Abstract
Zinc (Zn) deficiency can severely inhibit plant growth, yield, and enzymatic activities. Zn plays a vital role in various enzymatic activities in plants. Arbuscular mycorrhizal fungi (AMF) play a crucial role in improving the plant’s Zn nutrition and mitigating Zn stress effects on plants. The current study was conducted to compare the response of inoculated and non-inoculated maize (YH 1898) in the presence of different levels of zinc under greenhouse conditions under a Zn deficient condition. There were two mycorrhizal levels (i.e., M + with mycorrhizae, M- without mycorrhizae) and five Zn levels (i.e., 0, 1.5, 3, 6, and 12 mg kg-1), with three replicates following completely randomized design. At the vegetative stage (before tillering), biochemical, physiological, and agronomic attributes were measured. The results showed that maize plants previously inoculated with AMF had higher gaseous exchange traits, i.e., a higher stomatal conductance rate, favoring an increased photosynthetic rate. Improvement in antioxidant enzyme activity was also observed in inoculated compared to non-inoculated maize plants. Moreover, AMF inoculation also played a beneficial role in nutrients availability and its uptake by plants. Higher Zn12 (12 mg Zn kg-1 soil) treatment accumulated a higher Zn concentration in soil, root, and shoot in AMF-inoculated than in non-inoculated maize plants. These results are consistent with mycorrhizal symbiosis beneficial role for maize physiological functioning in Zn deficient soil conditions. Additionally, AMF inoculation mitigated the stress conditions and assisted nutrient uptake by maize.
Similar content being viewed by others
Introduction
Zinc (Zn) deficiency is widespread in arid and semi-arid regions, especially in alkaline calcareous soils, because of a high Zn fixation rate and Zn low mobility in such soil conditions1,2. Although a sufficient amount of Zn is present in the soil, only a small quantity of Zn is available for plant uptake. Zinc is mainly immobile in the soil as it is highly adsorbed to the surface of soil colloids3. Zinc deficiency is further aggravated by high soil pH, high soil Ca contents, mono-cropping, intensive agriculture and over the use of fertilizers. For instance, Rafique et al.4 reported that 80% of Pakistan's rainfed soil area is deficient in Zn due to high soil pH. Zinc deficiency of Zn in the surface layer of soils is widespread and its deficiency in plant tissues severely impacts their growth and reduces crop yields. Reduced plant concentrations of Zn stimulates reactive oxygen species (ROS) in plant tissues5. These ROS disrupt cell membranes and hinder normal cell functioning6. The ROS includes hydrogen peroxide and superoxide radicals, which cause extensive damage to plant tissue. On the other hand, Zn is involved in the gene expression, detoxification of ROS and activation of antioxidant enzyme production, including superoxide dismutase (SOD), peroxidase (PO) and catalase (CAT)7.
Most scientists suggest organic amendments, i.e., biochar3,8,9,10,11,12,13,14, biofertilizer15,16,17,18,19,20,21,22,23,24,25,26,27,28, humic substances, and organic matter29,30,31, to overcome the problem of abiotic and biotic stresses in crops. Arbuscular mycorrhizal fungi are well-known biofertilizers that protect host plants from biotic and abiotic stress by improving plant nutrition, especially P and Zn8,9. Extraradical hyphae of fungi explore the rhizosphere and bulk soil and enhance the uptake of nutrients with a low mobility rate like P, Zn and Cu. Most of the plants are colonized through AMF by Following two nutrients pathways. 1). Epidermis of roots, which is a direct pathway of uptake. 2). The formation of a fungal structure that designed mycorrhizal pathway uptake10.
Inoculation of mycorrhiza played an imperative role in the improvement of the chlorophyll contents of the plant. It also improved carotenoids contents end modified the synthesis of antioxidant enzymes, which regulates the metabolic activities under stress conditions11. Colonization of mycorrhiza increases the area under the rhizosphere through better branching of plant roots. Improvement in the branch also increases the uptake of water and nutrients, due to which growth and productivity of crops are increased12,13,14. Some studies have reported that mycorrhizal infection was sensitive to external P application, and thus, it affected the availability of other nutrients15,16. However, it was also reported that mycorrhizal fungi increased the uptake of Cu and contributed a 62% increase in Cu uptake irrespective of external P application17. Similarly, studies have highlighted the mycorrhizal fungi effect on Zn nutrition7,18. Therefore, instead of using inorganic sources for mitigating Zn deficiency, the use of mycorrhizal fungi as a bio-fertilizer may be an intriguing way of addressing Zn deficiency.
Subramanian et al.7 reported that mycorrhizal fungi trigger antioxidants that enable plants to survive in low Zn growing medium like Zn deficient soil. Studies have shown that mycorrhiza inoculated plants possess a high concentration of antioxidant enzymes above and below ground parts19. The AM fungi can increase the plant sugar and leaf proline contents and maintain plant cells' osmotic potential, protecting the plant’s enzymatic activities. AM fungi also increased the acid phosphatase activity in roots, leading to the improved nutritional value of inoculated plants20. Moreover, AM fungi also increased Zn and P concentrations in plant tissues, thereby positively affecting plant physiological processes such as photosynthesis, respiration, and stomatal conductance. Zinc is involved in chlorophyll synthesis; therefore, improved Zn nutrition in mycorrhizal plants stimulates chlorophyll synthesis21. So far, scientists have worked on AMF and Zn application solely under Zn deficiency to improve growth and yield in maize. However, the current study will cover the knowledge gap with the novel aspect of AMF and Zn application rates influence on antioxidants and physiological attributes of maize under Zn deficient. That’s why the current study was conducted to explore the efficacy of AMF for better uptake of Zn in maize in Zn deficient soil. It is hypothesized that AMF has the potential to mitigate Zn deficiency by unbalanced Zn application in maize.
Materials and methods
Experimental soil and location
The soil from the experimental site was aridisol, clay loam in texture, alkaline in pH (8.3), and contained low organic matter (0.58%)22. The available phosphorus level was 6.4 mg/kg extracted with NaHCO323, the potassium level was 225 mg/kg24, and the Zn level was 0.45 mg/kg25. The soil was taken from field latitude 29.92 and longitude 71.52.
Experimental site and design
A pot experiment was conducted in loamy soil to evaluate Zn deficiency effects on a maize crop's physiological functioning at the research area of the Department of Soil Science Bahauddin Zakaria University, Multan Pakistan. The study was arranged according to a completely randomized design (CRD).
Pot preparation and treatments
For this purpose, clay pots with 10 kg soil were filled with a mixture of soil and sand in a 4:1 ratio; the purpose of adding sand is to provide a mixture from which roots can be easily extracted. Plants were grown for a total period of 45 days after sowing. The treatments were two mycorrhizal levels (i.e., M+ with mycorrhizae, M- without mycorrhizae) and five Zn levels (i.e., 0, 1.5, 3, 6, and 12 mg/kg), with the entire experiment being repeated three times. The required quantity of Zn was applied using analytical grade zinc heptahydrate (ZnSO4·7 H2O).
Fertilizer application and AMF inoculation
Nitrogen fertilizer (227.24 kg ha-1) was applied in three separate aliquots. Half of P (71.63 kg ha-1) and full recommended dose of K (91.93 kg ha-1) were applied as a basal dose at the time of sowing. The AM inoculum containing a consortium of Glomus species, i.e., mycorrhizal inoculum Clonex® (Root Maximizer; 5711 Enterprise Drive, Lansing, MI, USA) having 158 propagules gram-1 was applied at 25 g inoculum per pot. Thiophanate methyl fungicide was applied to the control treatments at 50 mg kg-1 fortnight-1 pot-1 to control the root mycorrhizal colonization.
Seeds sowing and irrigation
Five maize seeds (YH 1898 cv.) were sown per pot. After 20 days of growth, only 2 healthy plants per pot were maintained. The selection was made based on visual observations. The moisture content in each pot was maintained at 60% of the total WHC.
Harvesting
At the vegetative stage (before tillering), biochemical, physiological, and agronomic attributes were measured.
Tissue and soil analysis
Fresh plant leaves and roots were excised, rinsed with water and oven-dried at 72ºC for 24 h. Plant leaf and fresh root weights and dried weights were recorded. Total N was determined by the calorimetric method26. Phosphorus was analyzed using the malachite green method27. Potassium was determined by flame photometer28. Pre- and post-treatment soil samples were collected to observe the nutrient status of the soil. Soil zinc was extracted by the DTPA extraction method and analyzed using an Atomic Absorption Spectrophotometer (AAS)25. The soil pH29 and EC30 were determined in soil water suspensions of 1:5 (soil:water) ratios using pH and EC meters.
Physiological parameter
The stomatal conductance, photosynthetic rate, and transpiration rate were measured using an IRGA (infrared gas analyzer). All physiological parameters were measured between 9 and 11 AM to avoid low humidity and high external temperature31.
Chlorophyll contents
Chlorophyll contents were determined by the procedure of Arnon32. The intensity of green color extract from fresh plant leaves was measured at 645 and 663 nm32.
*W = Fresh weight of leaves.
Enzymatic analysis
Determination of antioxidant enzymes was done by collecting fresh leaves, which were immediately kept in liquid nitrogen. Later, 1 g of sample was ground and completely homogenized in 50 mM phosphate.
Superoxide dismutase
This enzyme was measured by the method of Giannopolitis and Ries33. A mixture of 1 mL nitroblue tetrazolium (NBT) (50 mM), 500 μL ethylenediaminetetraacetic acid (EDTA) (75 mM), 1 mL riboflavin (1.3 μM), 950 μL sodium phosphate buffer (50 mM), and 0.5-mL methionine (13 mM) was prepared. A 50 μL sample extract was added, and the mixture was placed under fluorescent light for 5 min. The absorbance of the resultant solution was measured at 560 nm. The SOD activity was measured in IU min−1 mg−1 protein.
Catalase
A mixture of 0.9 mL H2O2 (5.9 mM) in 2 mL phosphate buffer (50 mM). was used to measure the catalase activity (CAT). 0.1 mL of an enzyme extract was mixed with the mixture. After 30 s the absorbance was measured at 240 nm and monitored continuously for 5 min to measure the disintegration of H2O2 by CAT activity34. The CAT activity was measured in μmol min−1 mg−1 protein.
Peroxidase
100 μL enzyme extract was added to a solution of 2 mL sodium phosphate buffer (50 mM), 400-μL guaiacol (20 mM), and 500 μL H2O2 (40 mM). The absorbance of this mixture was measured at an interval of 20 s for 5 min. The peroxidase (POD) concentration was calculated by the absorbance change34. The POD activity was measured in μmol min−1 mg−1 protein.
Total soluble proteins (TSP)
The method of Sambrook and Russell35 was used to determine the total soluble proteins, and the Bradford36 method was used to estimate the TSP. A 200 μL of either leaf or root extract was mixed into 780 μL DI water and 20 μL of Coomassie blue dye. The absorbance of this sample was measured at 595 nm. The TSP was expressed in mg mL−1.
Mycorrhizal colonization
The fine roots of maize plants were harvested and then washed with KOH (10% w/v)37. The root was then stained with a 0.05 trypan blue solution38. The gridline intersects method was used to search for the presence of AM structure in roots.
Statistical analysis
The data was analyzed using two factorial analysis of variance (ANOVA) and treatments were compared using the Least Significant Difference (LSD) test at p ≤ 0.0539 on Statistical Package Statistix 8.1 (Tallahassee Florida, USA). Correlation and principal component analysis (PCA) were performed using XLSTAT-2014.
Institutional, national, and international guidelines and legislation
Current experiment meets all international, national and/or institutional guidelines for experimental research and field studies on plants (either cultivated or wild), including the collection of plant material.
Relevant permits/permissions/licences
For collection of maize seeds all relevant permits or permissions have been obtained.
Ethics approval and consent to participate
We all declare that manuscripts reporting studies do not involve any human participants, human data, or human tissue. So, it is not applicable.
Complies with international, national and/or institutional guidelines
Current experiment meets all international, national and/or institutional guidelines.
Results
Colonization of mycorrhizal fungi
Data regarding mycorrhizal colonization was measured from the roots of both inoculated and un-inoculated maize plants. A significant (p ≤ 0.05) increase in mycorrhizal colonization was observed following inoculation irrespective of zinc levels. The inoculated roots showed colonization of mycorrhiza in the range of 60–76% whereas, un-inoculated roots had 24–27% root colonization. Maximum colonization was observed with the treatment of Zn12 as compared to the other treatments (Fig. 1).
Effect of arbuscular mycorrhizal fungi inoculation on mycorrhizal colonization by maize under zinc deficient soil conditions Zn0 (control), Zn1.5 (1.5 mg kg-1), Zn3 (3 mg kg-1), Zn6 (6 mg kg-1), Zn12 (12 mg kg-1), inoculated with AMF (M + grey), un-inoculated (M- white). Error bars represent standard error. Inoculated with AMF (M + grey); un-inoculated (M- white).
Influence of AMF on gaseous exchange traits
The stomatal conductance rate consistently increased with the increment in the zinc fertilization levels. A significant (p ≤ 0.05) difference was noticed among inoculated and non-inoculated maize plants. The highest stomatal conductance rate was measured from Zn12 inoculated with an increase of 12% compared to the control, whereas its counterpart showed the least stomatal conductance rate with the decrease of 36% in M- compared to the control (Fig. 2). Changes to the photosynthetic rate were similar to those observed for the stomatal conductance rate. The Zn12 (M +) showed the highest photosynthetic rate with an increase of 35%, while M- had a 6% decrease in photosynthetic rate. The remaining inoculated plants also had a higher photosynthetic rate at different zinc levels, showing significant (p ≤ 0.05) differences compared to non-inoculated plants. The M + plant with the highest zinc level (Zn12) tested in this study exhibited a higher transpiration rate with a 56% increase compared to the control (Zn0), while M- plants with Zn12 treatment exhibited a 77% decrease in transpiration rate compared to the Zn0 (Fig. 2).
Effect of arbuscular mycorrhizal inoculation on gaseous exchange traits of maize under zinc deficient soil conditions. (a) Photosynthetic rate (µmol m-2 s-1) (b) stomatal conductance (mol H2O m-2 s-1) (c) transpiration rate (mmol m-2 s-1). Zn0 (control), Zn1.5 (1.5 mg kg-1), Zn3 (3 mg kg-1), Zn6 (6 mg kg-1), Zn12 (12 mg kg-1), inoculated with AMF (M + grey), un-inoculated (M- white). Error bars represent standard error and asterisk (*) shows a significant difference (p ≤ 0.05) among treatments (Zn × AMF interaction).
Arbuscular mycorrhizal fungi effect on chlorophyll contents
This study also quantified the change in chlorophyll contents with the varying zinc levels with and without mycorrhizal inoculation; these values tend to increase more with mycorrhizal inoculation. All the chlorophyll contents (a, b, and total) indicated an increase in inoculated (M +) plants compared to M- plants. The chlorophyll a content was found to increase by 8% in Zn12, although a decrease of up to 10% was observed in non-inoculated plants. Likewise, the chlorophyll b content increased by 20% (Zn12) in M + plants and showed a 26% decrease in non-inoculated plants. Similar results were observed for total chlorophyll contents in that Zn12 (M +) had a 15% higher total chlorophyll contents, while Zn (M-) resulted in a decline of 6% (Fig. 3).
Effect of arbuscular mycorrhizal inoculation on chlorophyll contents of maize under zinc deficient soil conditions. (a) chlorophyll a content (mg g-1 fr. wt) (b) chlorophyll b contents (mg g-1 fr. wt) (c) total chlorophyll contents (mg g-1 fr. wt). Zn0 (control), Zn1.5 (1.5 mg kg-1), Zn3 (3 mg kg-1), Zn6 (6 mg kg-1), Zn12 (12 mg kg-1), inoculated with AMF (M + grey), un-inoculated (M- white). Error bars represent standard error and asterisk (*) shows a significant difference (p ≤ 0.05) among treatments (Zn × AMF interaction).
Antioxidant enzymes activity
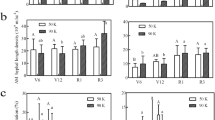
Mycorrhizal inoculated plants showed significantly (p ≤ 0.05) higher enzymatic activities than non-inoculated plants at each Zn level. The highest superoxide dismutase activity was observed in M + at Zn12 with an increase of 15%; M- also showed higher SOD activity by 16% compared to the control. The catalase activity was also higher with the higher level of zinc fertilization (Zn12). It showed a 19% increase in M + and a 20% increase in M- compared to their respective controls. Peroxidase also exhibited elevated enzyme activity. It was 30% higher in Zn12 with M + and 68% higher in M than their respective controls (Fig. 4). Mycorrhizal inoculation and Zn fertilization resulted in a significant (p ≤ 0.05) increase in maize plants' total soluble protein; these levels increased gradually with the increase in Zn levels regardless of M + and M- treatments. The highest TSP was recorded from the Zn12 in M + with a 17% increase whereas, M- showed a 36% increase in TSP (Fig. 4).
Effect of arbuscular mycorrhizal inoculation on antioxidant enzyme activity of maize under zinc deficient soil conditions. (a) superoxide dismutase (U g-1) (b) catalase (μmol H2O2 mg−1 protein) (c) peroxidase (U mg−1 protein), (d) total soluble protein (mg g -1). Zn0 (control), Zn1.5 (1.5 mg kg-1), Zn3 (3 mg kg-1), Zn6 (6 mg kg-1), Zn12 (12 mg kg-1), inoculated with AMF (M + grey), un-inoculated (M- white). Error bars represent standard error and asterisk (*) shows a significant difference (p ≤ 0.05) among treatments (Zn × AMF interaction).
Zinc concentration of the soil
The zinc concentration of zinc-deficient soil increased with the addition of zinc with mycorrhizal inoculation. The highest Zn accumulation from Zn12, increased by 41%; the lowest Zn accumulation was measured from Zn0 (control) with mycorrhizal inoculation. In non-mycorrhizal inoculation, the roots accumulation was also significantly higher under Zn12 compared to control maize plants. Zn12 also resulted in a higher uptake of the Zn in shoots, i.e., a 38% increase in shoots compared to the control. Similarly, with non-inoculated plants, Zn accumulation was 22% higher in shoots (Fig. 5).
Effect of arbuscular mycorrhizal inoculation on zinc concentration of maize under zinc deficient soil conditions. (a) zinc concentration in soil (mg kg−1) (b) zinc concentration in root (mg kg −1) (c) zinc concentration in shoot (mg kg −1). Zn0 (control), Zn1.5 (1.5 mg kg-1), Zn3 (3 mg kg-1), Zn6 (6 mg kg-1), Zn12 (12 mg kg-1), inoculated with AMF (M + grey), un-inoculated (M- white). Error bars represent standard error and asterisk (*) shows significant difference (p ≤ 0.05) among treatments (Zn × AMF interaction).
Phosphorus availability and uptake by maize
In the case of phosphorus availability in soil, Zn12 plants only showed a 2% increase in phosphorus accumulation in soil, whereas under M- plants exhibited a 6% increase compared to the control. Moreover, the phosphorus accumulation in shoots was also higher under M- conditions and Zn12, while M + showed 17% increase in shoot phosphorus levels. Finally, the higher application of zinc (Zn12) and mycorrhizal inoculation resulted in higher zinc availability in maize soil (Fig. 6).
Effect of arbuscular mycorrhizal inoculation on Phosphorus contents of maize under zinc deficient soil conditions. (a) phosphorus concentration in soil (mg kg−1) (b) phosphorus concentration shoot (mg kg −1). Zn0 (control), Zn1.5 (1.5 mg Kg-1), Zn3 (3 mg kg-1), Zn6 (6 mg kg-1), Zn12 (12 mg kg-1), inoculated with AMF (M + grey), un-inoculated (M- white). Error bars represent standard error and asterisk (*) shows significant difference (p ≤ 0.05) among treatments (Zn × AMF interaction).
Correlation analysis
The Pearson correlation coefficients (r) for the different physiological and biochemical parameters under zinc-deficient soil conditions are summarized in Table 1. All of the parameters showed a positive correlation. The photosynthetic rate showed a strong positive correlation with stomatal conductance, superoxide dismutase, total soluble protein, total chlorophyll, zinc, and phosphorus in soil. The antioxidant enzymes (SOD, CAT, POD) showed a strong positive correlation with zinc accumulation in the root, shoot and soil, and phosphorus accumulation in soil and maize shoots. Also, these antioxidant enzymes were positively correlated with the maize chlorophyll contents.
Principal component analysis
The interrelationship among selected zinc levels on zinc-deficient soil both with and without mycorrhizal inoculation were analysed by biplot principal component analysis (PCA), as shown in Fig. 7. This plot revealed that the first two components explained 94.54% of the variation (PC1 88.60%, and PC2 5.94%). The PCA biplot grouped the zinc levels based on maize performance observed for physiological and biochemical variables, showing that the Zn12 was the best performing situation for improving the maize's overall traits in this experiment. The Zn12 treatment also helped to improve the nutrient status of the soil. The Zn6 treatment also provided significant improvement in the measured characteristics. However, the Zn0, Zn1.5 and Zn3 treatments had a much smaller effect in significantly improving maize growth under zinc deficient soil conditions.
PCA biplot for different zinc levels under zinc deficient soil conditions. PCA biplot is a combination of score plot of zinc levels (represented in blue text) and loading plot of variables (represented by red vectors; black text). Photo, photosynthetic rate; Trans, transpiration rate; Conduc, stomatal conductance; SOD, superoxide dismutase; CAT, catalase; POD, peroxidase; TSP, total soluble protein; CHLA, chlorophyll a; CHLB, chlorophyll b; TCHL, total chlorophyll; ZR, zinc in roots; ZSH, zinc in shoot; ZS, zinc in soil; PS, phosphorus in soil; PSH, phosphorus in shoot.
Discussion
The current study has elaborated the beneficial role of AM colonization compared to non-mycorrhizal plants. Arbuscular mycorrhizal symbiosis alters plants' internal physiological conditions, consequently controlling the opening of stomata, one of the prime sites of photosynthesis40,41. A higher photosynthetic rate was attributed to the Zn12 (M +) condition compared to non-mycorrhizal plants in this study. Another study supported this result in which it was reported that regulation of abscisic acid and antioxidant enzyme activities are correlated with each other in altering stomatal behaviour42. Our results highlight AM symbiosis' importance that can increase the chlorophyll contents of maize in nutrient-deficient soils. Furthermore, mycorrhizal inoculation might enhance the magnesium uptake, which helps develop higher chlorophyll contents similar to what was observed in Zn12, ultimately resulting in a higher photosynthetic rate43. These findings agree with earlier studies' results44,45. Presence of AMF protects photosystem II during the photosynthesis that played a significant role in better uptake of nutrients and water. Balance uptake of water Regulate photosynthetic rate through improvement in transpiration rate in stomatal conductance. On the other hand, optimum uptake of zinc properly catalyzed the reactions Which facilitate the plants for better growth46,47,48,49.
Poor uptake of Zn facilitates the biosynthesis of reactive oxygen species (ROS) during photosynthetic electron transport50. However, antioxidants play a critical role in defense system of cells components such as proteins, membrane lipids, SH-containing enzymes, chlorophyll and DNA against ROS. The cysteine, histidine and glutamate or aspartate residues represent the most critical Zn binding sites in enzymes, DNA-binding proteins (Zn-®nger proteins) and membrane proteins6. Improvement in antioxidant enzyme activities were also observed in maize tissue samples of current study (Fig. 4). Since SOD is the main ROS scavenging enzyme that converts superoxide ion to H2O2 (which is also harmful to plant growth), the activity CAT and POD was also shown to be increased in our maize plants. In the present study, plants were exposed to artificial Zn deficient soil conditions, that might result in ROS species' overproduction. However, AM-inoculated maize plants produced higher antioxidant enzyme activity, favouring ROS stress molecule scavenging which diminished the oxidative burst6. Both enzyme activities were significantly (p ≤ 0.05) higher with AM inoculation than non-inoculated plants. It might be the reason that inoculated plants showed higher SOD activity in this study. Moreover, this enzyme activity also contributed to hyphal transport, which assists in diffusing essential micronutrients necessary for enzyme activities7. The AM role observed was supported by an earlier report that the vegetative part (mycelium) of mycorrhizal fungi in the rhizosphere enhance the availability of nutrients to roots.
The maize plant’s total soluble protein contents increased in inoculated and non-inoculated plants, although the increased level was highest with inoculated plants with a Zn dose of 6 mg kg-1 of soil. Our results regarding the increase in soluble proteins agree with previous reports7. In a separate study, mycorrhizal inoculation resulted in a soluble protein increase of 2–sixfold in clover plants51.
The higher availability of Zn in the soil might be due to the AM role in synthesizing amino acids (tryptophan and asparagine). Therefore, the current study showed that higher levels of Zn resulted in higher Zn availability in soil. Likewise, it allowed the plants a higher level of Zn uptake. Our results regarding higher zinc accumulation were consistent with the results of the earlier report of Marques et al.52 and are also confirmed by another study on maize, concluding that mycorrhizal inoculation renders a positive role in the accumulation of Zn under zinc-deficient soil53.
Phosphorus is a relatively immobile nutrient in soil yet its concentration in the soil increased with the elevated levels of Zn fertilization irrespective of mycorrhizal inoculation. However, in the case of Zn uptake in shoots, contrary results showed by inoculated maize plants. It might be the reason that M + assisted maize plants in the absorption of phosphorus by the roots. Numerous studies have reported AM positive role in phosphorus accumulation28,54. Moreover, increasing the level of Zn increased the root surface area of the plant with mycorrhizal colonization. It also secreted the glomalin protein that solubilizes the fixed phosphorus, resulting in a higher concentration of phosphorus in the soil.
Conclusions
The current study showed the beneficial role of arbuscular mycorrhizal inoculation for maize growth on zinc-deficient soil. It improved the maize's gaseous exchange traits, such as a higher photosynthetic rate and a higher zinc application level. The chlorophyll contents also improved with higher zinc fertilization and inoculation of maize plants. These favourable maize physiology changes were solely due to a higher nutrient availability under deficient soil conditions. Inoculated plants also showed a higher accumulation of nutrients and their availability in soil.
References
Alloway, B. J. Soil factors associated with zinc deficiency in crops and humans. Environ. Geochem. Health 31, 537–548 (2009).
Bibi, F. et al. Effect of various application rates of phosphorus combined with different zinc rates and time of zinc application on phytic acid concentration and zinc bioavailability in wheat. Agric. Nat. Resour. 54, 265–272 (2020).
Tahir, F. A., Ahamad, N., Rasheed, M. K. & Danish, S. Effect of various application rate of zinc fertilizer with and without fruit waste biochar on the growth and Zn uptake in maize. Int. J. Biosci. 13, 159–166 (2018).
Rafique, E., Rashid, A., Ryan, J. & Bhatti, A. U. Zinc deficiency in rainfed wheat in Pakistan: Magnitude, spatial variability, management, and plant analysis diagnostic norms. Commun. Soil Sci. Plant Anal. 37, 181–197 (2006).
Duc, N. H., Csintalan, Z. & Posta, K. Arbuscular mycorrhizal fungi mitigate negative effects of combined drought and heat stress on tomato plants. Plant Physiol. Biochem. 132, 297–307 (2018).
Cakmak, I. Tansley Review No. 111 Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol. 146, 185–205 (2000).
Subramanian, K. S., Tenshia, J. S. V., Jayalakshmi, K. & Ramachandran, V. Antioxidant enzyme activities in arbuscular mycorrhizal (Glomus intraradices) fungus inoculated and non-inoculated maize plants under Zinc Deficiency. Indian J. Microbiol. 51, 37–43 (2011).
Aslanpour, M., Baneh, H. D., Tehranifar, A. & Shoor, M. Effect of mycorrhizal fungi on macronutrients and micronutrients in the white seedless grape roots under the drought conditions. Int. Trans. J. Eng. Manag. Appl. Sci. Technol. 10, 397–408 (2019).
Wahid, F. et al. Sustainable management with mycorrhizae and phosphate solubilizing bacteria for enhanced phosphorus uptake in calcareous soils. Agriculture 10, 334 (2020).
Smith, S. E., Smith, F. A. & Jakobsen, I. Mycorrhizal fungi can dominate phosphate supply to plants irrespective of growth responses. Plant Physiol. 133, 16–20 (2003).
Latef, A. A. H. A. & Chaoxing, H. Arbuscular mycorrhizal influence on growth, photosynthetic pigments, osmotic adjustment and oxidative stress in tomato plants subjected to low temperature stress. Acta Physiol. Plant. 33, 1217–1225 (2011).
Alizadeh, O., Zare, M. & Nasr, A. H. Evaluation effect of mycorrhiza inoculate under drought stress condition on grain yield of sorghum (Sorghum bicolor). Adv. Environ. Biol. 5, 2361–2364 (2011).
Cavagnaro, T. R. et al. Arbuscular mycorrhizas, microbial communities, nutrient availability, and soil aggregates in organic tomato production. Plant Soil 282, 209–225 (2006).
Nunes, J. L. da S., de Souza, P. V. D., Marodin, G. A. B. & Fachinello, J. C. Effect of arbuscular mycorrhizal fungi and indolebutyric acid interaction on vegetative growth of ‘Aldrighi’ peach rootstock seedlings. Cienc. e Agrotecnologia 34, 80–86 (2010).
Davies, F. T., Puryear, J. D., Newton, R. J., Egilla, J. N. & Saraiva Grossi, J. A. Mycorrhizal fungi enhance accumulation and tolerance of chromium in sunflower (Helianthus annuus). J. Plant Physiol. 158, 777–786 (2001).
Kucey, R. M. N., Janzen, H. H. & Leggett, M. E. Microbially mediated increases in plant-available phosphorus. Adv. Agron. 42, 199–228 (1989).
Senovilla, M. et al. MtCOPT2 is a Cu+ transporter specifically expressed in Medicago truncatula mycorrhizal roots. Mycorrhiza 30, 781–788 (2020).
Coccina, A. et al. The mycorrhizal pathway of zinc uptake contributes to zinc accumulation in barley and wheat grain. BMC Plant Biol. 19, (2019).
Tu, J. L., Liu, X. M. & Xiao, J. X. Effects of arbuscular mycorrhizal inoculation on osmoregulation and antioxidant responses of blueberry plants. Bangladesh J. Bot. 48, 641–647 (2019).
Sato, T. et al. Secretion of acid phosphatase from extraradical hyphae of the arbuscular mycorrhizal fungus Rhizophagus clarus is regulated in response to phosphate availability. Mycorrhiza 29, 599–605 (2019).
Subramanian, K. S., Bharathi, C. & Jegan, A. Response of maize to mycorrhizal colonization at varying levels of zinc and phosphorus. Biol. Fertil. Soils 45, 133–144 (2008).
Walkley, A. & Black, I. A. An examination of the degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 37, 29–38 (1934).
Olsen, S., Cole, C., Watanabe, F. & Dean, L. Estimation of available phosphorus in soils by extraction with sodium bicarbonate. (United States Department of Agriculture, 1954).
Pratt, P. F. Potassium. in Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties, 9.2 (ed. Norman, A. G.) 1022–1030 (John Wiley & Sons, Ltd, 1965). https://doi.org/10.2134/agronmonogr9.2.c20.
Lindsay, W. L. & Norvell, W. A. A DTPA soil test for zinc, iron, manganese and copper. Soil Sci. Soc. Am. J. 42, 421–428 (1978).
Martin, F., Winspear, M. J., MacFarlane, J. D. & Oaks, A. Effect of methionine sulfoximine on the accumulation of ammonia in C3 and C4 leaves. Plant Physiol. 71, 177–181 (1983).
Ohno, T. & Zibilske, L. M. Determination of low concentrations of phosphorus in soil extracts using malachite green. Soil Sci. Soc. Am. J. 55, 892–895 (1991).
Ryan, M. H., McInerney, J. K., Record, I. R. & Angus, J. F. Zinc bioavailability in wheat grain in relation to phosphorus fertiliser, crop sequence and mycorrhizal fungi. J. Sci. Food Agric. 88, 1208–1216 (2008).
Thomas, G. W. Soil pH and soil acidity. In Methods of Soil Analysis, Part 3: Chemical Methods vol. 5 475–490 (John Wiley & Sons, 1996).
Rhoades, J. D. Salinity: Electrical Conductivity and Total Dissolved Solids. in Methods of Soil Analysis, Part 3, Chemical Methods (eds. D.L. Sparks et al.) vol. 5 417–435 (Soil Science Society of America, 1996).
Danish, S. & Zafar-ul-Hye, M. Co-application of ACC-deaminase producing PGPR and timber-waste biochar improves pigments formation, growth and yield of wheat under drought stress. Sci. Rep. 9, 5999 (2019).
Arnon, D. I. Copper enzymes in isolated chloroplasts Polyphenoloxidase in Beta vulgaris. Plant Physiol. 24, 1–15 (1949).
Giannopolitis, C. N. & Ries, S. K. Superoxide dismutases: I Occurrence in higher plants. Plant Physiol. 59, 309–314 (1977).
Chance, B. & Maehly, A. C. Assay of catalases and peroxidases. Methods Enzymol. 2, 764–775 (1955).
Sambrook, J. & Russell, D. W. In vitro mutagenesis using double-stranded DNA templates: selection of mutants with DpnI. Cold Spring Harb. Protoc. 2, 13–19 (2006).
Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein using the principle of protein dye binding. Anal. Biochem. 72, 248–254 (1976).
Phillips, J. M. & Hayman, D. S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 55, 158–IN18 (1970).
Vierheilig, H., Coughlan, A. P., Wyss, U. & Piché, Y. Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Appl. Environ. Microbiol. 64, 5004–5007 (1998).
Steel, R. G., Torrie, J. H. & Dickey, D. A. Principles and Procedures of Statistics: A Biometrical Approach. (McGraw Hill Book International Co., 1997).
Shahzad, S., Khan, M. Y., Zahir, Z. A., Asghar, H. N. & Chaudhry, U. K. Comparative effectiveness of different carriers to improve the efficacy. Pakistan J. Bot. 49, 1523–1530 (2017).
Lawson, T. Guard cell photosynthesis and stomatal function. New Phytol. 181, 13–34 (2009).
Goicoechea, N., Antolín, M. C. & Sánchez-Díaz, M. Gas exchange is related to the hormone balance in mycorrhizal or nitrogen-fixing alfalfa subjected to drought. Physiol. Plant. 100, 989–997 (1997).
Mathur, N. & Vyas, A. Biochemical changes in Ziziphus xylopyrus by VA mycorrhizae. Bot. Bull. Acad. Sin. 37, 209–212 (1996).
Sannazzaro, A. I., Ruiz, O. A., Albertó, E. O. & Menéndez, A. B. Alleviation of salt stress in Lotus glaber by Glomus intraradices. Plant Soil 285, 279–287 (2006).
Colla, G. et al. Alleviation of salt stress by arbuscular mycorrhizal in zucchini plants grown at low and high phosphorus concentration. Biol. Fertil. Soils 44, 501–509 (2008).
Yang, Y. et al. The combined effects of arbuscular mycorrhizal fungi (AMF) and lead (Pb) stress on Pb accumulation, plant growth parameters, photosynthesis, and antioxidant enzymes in Robinia pseudoacacia L. PLoS One 10, e0145726 (2015).
Lu, C. & Vonshak, A. Effects of salinity stress on photosystem II function in cyanobacterial Spirulina platensis cells. Physiol. Plant. 114, 405–413 (2002).
Chaves, M. M., Flexas, J. & Pinheiro, C. Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann. Bot. 103, 551–560 (2009).
Toler, H. D., Morton, J. B. & Cumming, J. R. Growth and metal accumulation of mycorrhizal sorghum exposed to elevated copper and zinc. Water. Air. Soil Pollut. 164, 155–172 (2005).
Marschner, H. & Cakmak, I. High Light Intensity Enhances Chlorosis and Necrosis in Leaves of Zinc, Potassium, and Magnesium Deficient Bean (Phaseolus vulgaris) Plants. J. Plant Physiol. 134, 308–315 (1989).
Arines, J., Palma, J. M. & Viarino, A. Comparison of protein patterns in non-mycorrhizal and vesicular–arbuscular mycorrhizal roots of red clover. New Phytol. 123, 763–768 (1993).
Marques, A. P. G. C., Oliveira, R. S., Rangel, A. O. S. S. & Castro, P. M. L. Application of manure and compost to contaminated soils and its effect on zinc accumulation by Solanum nigrum inoculated with arbuscular mycorrhizal fungi. Environ. Pollut. 151, 608–620 (2008).
Zhang, W. et al. Zinc uptake by roots and accumulation in maize plants as affected by phosphorus application and arbuscular mycorrhizal colonization. Plant Soil 413, 59–71 (2017).
Cavagnaro, T. R. The role of arbuscular mycorrhizas in improving plant zinc nutrition under low soil zinc concentrations: a review. Plant Soil 304, 315–325 (2008).
Acknowledgements
We acknowledge Directorate of Research Bahauddin Zakariya University Multan, Pakistan for facilitating us for research. The study was also supported by Researchers Supporting Project number (RSP-2021/257), King Saud University, Riyadh, Saudi Arabia.
Funding
We are thankful to Researchers Supporting Project number (RSP-2021/257), King Saud University, Riyadh, Saudi Arabia for their financial support.
Author information
Authors and Affiliations
Contributions
Conceptualization, A.S.; M.A.A.; N.A.; methodology, A.S.; M.A.A.; N.A.; software, A.S.; M.A.A.; N.A.; validation, A.S.; M.A.A.; N.A.; formal analysis, A.S.; M.A.A.; N.A.; investigation, A.S.; M.A.A.; N.A.; resources, A.S.; M.A.A.; N.A.; data curation, A.S.; M.A.A.; N.A.; writing—original draft preparation, S.D.; S.F.; R.D.; B.R.G.; writing—review and editing, S.D.; S.F.; R.D.; B.R.G.; M.J.A.; O.N.; M.H.u.R.; supervision, M.A.A.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saboor, A., Ali, M.A., Danish, S. et al. Effect of arbuscular mycorrhizal fungi on the physiological functioning of maize under zinc-deficient soils. Sci Rep 11, 18468 (2021). https://doi.org/10.1038/s41598-021-97742-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-97742-1
This article is cited by
-
Alleviation of salinity stress by EDTA chelated-biochar and arbuscular mycorrhizal fungi on maize via modulation of antioxidants activity and biochemical attributes
BMC Plant Biology (2024)
-
Foliar-Applied Zinc is Beneficial to Growth, Grain Yield, and Quality of Standard and Ancient Wheats Grown under Saline and Non-Saline Conditions
International Journal of Plant Production (2024)
-
Modulation of sunflower growth via regulation of antioxidants, oil content and gas exchange by arbuscular mycorrhizal fungi and quantum dot biochar under chromium stress
BMC Plant Biology (2023)
-
Mycorrhizal inoculation under water stress conditions and its influence on the benefit of host microbe symbiosis of Terminalia arjuna species
Bulletin of the National Research Centre (2023)
-
γ-Aminobutyric acid (GABA) and ectoine (ECT) impacts with and without AMF on antioxidants, gas exchange attributes and nutrients of cotton cultivated in salt affected soil
BMC Plant Biology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.