Abstract
Vitamin D deficiency during critical periods of development could lead to persistent brain alterations. We aimed to assess the association between maternal vitamin D3, the major circulatory form of vitamin D, at pregnancy and neurodevelopmental outcomes during childhood, namely: behavioural problems, Attention Deficit and Hyperactivity Disorder (ADHD) and Autism Spectrum Disorder (ASD) symptoms, and social competence. This study included 2,107 mother-child pairs of a Spanish population-based birth cohort. Maternal plasma vitamin D3 was measured in pregnancy. The outcomes were measured through questionnaires at 5, 8, 14, and 18 years old. We ran multivariate regression models adjusted for potential confounding variables. We found that per each 10 ng/mL increment of maternal vitamin D3, children obtained higher social competence scores (coefficient = 0.77; 95% CI = 0.19, 1.35) at 5 years old. However, we observed null associations between maternal vitamin D3 and total behavioural problems and ADHD and ASD symptoms in children from 5 to 18 years old. Further studies carried out in countries where the population is exposed to lower vitamin D levels are needed.
Similar content being viewed by others
Introduction
Vitamin D is a hormone that is involved in the formation of bone and in the regulation of several functions throughout the body, including the central nervous system function1. There are two forms of vitamin D, depending on the source: D2, of plant origin, and D3, of animal origin1. The majority of circulating vitamin D is in D3 form2,3, which is mainly produced photochemically in the epidermis by action of ultraviolet light1,4 and a small proportion comes from diet, principally from fish, eggs, and milk5. Vitamin D from the skin and diet is metabolized in the liver to 25-hydroxy-vitamin D (25[OH]D), the circulating form, which is one of the most stable vitamin D biomarkers, and in the kidney to 1,25-(OH)D, the active form1. During gestation, the foetus is entirely dependent on the maternal supply of 25(OH)D6.
Vitamin D modulates the central nervous system function mainly through its nuclear hormone receptor, the vitamin D receptor, which is expressed in neuronal and glial cells in almost all regions of the central nervous system7. It controls neuronal differentiation and maturation, regulates the genetic expression of neurotransmitters, and it has neuroprotective properties and antioxidant effects1. Vitamin D deficiency is a public health problem worldwide, particularly for darker skinned individuals living further away from the equator8. If this deficiency occurs during critical periods of development, it could lead to changes that persist through adulthood, and increases the risk of different psychiatric and neurological disorders, such as schizophrenia, multiple sclerosis, dementia, cognitive decline, Parkinson disease, and depression9. Some studies have investigated the association between vitamin D concentrations during pregnancy and neurodevelopmental outcomes, such as behavioural problems and social competence, in the offspring. Three independent studies observed no associations between maternal 25(OH)D measured in blood at 18, 26, and 33 weeks of pregnancy, respectively, and behavioural problems during childhood9,10,11. However, a recent study observed that higher maternal 25(OH)D concentration (>20.4 ng/mL) at week 13 of pregnancy was related to a lower number of behavioural problems and attention deficit and hyperactivity disorder (ADHD) symptoms at 4 years old12. Two other studies, one of them developed in our cohort, the Spanish population-based birth cohort INMA (INfancia y Medio Ambiente [Childhood and Environment]), also reported negative associations between prenatal 25(OH)D3 and ADHD symptoms at preschool age13,14. The evidence for an association between prenatal levels of 25(OH)D and autism spectrum disorder (ASD) symptoms is inconsistent, while some studies found negative relationships6,15,16,17,18,19, others obtained null results20,21. Nevertheless, a recently conducted meta-analysis showed negative associations between prenatal 25(OH)D3 and both ADHD and ASD symptoms22. Moreover, low concentrations of 25(OH)D during pregnancy have shown to be associated with delayed social development in toddlers23,24. Unlike the other mentioned outcomes, this domain is not considered a clinical phenotype, however, it encompasses several aspects of adaptive behavioural development with long-term consequences, such as unemployment, mental health problems, marital difficulties, delinquency, or violence25.
Further research on the association between prenatal 25(OH)D concentration and behaviour during childhood is needed. This study aims to assess the association between maternal circulating vitamin D3 (25[OH]D3) in pregnancy, when key neuronal maturation processes take place in the foetus, and behavioural problems, ADHD and ASD symptoms, and social competence from 5 to 18 years old in the INMA cohort, which is based in different regions of Spain.
Results
The characteristics of the entire sample and by the three clinically relevant categories of maternal 25(OH)D3 concentration in plasma during pregnancy are described in Supplementary Table S1. The study population included 50.8% male children and 56.4% firstborn. Ninety-four percent of the mothers were born in Spain, and maternal mean age was 30.7 (4.2) years old. Twenty-five percent of the mothers were overweight or obese before pregnancy, 25.8% had a low education level (primary or less) and 34% had high education level (university). Half of the mothers had a manual occupation, 17% of the mothers were smokers during the first trimester of pregnancy, and 29% reported that their partners smoked at home. Eighteen percent of the women (n = 372) had deficient concentrations of 25(OH)D3 (<20 ng/mL) during pregnancy. The highest concentrations of circulating 25(OH)D3 were observed in Valencia (mean [SD] 32.8 [10.8] ng/mL), and the lowest, in Asturias (mean [SD] 28 [10.2] ng/mL). Higher concentrations of 25(OH)D3 were detected in women from high education level and from managers/technicians occupation category, and those born in Europe, including Spain, as opposed to those women born in Latin America. Lower 25(OH)D3 concentrations were measured in younger women and in women with pre-pregnancy overweight or obesity, primiparous, active smokers and with partners smoking at home during pregnancy.
Behavioural problems, ADHD symptoms, ASD symptoms, and social competence were assessed using different instruments and at different children’s ages in each of the regions (Supplementary Table S2). Supplementary Table S3 shows the distribution of the outcomes included in this study according to the categories of maternal 25(OH)D3. Children prenatally exposed to deficient concentrations of 25(OH)D3 (<20 ng/mL) showed more ASD symptoms, more total behavioural problems, and less social competence at 5 years old, as compared to their peers exposed to higher concentrations. This group of children with low concentrations of 25(OH)D3 during gestation had more ADHD symptoms at 8 years old and more total behavioural problems at 8 and at 14 years old. However, this pattern was not observed at 18 years old.
Table 1 shows the associations between prenatal 25(OH)D3 concentrations and the neurodevelopmental outcomes. Regarding the outcomes measured at 5 years old, the regression models indicated that sufficient 25(OH)D3 concentrations during pregnancy (≥30 ng/ml) were associated with lower ASD symptoms (coefficient = −0.54; 95% CI = −1.01, −0.07) compared to deficient 25(OH)D3 concentrations, and that insufficient concentrations (20–29.9 ng/ml) were associated with lower total behavioural problems measured with the Strengths and Difficulties Questionnaire (SDQ) (coefficient = −1.65; 95% CI = −3.27, −0.03) compared to deficient 25(OH)D3 concentrations, although these associations were no longer present in the fully adjusted models. The association with social competence remained after adjusting for potential confounding variables, using the exposure variable as continuous and categorical forms. Per each 10 ng/mL increment of maternal 25(OH)D3 concentration in pregnancy, children obtained 0.77 (95% CI = 0.19, 1.35) points more of social competence. Similarly, the score was 2.14 (95% CI = 0.47, 3.82) points higher in children with sufficient prenatal concentrations of 25(OH)D3 versus children with deficient concentrations. A positive association was also detected in the insufficient category (coefficient = 2.09; 95% CI = 0.33, 3.84), which was not observed in the fully adjusted model. The associations observed between maternal 25(OH)D3 in pregnancy, treated as continuous and categorical variable, and total behavioural problems and ADHD symptoms in the offspring at 8, 14, and 18 years old were almost null. We observed that insufficient 25(OH)D3 concentrations (20–29.9 ng/mL) category was associated with lower total behavioural problems score measured with the Child Behaviour Checklist (CBCL) (Incidence Rate Ratio [IRR] = 0.86; 95% CI = 0.76, 0.97), compared to de deficiency category (<20 ng/ml), but this association was no longer found after adjusting for potential confounding variables.
As sensitivity analysis, no associations were detected between child 25(OH)D3 concentrations and the outcomes in the subsample of children with available child 25(OH)D3 concentrations. The association between prenatal 25(OH)D3 and child social competence remained when we restricted the analysis to the sample of children with both prenatal and child 25(OH)D3 data available (Supplementary Table S4).
Discussion
We explored the associations between maternal 25(OH)D3 concentration during pregnancy and a wide range of behavioural problems, including ADHD and ASD symptoms, and social competence in children at different ages using data from a population-based birth cohort located in five regions across Spain. Our findings indicated a positive association between prenatal 25(OH)D3 concentrations and social competence at 5 years of age. However, we observed null associations of prenatal 25(OH)D3 concentration with total behavioural problems, ADHD and ASD symptoms in children from 5 to 18 years old. Sensitivity analyses showed no associations between the concentrations of 25(OH)D3 in children and the outcomes.
In our study, we observed a weak negative association between prenatal 25(OH)D3 and ASD symptoms at 5 years of age that was not significant upon adjustment for potential confounding variables. These results are in line with two Californian studies20,21. On the contrary, previous research carried out in Australian15 and Dutch birth cohort studies6,19 found stronger associations with this outcome. The Australian study reported an association between lower mid-gestation 25(OH)D concentrations and higher scores on the Attention Switching subscale of the Autism-Spectrum Quotient15. In the Dutch studies, infants exposed to persistent low 25(OH)D concentrations (<7.9 ng/mL) from mid-gestation until birth showed more autism-related traits at 6 years old19, and mid-gestational 25(OH)D deficiency (<7.9 ng/mL) was associated with an increased risk of ASD diagnosis at 9 years old6. The different findings obtained could be explained by the lower levels of 25(OH)D in Dutch pregnant women, as compared to the Spanish sample. Other plausible explanations are the different pregnancy periods at blood collection and the different instruments used to assess ASD symptoms. The instrument used in the Dutch studies, the Social Responsiveness Scale, is particularly focused on measuring the social deficits associated with ASD26. This suggests that prenatal vitamin D could be more related to the social dimension of ASD. The previously reported positive association between prenatal vitamin D and social development in toddlers23,24 together with our findings on social competence at age 5 years reinforces this hypothesis. In our study, we obtained a relatively low effect estimates for the association between prenatal vitamin D levels and social competence. However, this effect size is in line with other studies assessing other exposures, such as head circumference27, child salivary cortisol levels at 14 months of age28, and maternal urinary phthalate metabolite levels during pregnancy29. Furthermore, although it might not be relevant at a clinical level, the observed effect may have an impact at a population level. If the whole population is exposed to low levels of vitamin D, the distribution of social competence scores would likely move to the left, and the prevalence of children with low social competence would increase substantially. This increase of social competence problems may have a negative impact on the community in terms of unemployment, mental health problems or delinquency25.
We observed no associations between the concentrations of 25(OH)D3 in children and the assessed outcomes in a subsample, while the association remained between prenatal 25(OH)D3 and social competence in the same group. This finding suggests that the levels of 25(OH)D3 influence child social development exclusively during the prenatal period. The biological mechanisms by which maternal vitamin D concentration during pregnancy affect the brain development in humans are still unclear. However, some animal studies observed that 25(OH)D3 deficiency during late gestation was associated with brain morphological alterations in the offspring, such as larger lateral ventricles, thinner cortex, and higher cell proliferation30, as well as hyperlocomotion at adulthood31. Although the most active period of foetal brain growth lies in the third trimester of pregnancy, key development processes, such as neuronal migration, start at week 6 of gestation and usually end around 24 weeks, and vitamin D is involved in the modulation of neuronal migration, dendritic spine morphology, and neuronal connectivity1. Therefore, the association we observed between 25(OH)D3 concentration in pregnancy and child social competence could be due to alterations in these neuronal processes.
The null associations detected between maternal 25(OH)D3 concentration in pregnancy and total behavioural problems in the offspring are consistent with some previous studies9,10,11. However, Daraki et al.12 found that higher 25(OH)D levels (>20.4 ng/mL) were related to decreased number of total behavioural problems in 4-year-old children12. In this study, carried out in a Greek cohort, almost two-thirds of pregnant women (n = 313) had 25(OH)D deficiency (<15.7 ng/mL), which seemed to be explained by darker skin pigmentation, poor dietary vitamin D intake, veiled clothing reducing sunshine exposure, and increased prevalence of obesity. This high prevalence of 25(OH)D deficiency could explain the different results obtained between this study and ours.
Regarding ADHD symptoms, we found that, despite the associations already observed between prenatal 25(OH)D3 and ADHD at preschool age in our cohort13 and in the Danish14 and the Greek12 cohorts, these associations did not remain at 8 and 18 years old. A possible explanation is that prenatal 25(OH)D3 concentrations do not have permanent effects on the brain development, but other factors could modulate these effects across the childhood and adolescence, exerting much more influence on ADHD symptoms at 8 and 18 years old than prenatal 25(OH)D3. Alternative explanations to the inconsistent findings between preschool age and older age in our cohort include the different instruments used in both periods and the different person reporting ADHD symptoms. At ages 4–5 years, we administered the ADHD Criteria of Diagnostic and Statistical Manual of Mental Disorders (ADHD-DSM-IV) form to the teachers, while at 8 and 18 years old the parents filled in the Conners’ Parent Rating Scale-Revised (short form) (CPRS-R [S]). It is known that agreement between parents and teachers regarding child ADHD symptoms is relatively poor32. However, previous research performed in a Scandinavian study using clinical ADHD diagnosis also reported null associations between prenatal 25(OH)D concentrations and ADHD at older ages33,34, which supports the hypothesis about the reversibility of the prenatal vitamin D deficiency effects on brain development.
This study has some limitations. First, we only used D3 form of vitamin D, however this is the major circulatory form and is commonly used in endocrinology studies3,30,31. Second, a single 25(OH)D3 measurement per subject was obtained, so it was not possible to compare different windows of susceptibility to 25(OH)D3 deficiency during pregnancy. Third, the heterogeneity in the assessment periods between regions complicated the interpretation of the results. Due to the small sample size of adolescents in our study, results on long-term associations between prenatal 25(OH)D3 concentrations and behavioural problems at 14 and 18 years old should be taken with caution. Moreover, given the prospective nature of the study, we had some loss of participants across follow ups. This limitation was minimized by applying inverse probability weighting in all the analyses. We also performed several tests that could have increased the risk of chance findings or Type I errors, however the association we observed was robust, given the consistency in the direction of the associations between the models, unadjusted and adjusted, and using the exposure as continuous and as categorical variable, and in line with previous literature. In addition, lack of information on parental behavioural problems during pregnancy could have resulted in some residual confounding.
The main strengths of this study include its population-based, prospective design, and large sample size located in heterogeneous geographical areas. Therefore, the conclusions of the study are generalizable to the general population. Several methodological aspects are also considered major strengths of the study, such as the inclusion of important confounding variables in the statistical models, the multiple imputation of missing covariate values, the use of inverse probability weighting to control the potential selection bias induced by loss of follow-up. Finally, the inclusion of several behavioural problems domains and ages conferred a wide view of the role of prenatal 25(OH)D3 on neurodevelopmental alterations, not restricted to specific types of behavioural problems or focused on single age periods.
In conclusion, we observed that prenatal 25(OH)D3 was related to social skills at 5 years of age. On the contrary, we found no evidence for the association between prenatal 25(OH)D3 and several behavioural problems across childhood including ADHD and ASD symptoms. Further studies carried out in countries where the population is exposed to lower vitamin D levels are needed.
Methods
Study population
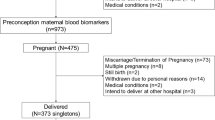
This study was embedded in the INMA Project, a population-based birth cohort based in different regions across Spain35. For the current study, we used data from Menorca (40°N), Valencia (39°N), Sabadell (41°N), Asturias (43°N), and Gipuzkoa (43°N) regions. A total of 3,126 pregnant women were recruited at their first routine specialized antenatal care visit (10–13 weeks of gestation) in the main public hospital or reference health centre. The recruitment took place between February 1997 and September 1998 in Menorca, and between November 2003 and February 2008 in the other regions. Since then, we collected data from the participants every two years approximately. The 89.3% (2,792) of the baseline sample had 25(OH)D3 data available. A total of 2,107 mother-child pairs, who had 25(OH)D3 measured and at least one of the behavioural assessments available, were included in this study, representing the 67.4% of the initial sample (Fig. 1).
This study was approved by the Clinical Research Ethical Committees of the Asturias, Donostia (Gipuzkoa), and La Fe (Valencia) Hospitals, and the Medical Assistance Municipal Institute (Barcelona), all research was performed in accordance with the relevant guidelines and regulations, and written informed consent was obtained from all participants for each phase.
25(OH)D3 assessment
A single maternal blood specimen was drawn during pregnancy (mean [SD] 13.3 [2.8] weeks of gestation). Most blood draws (78.6%) were done during the second trimester of pregnancy, with 20.6% during the first trimester, and 0.8% during the third trimester. We also measured child plasma concentrations of 25(OH)D3 in a subgroup of 808 participants (mean [SD] 4.5 [0.2] years old). Samples were processed immediately and stored from −70 to −80 °C until analysis. Plasma concentrations of 25(OH)D3 were quantified by high-performance liquid chromatography method using a BioRAD kit according to Clinical and Laboratory Standard Institute protocols36. Detection limit was 5 ng/ml, and interassay coefficient of variation was 4.5%. The assay was validated by German Programes of External Evaluation of Quality (DGKL-RFB-Referenzinstitut für Bionalytik), and results were satisfactory in 100% of the cases.
Neurodevelopmental outcomes assessment
ASD symptoms were measured through the Childhood Autism Spectrum Test (CAST)37. A psychologist administered this 37-item questionnaire to the child’s parents. The CAST identifies subtle manifestations of autism spectrum conditions (social impairments, communication impairments, and repetitive or stereotyped behaviours). The total score ranges from 0 to 31 points.
Social competence was reported by teachers using the California Preschool Social Competence Scale (CPSCS)38. The CPSCS consists of 30 items that result into scores for five subscales (considerateness, task orientation, extraversion, verbal facility, and response to unfamiliar) and a general social competence score. The scores were standardized to a mean of 100 and a standard deviation of 15.
Behavioural problems were reported by parents using SDQ39 and CBCL40. The SDQ consists of 25 items with five for each dimension: emotional problems, conduct problems, hyperactivity/inattention problems, peer/social problems, and pro-social behaviour. The sum of the four problematic scores (excluding pro-social behaviour) yields a total behavioural problems score, ranging from 0 to 40. Regarding the CBCL, we used the version for children between 6 and 18 years old, comprised by 113 items that measure nine dimensions: anxious/depressed, withdrawn/depressed, somatic complaints, social problems, thought problems, attention problems, rule-breaking behaviour, aggressive behaviour, and other problems. The sum of all subscales’ scores yields a total behavioural problems score, ranging from 0 to 240.
ADHD symptoms were reported by parents using the CPRS-R (S)41. This questionnaire comprises 27 items that result into scores for three subscales (oppositional, cognitive problems/inattention, hyperactivity), and a general ADHD index, ranging from 0 to 36.
Covariates assessment
During the first trimester of pregnancy, we used questionnaires to collect maternal background information, such as age, country of birth (Spain, Latin America, Europe, and other country), education level (primary or lower, secondary, and university degree), social class (managers/technicians, non-manual, and manual occupation)42, parity (0, 1, 2 or more), and pre-pregnancy body mass index (BMI) based on measured height at recruitment and pre-pregnancy self-reported weight (kilograms per square meter, kg/m2; underweight [<18.5], normal weight [18.5, <25], overweight [25, <30], and obese [≥30]). We collected information on active smoking and partner smoking at home during the first trimester of pregnancy.
Statistical analysis
The concentrations of 25(OH)D3 are highly dependent on the season when they are measured. To take into consideration the seasonal distribution of 25(OH)D3 and to reduce the influence of competing exposures not related to 25(OH)D3 status (e.g. winter infection, melatonin-related exposures, etc.), we deseasonalized the variable. We first tested seasonality of 25(OH)D3 concentration by fitting the data to a sine function with a period of 12 months in a nonlinear regression cosinor model43. We subtracted the predicted concentrations based on month at blood collection from the actual observed value for each subject. In our analyses, we used the residuals derived from the sinusoidal model, centred to the overall mean, as the exposure variable. Maternal and child 25(OH)D3 concentrations were treated as continuous (effect per 10 ng/ml increment) and as categorical divided into clinically relevant categories (<20 ng/ml [deficiency, reference group], 20 to 29.9 ng/ml [insufficiency], and ≥30 ng/ml [sufficiency]). Although there is no absolute consensus regarding the cut-offs that should be used, most experts agree on these categories44.
We ran linear regression models to examine the association between maternal 25(OH)D3 concentration in pregnancy and ASD symptoms, social competence, and total behavioural problems measured with the SDQ, as continuous scores. Negative binomial regression models were used for total behavioural problems measured with the CBCL and ADHD symptoms as continuous scores. Regression models were performed unadjusted, minimally adjusted (for region), and fully adjusted (for maternal age, maternal education level, maternal occupation, maternal country of birth, maternal smoking during pregnancy, and partner smoking at home during pregnancy). The confounding variables were defined based on scientific literature and on availability of data within the INMA Project.
We performed multiple imputations of missing values using chained equations to impute missing potential confounding variables among all participants with available data on the exposure and the outcomes. We generated and separately analysed twenty-five completed data sets and we combined the results using the standard Rubin’s rules45, assuming missing at random data.
Children included in the analysis (n = 2,107) were more likely to have parents from a higher socioeconomic position compared to children that were not included due to missing data in either the exposure or one of the outcomes (n = 1,019). We therefore applied inverse probability weighting (IPW) to correct for selection bias that potentially arises when only population with available exposure and outcome data is included as compared to the full initial cohort recruited at pregnancy46.
As sensitivity analyses, we repeated all the models using child 25(OH)D3 concentration as exposure variable, instead of maternal concentrations during pregnancy, in order to assess the associations between postnatal 25(OH)D3 and the outcomes.
We performed all the statistical analyses using Stata 12.0 (Stata Corporation, College Station, Texas).
Data Availability
The datasets generated during and/or analysed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
DeLuca, G. C., Kimball, S. M., Kolasinski, J., Ramagopalan, S. V. & Ebers, G. C. Review: the role of vitamin D in nervous system health and disease. Neuropathol. Appl. Neurobiol. 39, 458–484 (2013).
Morales, E. et al. Circulating 25-hydroxyvitamin D3 in pregnancy and infant neuropsychological development. Pediatrics 130, e913–920 (2012).
Eyles, D. W., Burne, T. H. J. & McGrath, J. J. Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front. Neuroendocrinol. 34, 47–64 (2013).
Holick, M. F. Photosynthesis of vitamin D in the skin: effect of environmental and life-style variables. Fed. Proc. 46, 1876–1882 (1987).
Schmid, A. & Walther, B. Natural vitamin D content in animal products. Adv. Nutr. Bethesda. Md 4, 453–462 (2013).
Vinkhuyzen, A. A. E. et al. Gestational vitamin D deficiency and autism spectrum disorder. BJPsych Open 3, 85–90 (2017).
Eyles, D. W., Smith, S., Kinobe, R., Hewison, M. & McGrath, J. J. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J. Chem. Neuroanat. 29, 21–30 (2005).
Lips, P. Worldwide status of vitamin D nutrition. J. Steroid Biochem. Mol. Biol. 121, 297–300 (2010).
Gale, C. R. et al. Maternal vitamin D status during pregnancy and child outcomes. Eur. J. Clin. Nutr. 62, 68–77 (2008).
Whitehouse, A. J. O. et al. Maternal serum vitamin D levels during pregnancy and offspring neurocognitive development. Pediatrics 129, 485–493 (2012).
Keim, S. A., Bodnar, L. M. & Klebanoff, M. A. Maternal and cord blood 25(OH)-vitamin D concentrations in relation to child development and behaviour. Paediatr. Perinat. Epidemiol. 28, 434–444 (2014).
Daraki, V. et al. High maternal vitamin D levels in early pregnancy may protect against behavioral difficulties at preschool age: the Rhea mother-child cohort, Crete, Greece. Eur. Child Adolesc. Psychiatry 27, 79–88 (2018).
Morales, E. et al. Vitamin D in Pregnancy and Attention Deficit Hyperactivity Disorder-like Symptoms in Childhood. Epidemiol. Camb. Mass 26, 458–465 (2015).
Mossin, M. H. et al. Inverse associations between cord vitamin D and attention deficit hyperactivity disorder symptoms: A child cohort study. Aust. N. Z. J. Psychiatry 51, 703–710 (2017).
Whitehouse, A. J. O. et al. Maternal vitamin D levels and the autism phenotype among offspring. J. Autism Dev. Disord. 43, 1495–1504 (2013).
Fernell, E. et al. Autism spectrum disorder and low vitamin D at birth: a sibling control study. Mol. Autism 6, 3 (2015).
Chen, J., Xin, K., Wei, J., Zhang, K. & Xiao, H. Lower maternal serum 25(OH) D in first trimester associated with higher autism risk in Chinese offspring. J. Psychosom. Res. 89, 98–101 (2016).
Magnusson, C. et al. Maternal vitamin D deficiency and the risk of autism spectrum disorders: population-based study. BJPsych Open 2, 170–172 (2016).
Vinkhuyzen, A. A. E. et al. Gestational vitamin D deficiency and autism-related traits: the Generation R Study. Mol. Psychiatry 23, 240–246 (2018).
Schmidt, R. J., Niu, Q., Eyles, D. W., Hansen, R. L. & Iosif, A.-M. Neonatal vitamin D status in relation to autism spectrum disorder and developmental delay in the CHARGE case-control study. Autism Res. Off. J. Int. Soc. Autism Res. 12, 976–988 (2019).
Windham, G. C. et al. Newborn vitamin D levels in relation to autism spectrum disorders and intellectual disability: A case-control study in california. Autism Res. Off. J. Int. Soc. Autism Res. 12, 989–998 (2019).
García-Serna, A. M. & Morales, E. Neurodevelopmental effects of prenatal vitamin D in humans: systematic review and meta-analysis. Mol. Psychiatry, https://doi.org/10.1038/s41380-019-0357-9 (2019).
Darling, A. L. et al. Association between maternal vitamin D status in pregnancy and neurodevelopmental outcomes in childhood; results from the Avon Longitudinal Study of Parents and Children (ALSPAC). Br. J. Nutr. 117, 1682–1692 (2017).
Chawla, D. et al. Early prenatal vitamin D concentrations and social-emotional development in infants. J. Matern.-Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obstet. 32, 1441–1448 (2019).
Forns, J. et al. A conceptual framework in the study of neuropsychological development in epidemiological studies. Neuroepidemiology 38, 203–208 (2012).
Goldstein, S., Naglieri, J.A. & Ozonoff, S. Assessment of Autism Spectrum Disorders, First Edition. (Guilford Press, 2008).
Ferrer, M. et al. Head circumference and child ADHD symptoms and cognitive functioning: results from a large population-based cohort study. Eur. Child Adolesc. Psychiatry 28, 377–388 (2019).
Andiarena, A. et al. Evening salivary cortisol and alpha-amylase at 14months and neurodevelopment at 4years: Sex differences. Horm. Behav. 94, 135–144 (2017).
Gascon, M. et al. Prenatal exposure to bisphenol A and phthalates and childhood respiratory tract infections and allergy. J. Allergy Clin. Immunol. 135, 370–378 (2015).
Eyles, D., Brown, J., Mackay-Sim, A., McGrath, J. & Feron, F. Vitamin D3 and brain development. Neuroscience 118, 641–653 (2003).
O’Loan, J. et al. Vitamin D deficiency during various stages of pregnancy in the rat; its impact on development and behaviour in adult offspring. Psychoneuroendocrinology 32, 227–234 (2007).
Mitsis, E. M., McKay, K. E., Schulz, K. P., Newcorn, J. H. & Halperin, J. M. Parent-teacher concordance for DSM-IV attention-deficit/hyperactivity disorder in a clinic-referred sample. J. Am. Acad. Child Adolesc. Psychiatry 39, 308–313 (2000).
Strøm, M. et al. Vitamin D measured in maternal serum and offspring neurodevelopmental outcomes: a prospective study with long-term follow-up. Ann. Nutr. Metab. 64, 254–261 (2014).
Gustafsson, P. et al. Vitamin D Status at Birth and Future Risk of Attention Deficit/Hyperactivity Disorder (ADHD). PloS One 10, e0140164 (2015).
Guxens, M. et al. Cohort Profile: the INMA–INfancia y Medio Ambiente–(Environment and Childhood) Project. Int. J. Epidemiol. 41, 930–940 (2012).
Instruction manual BIO-RAD. 25(OH)-viamin D3 by HPLC. (Laboratories GmbH, 2003).
Baron-Cohen, S. et al. Prevalence of autism-spectrum conditions: UK school-based population study. Br. J. Psychiatry J. Ment. Sci. 194, 500–509 (2009).
Levine, S., Elzey, F. & Lewis, M. Manual: California Preschool Social Competency Scale. (Consulting Psychologists Press, 1969).
Goodman, R. The Strengths and Difficulties Questionnaire: a research note. J. Child Psychol. Psychiatry 38, 581–586 (1997).
Achenbach, T. M. & Rescorla, L. A. Manual for the ASEBA School-Age Forms and Profiles. (University of Vermont, Research Center for Children, Youth, and Families, 2001).
Conners, C. K. Conners’ Rating Scales-Revised technical manual. (Multi-Health Systems, 1997).
Domingo-Salvany, A., Regidor, E., Alonso, J. & Alvarez-Dardet, C. Proposal for a social class measure. Working Group of the Spanish Society of Epidemiology and the Spanish Society of Family and Community Medicine. Aten. Primaria 25, 350–363 (2000).
Barnett, A. G. & Dobson, A. J. Analysing Seasonal Health Data. (Springer-Verlag, 2010).
Holick, M. F. Vitamin D status: measurement, interpretation, and clinical application. Ann. Epidemiol. 19, 73–78 (2009).
Royston, P. Multiple imputation of missing values. Stata J. 4, 227–241 (2004).
Weisskopf, M. G., Sparrow, D., Hu, H. & Power, M. C. Biased Exposure-Health Effect Estimates from Selection in Cohort Studies: Are Environmental Studies at Particular Risk? Environ. Health Perspect. 123, 1113–1122 (2015).
Acknowledgements
The authors are grateful to all the teachers, parents and children who have participated in the INMA project for their generous collaborative efforts. We thank all the investigators who have collected information and samples from the participants. Menorca: This study was funded by grants from Instituto de Salud Carlos III (Red INMA G03/176; CB06/02/0041; 97/0588; 00/0021-2; PI061756; PS0901958; PI14/00677 incl. FEDER funds), CIBERESP, Beca de la IV convocatoria de Ayudas a la Investigación en Enfermedades Neurodegenerativas de La Caixa, and EC Contract No. QLK4-CT-2000-00263. Sabadell: This study was funded by grants from Instituto de Salud Carlos III (Red INMA G03/176; CB06/02/0041; PI041436; PI081151 incl. FEDER funds; CPII/00018), CIBERESP, Generalitat de Catalunya-CIRIT 1999SGR 00241, Generalitat de Catalunya-AGAUR 2009 SGR 501, Fundació La marató de TV3 (090430), EU Commission (261357). ISGlobal is a member of the CERCA Programme, Generalitat de Catalunya. Valencia: This study was funded by Grants from UE (FP7-ENV-2011 cod 282957 and HEALTH.2010.2.4.5-1), Spain: ISCIII (G03/176; FIS-FEDER: PI11/01007, PI11/02591, PI11/02038, PI12/00610, PI13/1944, PI13/2032, PI14/00891, PI14/01687, PI16/1288, and PI17/00663; Miguel Servet-FEDER CP11/00178, CP15/00025, and MSII16/00051), Generalitat Valenciana: FISABIO (UGP 15-230, UGP-15-244, and UGP-15-249), and Alicia Koplowitz Foundation 2017. Asturias: This study was funded by grants from Instituto de Salud Carlos III (Red INMA G03/176 and CB06/02/0041), FIS-PI042018, FIS-PI09/02311, FIS-PI13/02429, FIS-PI18/00909, CIBERESP, Obra Social Cajastur/Fundación Liberbank and UNIVERSIDAD DE OVIEDO. Gipuzkoa: This study was funded by grants from Instituto de Salud Carlos III (FIS-PI06/0867, FIS-PI09/00090 and FIS-PI13/02187), CIBERESP, Department of Health of the Basque Government (2005111093, 2009111069, 2013111089 and 2015111065), and the Provincial Government of Gipuzkoa (DFG06/002, DFG08/001 and DFG15/221) and annual agreements with the municipalities of the study area (Zumarraga, Urretxu, Legazpi, Azkoitia y Azpeitia y Beasain).
Author information
Authors and Affiliations
Contributions
M.L.V., J.S. and M.G. designed research and interpreted the results. M.L.V. analysed the data and wrote the manuscript. N.L., L.G., C.R.D., M.V., A.T., S.L., M.T. and J.I. coordinated and supervised data collection. M.E.S.-T. was responsible of plasma 25(OH)D3 measurement. All authors critically reviewed the manuscript and approved the submitted version.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
López-Vicente, M., Sunyer, J., Lertxundi, N. et al. Maternal circulating Vitamin D3 levels during pregnancy and behaviour across childhood. Sci Rep 9, 14792 (2019). https://doi.org/10.1038/s41598-019-51325-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-51325-3
This article is cited by
-
Maternal vitamin D during pregnancy and offspring autism and autism-associated traits: a prospective cohort study
Molecular Autism (2022)
-
A profile and review of findings from the Early Markers for Autism study: unique contributions from a population-based case–control study in California
Molecular Autism (2021)
-
Vitamin D, pregnancy and caries in children in the INMA-Asturias birth cohort
BMC Pediatrics (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.