to reduced plant nutrient uptake, prolonged maturity stage10,11, and affects enzyme activity and many signal transduction cascades12. However, the primary source of P fertilizer (phosphate rock) for plant growth is a finite resource, and thus is becoming depleted over time because of demand from the agricultural sector13,14. To effectively manage the available levels of soil Zn and P, an advanced understanding of plant root uptake capacity of nutrients is required, in particular, of plant membrane transporters related to P and Zn transport15. Additionally, it is important to understand factors that affect P and Zn transport gene regulation, such as associations with arbuscular mycorrhizal fungi (AMF), which can enhance plant nutrient uptake16.
AMF form associations with more than 80% of flowering plant species, and form part of the function of plant root systems17,18. AMF colonise the root cortex, and can extend their hyphal network into the surrounding soil environment19,20. These external hyphae contribute to plant uptake of P and Zn, as well as other mineral nutrient including iron (Fe), calcium (Ca), and copper (Cu)18. Previous research has demonstrated that AMF can enhance plant Zn uptake, sometimes leading to a boost in plant growth and Zn concentration in plant tissue21,22. Furthermore, P concentration in the shoots and roots of mycorrhizal plants can be significantly higher than in non-mycorrhizal plants grown under soil P deficiency23,24,25,26. Additionally, AMF can reduce plant heavy metal uptake (Zn, copper (Cu), lead (Pb), arsenic (As)) in contaminated soils, thereby protecting plants from toxic effects27,28. Studies on red clover and tomato, respectively, have established that Zn content uptake in shoots and roots decreased substantially under high soil Zn concentration when plants were colonised by AMF29,30.
There are two pathways for plant uptake of nutrients from soil: via root epidermal cells (direct pathway; DPU), and via associations with arbuscular mycorrhizal fungi (mycorrhizal pathway; MPU). In plants, Zn is taken up from the rhizosphere as Zn2+ via ZIP (Zrt, Irt-like Protein) membrane transporters31. Some ZIP transporters have the potential to also transport Fe2+ and Mn2+ 31,32. Several studies have focused on characterising the ZIP transporter family in different plant species, including barley32,33,34,35, rice36, potato, and Arabidopsis thaliana31. In Medicago truncatula (Medicago), four ZIP transporters - MtZIP1, MtZIP2, MtZIP5 and MtZIP6 - are able to transport Zn2+, as confirmed by yeast complementation assays, have been identified37,38. As such, Medicago provide a model plant species for studies of mycorrhizal impacts on plant Zn (and P, see below) nutrition. Additionally, MtZIP2 has been localised to the plasma membrane in onion epidermal cells39. Further work is required to discover whether any of these ZIP transporters are implicated in the mycorrhizal transport of Zn into plants (via the MPU).
In terms of plant P uptake, the phosphate transporters (PTs) MtPT1, MtPT2, and MtPT3 are involved in the DPU for P uptake, and are closely related to low-affinity P transporters, belonging to the Pht1 family40,41. The genes encoding these PTs are generally highly expressed when plants are growing under low soil P conditions. However, in roots colonised by AMF, MtPT1 and MtPT2 are down-regulated significantly as the symbiosis develops26,42,43. Furthermore, one of the PTs in M. truncatula (MtPT4) has been demonstrated to be a mycorrhiza-induced phosphate transporter44. MtPT4 is expressed exclusively in mycorrhizal roots, and specifically, in cells containing arbuscules44. Loss of MtPT4 function in plants leads to impairment of the mycorrhizal symbiosis, because it causes mature arbuscules to degenerate, resulting in premature arbuscular death45,46. In addition, MtPT8 from the Pht1 family has also been identified to be induced upon formation of the mycorrhizal symbiosis, and contribute to the uptake of P ions released by the fungal membranes47,48,49.
A phosphate starvation-induced (PSI) gene in M. truncatula, MT426,50, has been shown to be involved in P accumulation in plants, and is down-regulated in response to both P fertilisation and to mycorrhizal colonization51. More recently, another gene (LysoPhosphatidylCholine AcylTransferase 1; AtLPCAT1) has been demonstrated to be involved in the accumulation of P in the shoot under soil Zn deficiency in A. thaliana (a non-mycorrhizal species)52. Therefore, these genes are also of interest with regards to plant responses to P and Zn deficiency in the present study.
In M. truncatula, there is evidence that mycorrhizal inoculation affects plant P and Zn uptake at different soil P concentrations, and at a range of soil Zn concentrations, ranging from deficient to toxic30,53,54. However, there presently exists a lack of research focusing on the three-way interaction between soil P and Zn availabilities and AMF inoculation, that also addresses potential underlying molecular mechanisms. Therefore, the current study aimed to link the mycorrhizal effects under variable P and Zn soil conditions, on whole plant physiology, with expression of molecular markers for Zn and P uptake. Specifically, there were several hypotheses related to the experiment:
-
1.
That the effects of soil Zn and P availability were interactive with AMF function, and
-
2.
That the effects of AMF on plant nutrition and gene expression could be linked:
-
a.
In relation to the dual role of AMF at low and high soil Zn availability, and
-
b.
In relation to the role of AMF in improving P uptake at low soil P availability
-
a.
Material and Methods
Field soil was collected from the Mallala region of South Australia, and had a pH of 7.1 and plant-available (Colwell) P concentration of 22 mg kg−1. Soil was sieved to <2 mm, and both fine sand and soil were autoclaved twice, then dried, before being mixed in a ratio of 9:1 sand/soil53. The sand/soil mix was further mixed with 10% (140 g per pot) of either a Rhizophagus irregularis WFVAM10 or a non-mycorrhizal mock inoculum (see below) to a total mass of 1.4 kg per pot. The R. irregularis inoculum was added as a mixture of dry soil, spores, external AMF hyphae and root fragments of Trifolium subterraneum L. (clover) cv. Mt. Barker pot cultures. The control, a mock inoculum, was a mixture of dry soil and root fragments of Trifolium subterraneum L. (clover) cv. Mt. Barker pots that had not been inoculated with AMF.
To establish soil Zn addition treatments, ZnSO4.7H2O solution was added to the soil/sand mix at the rates of 0, 5, 10, and 20 mg Zn kg−1 soil. This resulted in four soil Zn treatments, with DTPA-extractable Zn concentrations of 0.3, 4.0, 5.8, and 15.0 mg Zn kg−1, referred to hereafter as Zn0, Zn5, Zn10, and Zn20, respectively. Phosphorus treatments were established by adding K2HPO4 solution to the soil at concentrations of 0, 20, and 50 mg P kg−1 soil, resulting in three plant-available (Colwell) P concentrations: 4.4, 13.8, and 31.8 mg P kg−1, referred to hereafter as P0, P20, and P50, respectively. Plastic, non-draining pots were then filled with the prepared soils. Each soil Zn addition treatment, soil P addition treatment, and AMF treatment were combined in a factorial manner, so that in total there were 24 treatments, each with five biological replicates, giving a total of 120 pots.
Medicago truncatula cv. Jemalong A17 seeds were scarified using fine sandpaper, then surface-sterilised for 5 minutes by shaking in 10% sodium hypochlorite solution (NaClO). Then, seeds were washed and rinsed with reserve osmosis (RO) water before being placed onto moist filter paper in a Petri dish. The Petri dishes then were sealed with Parafilm, covered with aluminium foil and kept at 4 °C for four days. Following that, dishes were transferred to the bench and left covered at room temperature for one day, before being uncovered, unsealed, and left for a further three days while being provided water daily. When green cotyledons had emerged, seedlings were transplanted to the prepared soils (one plant per pot) following Watts-Williams, et al.53.
The M. truncatula plants were grown in a controlled environment glasshouse at the University of Adelaide, Waite campus, between March-April 2018 (Austral Autumn). Plants were watered three to four times per week (based on plant demand) with RO water to 10% of the soil weight. They also were nutritionally supplemented once per week with 10 mL of a modified Long-Ashton solution that omitted Zn and P53. Furthermore, to avoid Rhizobia bacteria forming nodules, plants also were supplemented with nitrogen as NH4NO3, solution to a total of 80 mg N per plant over the growing period. Plant position on the glasshouse bench was randomised, and re-randomised once per week.
All plants were destructively harvested 36 days post-transplantation. Shoots were cut at the soil level, then were weighed for fresh shoot biomass. Roots were washed thoroughly of soil and were then weighed for fresh root biomass, before subsamples were taken for mycorrhizal colonisation and gene expression analysis. The root subsamples were immediately flash frozen in liquid nitrogen and then stored at −80 °C (for gene expression studies; see below), or placed into 50% ethanol (EtOH) solution (for quantification of mycorrhizal colonisation; see below). Nodulation was observed in only three plants, indicating that N fertilisation was generally effective at suppressing nodulation by rhizobia. Both fresh shoot and remaining fresh root biomass were then dried at 60 °C for at least 48 hours before dry biomass was determined. Next, dry shoot biomass was ground to homogenise, then a weighed sub-sample was used for digestion using a 4:1 (v/v) mix of nitric acid (HNO3) and hydrogen peroxide (H2O2). Acid digests were then diluted with RO water, before being analysed for nutrient concentrations (including Zn, P and other nutrients) by Inductively Coupled Plasma-Atomic Emission Spectroscopy analytical technique (ICP-AES; Avio 200 ICP Optical Emission Spectrometer).
Fresh root subsamples stored in 50% EtOH were rinsed in RO water and then cleared by submerging in 10% (w/v) potassium hydroxide (KOH) at room temperature for seven days. The cleared roots were then rinsed again with RO water before being stained in a solution of 5% of ink in vinegar53,55 at 60 °C for 10 minutes. The root samples were then rinsed and de-stained in RO water for 24 hours, before being transferred to a 50% glycerol solution for storage and microscope assessment. Mycorrhizal colonisation was determined on the stained root samples under a dissection microscope using the gridline intersect method56.
For gene expression analysis, transcript levels of chosen M. truncatula ZIPs, PTs, and other genes were determined by quantitative real-time PCR (qPCR). The MtLPCAT1 gene was determined as the nearest M. truncatula orthologue of the AtLPCAT1 gene sequences by BLAST (https://phytozome.jgi.doe.gov/pz/portal.html#!search?show=BLAST; last accessed 15 October, 2018). The flash-frozen root subsamples were ground to a fine powder under liquid nitrogen. RNA was then extracted using a Spectrum Plant Total RNA kit (Sigma) including an on-column DNase treatment step (Sigma). Following this, the RNA yield and quality was quantified by Nanodrop, and the iScript cDNA synthesis kit (Bio-Rad) was used to synthesise cDNA from 800 ng of RNA. The expression of MtZIP genes, MtPT genes, and other genes-of-interest, as well as fungal α-tubulin gene (a marker gene for R. irregularis biomass in roots), were quantified by qPCR (QuantStudio 12 K Flex Real-Time PCR system, Applied Biosystems), using forward and reverse primers designed to target the specific genes (Supplementary Information Table 1). The primer pair for MtASPP amplification spanned an exon-exon boundary in the gene and were thus used to confirm there was no genomic DNA contamination following RNA extraction. Expression of each gene-of-interest was then normalised to the geometric mean of the expression of three M. truncatula housekeeping genes.
Data were checked for the assumption of normal distribution using Genstat (19th edition), and any non-normal data (p < 0.05) were log- or square root-transformed to conform to the assumption of normality. In Figures, presented values are non-normalised data. For the physiological and gene expression data, the response variables were subjected to three-factor analysis of variance (ANOVA), with Mycorrhiza, Zn, and P as treatment factors. Following ANOVA, the mycorrhizal and control treatment means were compared at each soil Zn and P treatment, respectively, by a Student’s t-test. For response variables where there was only mycorrhizal data (% mycorrhizal colonisation, MtPT4, MtPT8, and R. irregularis fungal α-tubulin expression), two-way ANOVA was applied, with P and Zn treatments the factors. Following ANOVA, where there was a significant main effect or interaction, comparisons between treatment means were made using Tukey’s honestly significant difference (HSD) post hoc test. The relationships between R. irregularis fungal α-tubulin and MtPT4 expression were analysed by regression in the mycorrhizal samples only. All statistical analyses were performed using Genstat (19th Edition) or Microsoft Excel 2016 (Office 365 ProPlus).
Results
Mycorrhiza, zinc and phosphorus interact to affect plant biomass and nutrition (Hypothesis 1)
In general, shoot dry weight (SDW) and root dry weight (RDW) increased dramatically with increasing soil P addition, with mean SDW values ranging from 0.042 g (mock-inoculated P0, Zn5) to 1.977 g (AMF-inoculated P50, Zn20) (Fig. 1a). In terms of AMF-inoculation, the SDW of the mycorrhizal plants were greater than the mock-inoculated plants when no P was applied, except in the Zn10 treatment; a similar result was found for the RDW (see Table 1 for ANOVA outcomes; Fig. 1b). Regardless of P application, the inoculated plants were also larger in terms of both shoots and roots, when no Zn was applied to the soil.
Shoot dry weights (a) and root dry weights (b) of Medicago truncatula plants inoculated with the AMF R. irregularis (grey bars) or mock-inoculated (white bars) and grown at different soil Zn (Zn0-Zn20) and P (P0-P50) concentrations. Values are mean ± SEM, n = 5. Means labelled with the same letter were not significantly different at the P < 0.05 level (Tukey’s HSD), see Table 1 for details of ANOVA results.
Shoot P concentration (Fig. 2a) and shoot P contents (Fig. 2b) revealed similar statistical trends, and increased substantially with increasing soil P addition. Furthermore, shoot P concentration was significantly higher in the mycorrhizal plants than in the mock-inoculated plants when soil P was limiting (P0). Shoot Zn concentration (Fig. 3a) was similar between the mycorrhizal and mock-inoculated plants in all treatments, except for in the Zn20 P20 treatment where it was higher in the mock-inoculated plants. Shoot Zn contents (Fig. 3b), revealed an effect of increasing soil Zn, but was not affected by mycorrhizal inoculation.
Shoot P concentration (a) and shoot P contents (b) in Medicago truncatula plants inoculated with the AMF R. irregularis (grey bars) or mock-inoculated (white bars), and grown at different soil Zn (Zn0-Zn20) and P (P0-P50) concentrations. Values are mean ± SEM, n = 5. Means labelled with the same letter were not significantly different at the P < 0.05 level (Tukey’s HSD), see Table 1 for details of ANOVA results.
Shoot Zn concentration (a) and shoot Zn contents (b) in Medicago truncatula plants inoculated with the AMF R. irregularis (grey bars) or mock-inoculated (white bars), and grown at different soil Zn (Zn0-Zn20) and P (P0-P50) concentrations. Values are mean ± SEM, n = 5. Means labelled with the same letter were not significantly different at the P < 0.05 level (Tukey’s HSD), see Table 1 for details of ANOVA results.
Mycorrhizal colonisation markers decrease under high phosphorus application
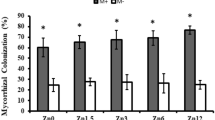
Mycorrhizal colonisation in AMF-inoculated roots was highest when Zn and P were the most limiting to the plant (P0, Zn0) (mean 75.8%; Fig. 4a), but decreased substantially with increasing soil P concentration to 8.7% in the P50, Zn5 treatment (see Table 2 for ANOVA outcomes). Mycorrhizal colonisation was also affected negatively by increasing soil Zn application, but only in the P0 and P50 treatments. The R. irregularis α-tubulin gene, a marker for live mycorrhizal fungal biomass in roots at the time of harvest, was expressed in a similar pattern to the root colonisation data (Fig. 4b) whereby expression decreased with increasing soil P application. There was no mycorrhizal colonisation observed in the roots of the mock-inoculated plants when examined by microscopy, nor expression of the R. irregularis α-tubulin gene in any of the mock-inoculated plants.
Root colonisation by arbuscular mycorrhizal fungal structures (a), and normalised expression of the AMF biomass marker gene α-tubulin (b) in the roots of Medicago truncatula plants inoculated with the AMF R. irregularis, and grown at different soil Zn (Zn0-Zn20) and P (P0-P50) concentrations. Values are mean ± SEM, n = 5. Means labelled with the same letter were not significantly different at the P < 0.05 level (Tukey’s HSD), see Table 2 for details of ANOVA results.
ZIP gene expression was modulated by mycorrhiza and Zn-deficiency (Hypothesis 2a)
The different ZIP genes were all affected by the Zn, P and AMF treatments in different ways, as follows. While the expression of MtZIP2 was primarily affected by the P and Zn application treatments (Fig. 5a), the expression of MtZIP5 and MtZIP6 expression were affected by the interaction between P, Zn, and mycorrhizal colonisation. Specifically, the expression of MtZIP2 increased between the lowest and the highest soil P treatments and, also increased between the lowest and highest soil Zn treatments. Expression of MtZIP5 was induced at the lowest Zn treatment (Zn0), and was also higher in the mycorrhizal plants than in mock-inoculated plants at Zn0 (Fig. 5b). Conversely, expression of MtZIP6 was significantly higher in the mock-inoculated plants than in the mycorrhizal plants in some treatments, but particularly where P and Zn additions were very low (Fig. 5c). The expression of MtZIP1 was analysed, but was very low and highly variable between biological replicates, so is not presented here.
Normalised expression of the Zn-transporting ZIP transporter genes: MtZIP2 (a), MtZIP5 (b), and MtZIP6 (c) in the roots of Medicago truncatula plants inoculated with the AMF R. irregularis (grey bars) or mock-inoculated (white bars), and grown at different soil Zn (Zn0-Zn20) and P (P0-P50) concentrations. Values are mean ± SEM, n = 5. Means labelled with the same letter were not significantly different at the P < 0.05 level (Tukey’s HSD), see Table 1 for details of ANOVA results.
Direct- and mycorrhizal-pathway PT genes display contrasting expression patterns (Hypothesis 2b)
The direct pathway PT gene MtPT1 was down-regulated in the mycorrhizal plants (Fig. 6a), as was the P-starvation-induced gene MtMT4 (Fig. 6b). The expression of both these genes was also repressed as soil P addition increased. Expression of MtLPCAT1 was affected by mycorrhizal inoculation, and was lower in the mock-inoculated plants than the mycorrhizal plants (pooling P and Zn treatments; Fig. 6c).
Normalised expression of the phosphate transporter gene MtPT1 (a), a phosphate starvation-induced (PSI) gene MtMT4 (presented on log-scale y-axis; b), and MtLPCAT1 (c) in the roots of Medicago truncatula plants inoculated with the AMF R. irregularis (grey bars) or mock-inoculated (white bars), and grown at different soil Zn (Zn0-Zn20) and P (P0-P50) concentrations. Values are mean ± SEM, n = 5. Means labelled with the same letter were not significantly different at the P < 0.05 level (Tukey’s HSD), see Table 1 for details of ANOVA results.
Finally, MtPT4 was expressed exclusively in the mycorrhizal roots, and there was a very clear reduction of expression as soil P addition increased (Fig. 7a), which correlated strongly and positively with R. irregularis α-tubulin expression (R2 = 0.8794) (Fig. 7b). In contrast, the expression of another AMF-induced PT, MtPT8, revealed a very different pattern to MtPT4, with increased expression between P0 and P20, and then decreased between P20 and P50 (Fig. 7c).
Normalised expression of the mycorrhiza-induced phosphate transporter (PT) gene MtPT4 (a) correlation between the expression of the R. irregularis biomass marker gene α-tubulin and MtPT4 (b), and expression of the mycorrhiza-induced PT MtPT8 (c) in the roots of Medicago truncatula inoculated with the AMF R. irregularis, and grown at different soil Zn (Zn0-Zn20) and P (P0-P50) concentrations. Values are mean ± SEM, n = 5. Means labelled with the same letter were not significantly different at the P < 0.05 level (Tukey’s HSD), see Table 2 for details of ANOVA results.
Discussion
Mycorrhizal plants are more tolerant of soil P and Zn deficiencies
The positive effects of forming mycorrhizal associations on plant growth are considered one of the main benefits of the symbiosis, and these effects have been studied extensively in various plant species57,58,59,60,61,62. In this study, the benefits of mycorrhizal fungal inoculation in terms of increased Medicago biomass were the greatest under low soil P concentration, which confirms earlier studies in this and other plant species13,30,63. In the two soil P addition treatments (P20, P50), there were smaller, albeit still positive, plant biomass responses to mycorrhizal inoculation. These smaller responses may be due to the sufficient levels of P in soil, which allowed plants to increase their biomass and nutrient uptake without the aid of mycorrhizal fungi. In an earlier study, it was demonstrated using radioisotope tracing that even where the biomass of mycorrhizal flax (Linum usitatissium) plants were not larger than mock-inoculated plants, the MPU was still active and transporting substantial amounts of P to the plant64. Thus, the growth response of a plant to mycorrhizal fungi, whether it is positive, neutral or negative, cannot be used to estimate the activity of the mycorrhizal pathway of P uptake. In the present study, the reduction in growth response was in line with the decreased percentage of mycorrhizal colonisation in the roots, when P was added to the soil, as demonstrated in previous studies in both the same plant and fungi species26 and in other species14,65,66,67,68. Consequently, the reduction in mycorrhizal colonisation and MGR with soil P addition points to a reduced contribution of P by the MPU; however, the actual activity of the MPU would need to be confirmed by radioisotope labelling.
With regards to soil Zn, colonisation by mycorrhizal fungi resulted in increased Medicago biomass under both soil Zn deficiency (Zn0) and soil Zn toxicity (Zn20); this result highlights the dual role of mycorrhizal fungi in ameliorating Zn stress for the host plant53,69,70. While the mycorrhizal plants thrived in soil with high levels of Zn addition (Zn20), the non-mycorrhizal plants experienced reduced growth and visual symptoms of Zn toxicity in the leaves, as in earlier study53. Additionally, at high soil P supply (P50), the non-mycorrhizal M. truncatula plants were not as affected by the high soil Zn concentration, and this ‘alleviation’ of Zn toxicity at high P availability is possibly a result of dilution of Zn in the plant tissues due to the greater P uptake and, thus, biomass of the plants63,71,72,73,74.
Medicago plants are more tolerant of Zn stress as deficiency or toxicity when colonised by AMF
In this study, there was evidence for the ‘dual roles’ of mycorrhizal fungi at low and at high soil Zn availabilities. When Zn was limiting to the plant, the mycorrhizal plants generally had higher shoot Zn contents; colonisation by R. irregularis also conferred a benefit to plants growing in toxic soil Zn conditions (Zn20), as indicated by the reduced shoot Zn concentration in the mycorrhizal plants at high soil P supply. Along with the increased biomass at Zn20 in the mycorrhizal plants at low soil P availability, these results support the hypothesis that mycorrhizal inoculation provides a ‘protective’ effect against high soil Zn availability. These results are in accordance with earlier studies exploring the benefits of mycorrhizal fungi in other plant species (red clover and white clover) in Zn-contaminated soil29,75. Additionally, other studies have indicated that under soil heavy metal contamination with high levels of plant-available soil P, mycorrhizal plants can increase their biomass, thus resulting in reduced uptake of heavy metals such as Zn, copper (Cu), and lead (Pb) into their tissues28,70,72,76.
Expression of ZIP genes may be linked to mycorrhizal Zn tolerance mechanisms
The ZIP membrane transporter gene family can transport Zn2+ from the rhizosphere into plants via the roots31. There are four characterised ZIP transporters (MtZIP1, MtZIP2, MtZIP5 and MtZIP6) that are able to transport Zn2+ in M. truncatula, as confirmed by yeast complementation assays37,38.
Inoculation with AMF induced the expression of MtZIP5 in Medicago roots when grown in Zn-deficient soil; this result has not to our knowledge been reported before. Given that the Medicago plants were growth-limited by Zn availability at Zn0, the induction of MtZIP5 in mycorrhizal roots at all Zn0 treatments may have helped to overcome the Zn limitation somewhat, as supported by the greater biomass and Zn concentration. Therefore, as a Zn transporter, MtZIP5 may have a direct or indirect association with the MPU of Zn uptake, and this novel result is deserving of further study.
The expression of MtZIP2 was down-regulated in the roots when inoculated with AMF, which is in line with an earlier study39. These results together suggest that MtZIP2 may not be directly involved in Zn uptake via the MPU. Similarly, the expression of the MtZIP6 gene was also found to be down-regulated by AMF. By contrast, in a previous study, MtZIP6 was highly up-regulated using the same plant and AMF species53; this difference may be due to differences in Zn and/or P availability in the two studies, or the different soil type used. The contrasting MtZIP6 results with previous work, highlight that the role of the MtZIP6 protein may not be one directly involved in the MPU.
While the present study has shown that the expression of a number of ZIP genes are modified by AMF inoculation, further studies are required that use knock-out mutant plants in conjunction with radioisotope tracing to elicit the function of ZIP transporters, and their potential role in Zn uptake via the mycorrhizal pathway.
Plant P nutrition was improved under P-deficiency and buffered under varying Zn-stress in mycorrhizal plants
The results of this study clearly confirm that mycorrhizal colonisation benefits Medicago plant tissue P concentration and contents at low soil P addition13,54,71,77. In addition, shoot P contents in mycorrhizal plants were maintained across the range of soil Zn availabilities (from deficient to toxic) at high soil P treatments (P20, P50). By contrast, the shoot P content in non-mycorrhizal plants was negatively influenced by soil Zn deficiency at high soil P concentration treatments, and is in agreement with a previous Zn-AMF interaction study in Medicago, up to 20 mg Zn kg−1 53. However, once soil Zn reached a highly toxic concentration (40 mg Zn kg−1) in Watts-Williams, et al.53, mycorrhizal colonisation no longer had the capability to maintain plant shoot P contents. This ‘buffering’ capability of mycorrhizal plants at varying soil Zn concentrations when P is not deficient could be considered a benefit of AMF beyond simply increased plant P concentration and/or contents at P-deficiency.
PT gene expression patterns are highly influenced by mycorrhizal colonisation
This study investigated the different roles of the phosphate (Pi) transport-related genes in M. truncatula plants and the effect of soil P availability and mycorrhizal inoculation. When plants are colonised by AMF, the expression of a number of membrane PT genes is modified. Specifically, two P transporters (MtPT4 and MtPT8) have been localised to the peri-arbuscular membrane (PAM), the site of plant-fungus nutrient exchange, and are directly implicated in the transport of P from fungus to the host plant44,48. In this study, MtPT4 was exclusively expressed in mycorrhizal plants and was down-regulated with increasing soil P availability, with no influence of soil Zn availability. The lack of effect of Zn availability on MtPT4 expression is in contrast with a previous study that reported that an increased expression of MtPT4 corresponded with an increase in soil Zn concentration53. The present study suggests that MtPT4 expression interacts with soil Zn availability under certain conditions only. Furthermore, in this study, the expression of MtPT4 was highly correlated with the expression of R. irregularis α-tubulin (a mycorrhizal fungal biomass marker gene), as demonstrated in earlier studies53,78. Therefore, the induced expression of MtPT4 at low soil P concentration may be due to the increased requirement for the MPU, and thus transport of P across the PAM, which in turn contributed to the higher plant P concentrations and contents in the mycorrhizal plants. Conversely, MtPT8, a second AMF-induced Pi transporter gene47,48, was highly expressed in the P20 treatment, where MtPT4 had reduced expression. This interplay between the expression of MtPT4 and MtPT8 has been discussed in previous studies48,49 in which MtPT4 was mutated, and this led to the compensation by MtPT8 expression, presumably to balance the P homeostasis in plants colonised by AMF. This compensation by MtPT8 in previous studies may also explain why MtPT4 and MtPT8 expression was dominant at different soil P availabilities in this study.
The expression of PT gene MtPT1 is a representative of the direct pathway of P uptake (DPU), and here was down-regulated in the mycorrhizal plants across different soil P and Zn availabilities, which is consistent with previous studies26,42. The expression of MtPT1 in non-mycorrhizal plants was particularly up-regulated when soil P was the most limiting to plants. This highlights again the important role that MtPT1 and other PTs may have in P uptake via the DPU in non-mycorrhizal plants, as they are relying on a single pathway of P uptake from the soil. Furthermore, the P starvation-induced gene MT450 was also down-regulated in the mycorrhizal plants at low soil P availability; at any higher levels of soil P addition the expression of MT4 was similar between mycorrhizal and non-mycorrhizal plants, presumably because the plant was not P-starved26,51,79. In summary, whereas MtPT4 and MtPT8 expression interacted with soil P availability in the mycorrhizal plants, MtPT1 and MtMT4 were likely important for P uptake and regulation in the non-mycorrhizal plants at low soil P availability.
Soil P and Zn availabilities strongly influence responses to mycorrhiza and gene expression
This study demonstrated that, aside from inoculation with mycorrhizal fungi, soil Zn and P availability also have a powerful impact on plant physiology and gene expression. For example, in addition to the down-regulation effects of AMF on the expression of MtZIP2, this gene was also up-regulated by increased soil Zn addition. This finding has also been documented in Burleigh, et al.39, in which the authors observed that MtZIP2 was up-regulated within roots by high Zn fertilisation, and was highest in roots exposed to a toxic level of soil Zn. Thus, it is possible that MtZIP2 is either expressed directly in response to high concentrations of soil Zn, or is stimulated by internal plant Zn concentrations. Given that both shoot Zn concentrations and the expression of MtZIP2 were generally lower in the mycorrhizal plants, the results presented here suggest that the second hypothesis is more plausible.
By contrast, expression of MtZIP5 in roots appeared to be soil Zn deficiency-induced, which has not been shown previously; this result suggests that a threshold in soil or plant Zn concentration exists between the Zn0 and Zn5 treatments, and thus that ZIP5 expression was reduced to a baseline level when the plant was not considered Zn-limited. A similar trend was also identified in barley whereby the expression of six HvZIP genes was increased by at least three-fold in Zn-deficient roots compared to the expression in Zn-sufficient plant roots34.
In a previous study, researchers observed that loss-of-function of LPCAT1 in Arabidopsis thaliana at soil Zn-deficient conditions lead to increased shoot P accumulation52. Therefore, this recent study hypothesised that the expression of the nearest orthologue of LPCAT1 in Medicago may be highly interactive with soil P and Zn concentration. However, the expression of this gene was not affected by the interaction between soil P and Zn availability, although expression was higher in mycorrhizal plants than in non-mycorrhizal plants across all soil Zn addition treatments. Therefore, the role of this gene in Medicago, and other species with the ability to form arbuscular mycorrhizal associations remains unclear, but we have in this study uncovered a potential interaction between the gene and mycorrhizal inoculation.
Conclusions and future research
This experiment presents, to our knowledge, the first attempt to link physiological and molecular markers of mycorrhizal associations pertaining to both Zn and P nutrition, in Medicago. The expression of MtZIP5 was induced both by mycorrhizal colonisation and low soil Zn availability. In contrast, MtZIP2 expression was up-regulated in non-mycorrhizal roots, and increased with soil Zn availability. In examining shoot biomass and Zn concentration, there was evidence of a ‘protective’ role of mycorrhizal fungi at high levels of soil Zn. Regarding PTs, MtPT4 and MtPT8 were up-regulated, and MtPT1 and MtMT4 down-regulated in mycorrhizal plants; the expression of all PTs was interactive with available soil P. The expression of both MPU- and DPU-related PTs likely conferred greater P uptake in the mycorrhizal plants when soil P was limiting. Further studies are necessary to understand the potential of mycorrhizal fungi and the role of ZIP transporters to improve the Zn nutrient uptake via the MPU.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Alloway, B. Soil factors associated with zinc deficiency in crops and humans. Environ. Geochem. Health 31, 537–548, https://doi.org/10.1007/s10653-009-9255-4 (2009).
Cakmak, I., McLaughlin, M. & White, P. Zinc for better crop production and human health. Plant Soil 411, 1–4, https://doi.org/10.1007/s11104-016-3166-9 (2017).
Cakmak, I. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant and Soil 302, 1–17, https://doi.org/10.1007/s11104-007-9466-3 (2008).
Lokuruka, M. N. I. Role of zinc in human health with reference to african elderly: A review. African Journal of Food, Agriculture, Nutrition and Development 12 (2012).
Gibson, R. Zinc deficiency and human health: etiology, health consequences, and future solutions. Plant Soil 361, 291–299, https://doi.org/10.1007/s11104-012-1209-4 (2012).
Singh, U. (eds Ummed editor Singh, C. S. editor Praharaj, S. S. editor Singh, & N. P. editor Singh) (Springer India: Imprint: Springer, 2016).
Humayan Kabir, A., Swaraz, A. M. & Stangoulis, J. Zinc‐deficiency resistance and biofortification in plants. J. Plant Nutr. Soil Sci. 177, 311–319, https://doi.org/10.1002/jpln.201300326 (2014).
Shahzad, Z., Rouached, H. & Rakha, A. Combating Mineral Malnutrition through Iron and Zinc Biofortification of Cereals. Comprehensive Reviews in Food Science and Food Safety 13, 329–346, https://doi.org/10.1111/1541-4337.12063 (2014).
Clemens, S. How metal hyperaccumulating plants can advance Zn biofortification. Plant Soil 411, 111–120, https://doi.org/10.1007/s11104-016-2920-3 (2017).
Kisko, M., Bouain, N., Rouached, A., Choudhary, S. P. & Rouached, H. Molecular mechanisms of phosphate and zinc signalling crosstalk in plants: Phosphate and zinc loading into root xylem in Arabidopsis. Environ. Exp. Bot. 114, 57–64, https://doi.org/10.1016/j.envexpbot.2014.05.013 (2015).
White, P. J. The Ecophysiology of Plant-Phosphorus Interactions. (Springer Netherlands, 2008).
Poirier, Y. & Bucher, M. P Transport and Homeostasis in Arabidopsis. The Arabidopsis Book 1, https://doi.org/10.1199/tab.0024 (2002).
Smith, S. E., Jakobsen, I., Gronlund, M. & Smith, F. A. Roles of arbuscular mycorrhizas in plant phosphorus nutrition: interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition.(Update on Arbuscular Mycorrhizas and Phosphorus Nutrition)(Report). Plant Physiol. 156, 1050, https://doi.org/10.1104/pp.111.174581 (2011).
Smith, S. E., Smith, F. A. & Jakobsen, I. Mycorrhizal fungi can dominate phosphate supply to plants irrespective of growth responses (1).(Scientific Correspondence). Plant Physiol. 133, 16, https://doi.org/10.1104/pp.103.024380 (2003).
Julian, I. S. et al. Using membrane transporters to improve crops for sustainable food production. Nature 497, 60, https://doi.org/10.1038/nature11909 (2013).
Cavagnaro, T. R. The role of arbuscular mycorrhizas in improving plant zinc nutrition under low soil zinc concentrations: a review. Plant Soil 304, 315–325, https://doi.org/10.1007/s11104-008-9559-7 (2008).
Malbreil, M., Tisserant, E., Martin, F. & Roux, C. Chapter Nine - Genomics of Arbuscular Mycorrhizal Fungi: Out of the Shadows. Vol. 70 (Elsevier Science & Technology, 2014).
Marschner, H. & Dell, B. Nutrient uptake in mycorrhizal symbiosis. Plant Soil 159, 89–102, https://doi.org/10.1007/BF00000098 (1994).
Johnson, N. C. & Gehring, C. A. Mycorrhizas-Chapter 4:Symbiotic Mediators of Rhizosphere and Ecosystem Processes. (Elsevier Inc., 2007).
Bago, B., Azcn-Aguilar, C., Goulet, A. & Pich, Y. Branched absorbing structures (BAS): a feature of the extraradical mycelium of symbiotic arbuscular mycorrhizal fungi. New Phytol. 139, 375–388, https://doi.org/10.1046/j.1469-8137.1998.00199.x (1998).
Ercoli, L., Schüßler, A., Arduini, I. & Pellegrino, E. Strong increase of durum wheat iron and zinc content by field-inoculation with arbuscular mycorrhizal fungi at different soil nitrogen availabilities. Plant Soil 419, 153–167, https://doi.org/10.1007/s11104-017-3319-5 (2017).
Watts-Williams, S., Smith, F., McLaughlin, M., Patti, A. & Cavagnaro, T. How important is the mycorrhizal pathway for plant Zn uptake? Plant Soil 390, 157–166, https://doi.org/10.1007/s11104-014-2374-4 (2015).
Ning, J. & Cumming, J. R. Arbuscular mycorrhizal fungi alter phosphorus relations of broomsedge (Andropogon virginicus L.) plants. J. Exp. Bot. 52, 1883–1891, https://doi.org/10.1093/jexbot/52.362.1883 (2001).
Stribley, D. P., Tinker, P. B. & Snellgrove, R. C. Effect of vesicular‐arbuscular mycorrhizal fungi on the relations of plant growth, internal phosphorus concentration and soil phosphate analyses. J. Soil Sci. 31, 655–672, https://doi.org/10.1111/j.1365-2389.1980.tb02112.x (1980).
Tawaraya, K., Hirose, R. & Wagatsuma, T. Inoculation of arbuscular mycorrhizal fungi can substantially reduce phosphate fertilizer application to Allium fistulosum L. and achieve marketable yield under field condition. Boilogy and Fertility of Soils 48, 839–843, https://doi.org/10.1007/s00374-012-0669-2 (2012).
Watts-Williams, S. J., Jakobsen, I., Cavagnaro, T. R. & Grønlund, M. Local and distal effects of arbuscular mycorrhizal colonization on direct pathway Pi uptake and root growth in Medicago truncatula. J. Exp. Bot. 66, 4061–4073, https://doi.org/10.1093/jxb/erv202 (2015).
Karimi, A., Khodaverdiloo, H., Sepehri, M. & Sadaghiani, M. R. Arbuscular mycorrhizal fungi and heavy metal contaminated soils. African Journal of Microbiology Research 5, 1571–1576 (2011).
Ferrol, N., Tamayo, E. & Vargas, P. The heavy metal paradox in arbuscular mycorrhizas: from mechanisms to biotechnological applications. Journal of experimental botany, erw403 (2016).
Li, X. & Christie, P. Changes in soil solution Zn and pH and uptake of Zn by arbuscular mycorrhizal red clover in Zn-contaminated soil. Chemosphere 42, 201–207, https://doi.org/10.1016/S0045-6535(00)00126-0 (2001).
Watts-Williams, S., Patti, A. & Cavagnaro, T. Arbuscular mycorrhizas are beneficial under both deficient and toxic soil zinc conditions. Plant Soil 371, 299–312, https://doi.org/10.1007/s11104-013-1670-8 (2013).
Guerinot, M. L. The ZIP family of metal transporters. BBA - Biomembranes 1465, 190–198, https://doi.org/10.1016/S0005-2736(00)00138-3 (2000).
Watts-Williams, S. J. & Cavagnaro, T. R. Arbuscular mycorrhizal fungi increase grain zinc concentration and modify the expression of root ZIP transporter genes in a modern barley (Hordeum vulgare) cultivar. Plant Sci. 274, 163–170, https://doi.org/10.1016/j.plantsci.2018.05.015 (2018).
Pedas, P., Schjoerring, J. K. & Husted, S. Identification and characterization of zinc-starvation-induced ZIP transporters from barley roots. Plant Physiol. Biochem. 47, 377–383, https://doi.org/10.1016/j.plaphy.2009.01.006 (2009).
Tiong, J. et al. Increased expression of six ZIP family genes by zinc (Zn) deficiency is associated with enhanced uptake and root‐to‐shoot translocation of Zn in barley (Hordeum vulgare. New Phytol. 207, 1097–1109, https://doi.org/10.1111/nph.13413 (2015).
Tiong, J. et al. H v ZIP 7 mediates zinc accumulation in barley (H ordeum vulgare) at moderately high zinc supply. New Phytologist 201, 131–143, https://doi.org/10.1111/nph.12468 (2014).
Ramesh, S. A., Shin, R., Eide, D. J. & Schachtman, D. P. Differential metal selectivity and gene expression of two zinc transporters from rice (1). Plant Physiol. 133, 126, https://doi.org/10.1104/pp.103.026815 (2003).
López-Millán, A.-F., Ellis, D. & Grusak, M. Identification and Characterization of Several New Members of the ZIP Family of Metal Ion Transporters in Medicago Truncatula. Plant Molecular Biology 54, 583–596, https://doi.org/10.1023/B:PLAN.0000038271.96019.aa (2004).
Stephens, B., Cook, D. & Grusak, M. Characterization of zinc transport by divalent metal transporters of the ZIP family from the model legume Medicago truncatula. BioMetals 24, 51–58, https://doi.org/10.1007/s10534-010-9373-6 (2011).
Burleigh, S., Kristensen, B. & Bechmann, I. A plasma membrane zinc transporter from Medicago truncatula is up-regulated in roots by Zn fertilization, yet down-regulated by arbuscular mycorrhizal colonization. Plant Mol. Biol. 52, 1077–1088, https://doi.org/10.1023/A:1025479701246 (2003).
Liu, J. et al. Closely Related Members of the Medicago truncatula PHT1 Phosphate Transporter Gene Family Encode Phosphate Transporters with Distinct Biochemical Activities. The Journal of biological chemistry 283, 24673–24681, https://doi.org/10.1074/jbc.M802695200 (2008).
Bucher, M., Rausch, C. & Daram, P. Molecular and biochemical mechanisms of phosphorus uptake into plants. Journal of Plant Nutrition and Soil Science 164, 209–217 (2001).
Chiou, T. J., Liu, H. & Harrison, M. J. The spatial expression patterns of a phosphate transporter (MtPT1) from Medicago truncatula indicate a role in phosphate transport at the root/soil interface. The Plant Journal 25, 281–293, https://doi.org/10.1046/j.1365-313x.2001.00963.x (2001).
Liu, H., Trieu, A. T., Blaylock, L. A. & Harrison, M. J. Cloning and characterization of two phosphate transporters from Medicago truncatula roots: Regulation in response to phospate and to colonization by arbuscular mycorrhizal (AM) fungi. Mol. Plant-Microbe Interact. 11, 14–22, https://doi.org/10.1094/MPMI.1998.11.1.14 (1998).
Harrison, M., Dewbre, G. & Liu, J. A phosphate transporter from Medicago truncatula involved in the acquisiton of phosphate released by arbuscular mycorrhizal fungi. Plant Cell 14, 2413–2429, https://doi.org/10.1105/tpc.004861 (2002).
Hélène, J., Penmetsa, R. V., Nadia, T., Douglas, R. C. & Maria, J. H. A Medicago truncatula phosphate transporter indispensable for the arbuscular mycorrhizal symbiosis. Proceedings of the National Academy of Sciences 104, 1720, https://doi.org/10.1073/pnas.0608136104 (2007).
Javot, H. et al. Medicago truncatula mtpt4 mutants reveal a role for nitrogen in the regulation of arbuscule degeneration in arbuscular mycorrhizal symbiosis. The Plant Journal 68, 954–965, https://doi.org/10.1111/j.1365-313X.2011.04746.x (2011).
Michael, R. B. & Janin, R. Roots - The Hidden Provider. (Frontiers Media SA, 2017).
Branscheid, A. Phosphate homeostasis and posttranscriptional gene regulation during arbuscular mycorrhizal symbiosis in Medicago truncatula Doctor of Philosophy thesis (2012).
Breuillin-Sessoms, F. et al. Suppression of arbuscule degeneration in Medicago truncatula phosphate transporter4 mutants is dependent on the ammonium transporter 2 family protein AMT2; 3. Plant Cell, tpc. 114.131144 (2015).
Burleigh, S. & Harrison, M. A novel gene whose expression in Medicago truncatula roots is suppressed in response to colonization by vesicular-arbuscular mycorrhizal (VAM) fungi and to phosphate nutrition. Plant Mol. Biol. 34, 199–208, https://doi.org/10.1023/A:1005841119665 (1997).
Burleigh, S. H. & Harrison, M. J. The down-regulation of Mt4-like genes by phosphate fertilization occurs systemically and involves phosphate translocation to the shoots. Plant Physiology 119, 241–248, https://doi.org/10.1104/pp.119.1.241 (1999).
Kisko, M. et al. LPCAT1 controls phosphate homeostasis in a zinc-dependent manner. Elife 7, e32077 (2018).
Watts-Williams, S. J., Tyerman, S. D. & Cavagnaro, T. R. The dual benefit of arbuscular mycorrhizal fungi under soil zinc deficiency and toxicity: linking plant physiology and gene expression. Plant Soil 420, 375–388, https://doi.org/10.1007/s11104-017-3409-4 (2017).
Watts-Williams, S. & Cavagnaro, T. Arbuscular mycorrhizas modify tomato responses to soil zinc and phosphorus addition. Biol. Fertility Soils 48, 285–294, https://doi.org/10.1007/s00374-011-0621-x (2012).
Vierheilig, H., Coughlan, A. P., Wyss, U. & Piche, Y. Ink and Vinegar, a Simple Staining Technique for Arbuscular-Mycorrhizal Fungi. Applied and Environmental Microbiology 64, 5004 (1998).
Giovannetti, M. & Mosse, B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 84, 489–500, https://doi.org/10.1111/j.1469-8137.1980.tb04556.x (1980).
Redon, P.-O., Béguiristain, T. & Leyval, C. Differential effects of AM fungal isolates on Medicago truncatula growth and metal uptake in a multimetallic (Cd, Zn, Pb) contaminated agricultural soil. Mycorrhiza 19, 187–195, https://doi.org/10.1007/s00572-009-0230-9 (2009).
Monzon, A. & Azcón, R. Relevance of mycorrhizal fungal origin and host plant genotype to inducing growth and nutrient uptake in Medicago species. Agriculture, Ecosystems and Environment 60, 9–15, https://doi.org/10.1016/S0167-8809(96)01066-3 (1996).
Martin, A. W. The role of arbuscular mycorrhizal fungi in sustainable tomato production Doctor of Philosophy thesis, The University of Adelaide (2007).
Borowicz, V. A. The impact of arbuscular mycorrhizal fungi on plant growth following herbivory: A search for pattern. Acta Oecologica (ACTA OECOL) 52, 1–9 (2013).
Dickson, S. Phosphate transfer efficiency of two arbuscular mycorrhizal fungi Doctor of Philosophy thesis, University of Adelaide (1999).
Bowles, T. M., Barrios-Masias, F. H., Carlisle, E. A., Cavagnaro, T. R. & Jackson, L. E. Effects of arbuscular mycorrhizae on tomato yield, nutrient uptake, water relations, and soil carbon dynamics under deficit irrigation in field conditions. Sci. Total Environ. 566, 1223–1234, https://doi.org/10.1016/j.scitotenv.2016.05.178 (2016).
Clark, R. B. & Zeto, S. K. Mineral acquisition by arbuscular mycorrhizal plants. J. Plant Nutr. 23, 867–902, https://doi.org/10.1080/01904160009382068 (2000).
Smith, S. E., Smith, F. A. & Jakobsen, I. Functional diversity in arbuscular mycorrhizal (AM) symbioses: the contribution of the mycorrhizal P uptake pathway is not correlated with mycorrhizal responses in growth or total P uptake. New Phytol. 162, 511–524, https://doi.org/10.1111/j.1469-8137.2004.01039.x (2004).
Wei, L. et al. Arbuscular mycorrhizal fungi in soil and roots respond differently to phosphorus inputs in an intensively managed calcareous agricultural soil. Report No. 2045–2322 (2016).
Xu, P. et al. Response of Soil Phosphorus Required for Maximum Growth of Asparagus officinalis L. to Inoculation of Arbuscular Mycorrhizal Fungi. Pedosphere 24, 776–782, https://doi.org/10.1016/S1002-0160(14)60064-3 (2014).
Nagy, R., Drissner, D., Amrhein, N., Jakobsen, I. & Bucher, M. Mycorrhizal phosphate uptake pathway in tomato is phosphorus‐repressible and transcriptionally regulated. New Phytol. 181, 950–959, https://doi.org/10.1111/j.1469-8137.2008.02721.x (2009).
Bruce, A., Smith, S. E. & Tester, M. The development of mycorrhizal infection in cucumber: effects of P supply on root growth, formation of entry points and growth of infection units. New Phytol. 127, 507–514, https://doi.org/10.1111/j.1469-8137.1994.tb03968.x (1994).
Chen, B. D., Li, X. L., Tao, H. Q., Christie, P. & Wong, M. H. The role of arbuscular mycorrhiza in zinc uptake by red clover growing in a calcareous soil spiked with various quantities of zinc. Chemosphere 50, 839–846, https://doi.org/10.1016/s0045-6535(02)00228-x (2003).
Miransari, M. Contribution of arbuscular mycorrhizal symbiosis to plant growth under different types of soil stress. Plant Biol. 12, 563–569, https://doi.org/10.1111/j.1438-8677.2009.00308.x (2010).
Smith, S. E., Jakobsen, I., Grønlund, M. & Smith, F. A. Roles of arbuscular mycorrhizas in plant phosphorus nutrition: interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant physiology 156, 1050, https://doi.org/10.1104/pp.111.174581 (2011).
Shetty, K. G., Hetrick, B. A. D. & Schwab, A. P. Effects of mycorrhizae and fertilizer amendments on zinc tolerance of plants. Environ. Pollut. 88, 307–314, https://doi.org/10.1016/0269-7491(95)93444-5 (1995).
Nielsen, J. & Jensen, A. Influence of vesicular-arbuscular mycorrhiza fungi on growth and uptake of various nutrients as well as uptake ratio of fertilizer P for lucerne (Medicago sativa). Plant and Soil 70, 165–172, https://doi.org/10.1007/BF02374777 (1983).
Zhu, Y. G., Smith, S. E. & Smith, F. A. Zinc (Zn)-phosphorus (P) interactions in two cultivars of spring wheat (Triticum aestivum L.) differing in P uptake efficiency. Ann. Bot. 88, 941–945, https://doi.org/10.1006/anbo.2001.1522 (2001).
Zhu, Y., Christie, P. & Scott Laidlaw, A. Uptake of Zn by arbuscular mycorrhizal white clover from Zn-contaminated soil. Chemosphere 42, 193–199, https://doi.org/10.1016/S0045-6535(00)00125-9 (2001).
Díaz, G., Azcón-Aguilar, C. & Honrubia, M. Influence of arbuscular mycorrhizae on heavy metal (Zn and Pb) uptake and growth of Lygeum spartum and Anthyllis cytisoides. Plant Soil 180, 241–249, https://doi.org/10.1007/BF00015307 (1996).
Deguchi, S., Uozumi, S., Touno, E., Kaneko, M. & Tawaraya, K. Arbuscular mycorrhizal colonization increases phosphorus uptake and growth of corn in a white clover living mulch system. Soil Sci. Plant Nutr. 58, 169–172, https://doi.org/10.1080/00380768.2012.662697 (2012).
Uhe, M., Hogekamp, C., Hartmann, R. M., Hohnjec, N. & Küster, H. The mycorrhiza-dependent defensin MtDefMd1 of Medicago truncatula acts during the late restructuring stages of arbusculecontaining cells. PloS one 13, https://doi.org/10.1371/journal.pone.0191841 (2018).
Branscheid, A. et al. Expression Pattern Suggests a Role of MiR399 in the Regulation of the Cellular Response to Local Pi Increase During Arbuscular Mycorrhizal. Symbiosis. Mol. Plant-Microbe Interact. 23, 915–926, https://doi.org/10.1094/mpmi-23-7-0915 (2010).
Acknowledgements
SJWW would like to acknowledge the University of Adelaide Ramsay Fellowship, SJWW and TDN the Australian Research Council Centre of Excellence in Plant Energy Biology (Grant number: CE140100008), and TDN the Australia Award Scholarship, for support. The authors thank Prof Mike McLaughlin for access to the ICP-AES, members of the Cavagnaro lab, Ms Wendy Sullivan and Ms Bogumila Tomczack for technical assistance, Profs Steve Tyerman and Diane Mather for their valuable discussions, Dr Julian Taylor, Dr Ute Baumann, Mr Juan Carlos Sanchez and Ms Jessica Scott for assistance with statistical analyses or English language proof-reading.
Author information
Authors and Affiliations
Contributions
T.D.N. and S.J.W.W. designed the experiment and carried out the sample and data analyses. T.D.N. prepared all of the figures, and all authors contributed to data interpretation. T.D.N. wrote the first draft of the manuscript and S.J.W.W. and T.R.C. edited the draft. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nguyen, T.D., Cavagnaro, T.R. & Watts-Williams, S.J. The effects of soil phosphorus and zinc availability on plant responses to mycorrhizal fungi: a physiological and molecular assessment. Sci Rep 9, 14880 (2019). https://doi.org/10.1038/s41598-019-51369-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-51369-5
This article is cited by
-
Root system architecture associated zinc variability in wheat (Triticum aestivum L.)
Scientific Reports (2024)
-
Arbuscular mycorrhizal fungi-based bioremediation of mercury: insights from zinc and cadmium transporter studies
International Journal of Environmental Science and Technology (2024)
-
Responses of Plant Growth to N or P Addition Vary With Mycorrhizal Strategy
Journal of Plant Growth Regulation (2024)
-
Nutrient distribution and bioaccumulation in silver birch (Betula pendula Roth) biomass grown in nutrient-poor soil
Trees (2024)
-
Influences of peanut hull-derived biochar, Trichoderma harzianum and supplemental phosphorus on hairy vetch growth in Pb- and Zn-contaminated soil
Environmental Geochemistry and Health (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.