Abstract
It is known that if unused drugs are improperly disposed, they can pollute the environment. Furthermore, researchers are still trying to find an environmentally friendly corrosion inhibitor. These factors lead to the possible application of unused pharmaceutical compounds as corrosion inhibitors. The feasibility of an anti-inflammatory, analgesic and antipyretic drug, ibuprofen, was evaluated as a potential copper corrosion inhibitor in synthetic acid rain solution. This investigation was performed by applying electrochemical and weight loss measurements and quantum chemical calculations. The results obtained by these techniques revealed the ability of ibuprofen to protect copper from corrosion. The inhibition efficiency of ibuprofen rises with increase in its concentration and can reach a value of 97.3%. The results of surface analysis of treated coupons by scanning electron microscopy and theoretical calculations are consistent with the experimental results.
Similar content being viewed by others
Introduction
Generally, several metals and alloys such as copper, brass, aluminum, tin and steel are used in various industrial fields. Copper possesses thermal and electrical conductivity so it is used in heat exchangers, and as a conductor in the electronic industry. In addition, copper is also used for coating sculptures and roofs. Regardless of the place of application, copper dissolution can occur in aggressive environments1,2,3. This has negative consequences for the properties of the metal, and can leads to significant economic losses. Based on many studies4,5,6,7, different corrosion inhibitors have been developed that can be used for the protection of copper. These inhibitors can be inorganic8,9 or organic compounds10,11. Due to the low inhibition efficiency of inorganic compounds9, different classes of organic compounds have been investigated for this purpose among which the most important are azole compounds. In the presence of azole and azole derivatives such as imidazole12,13,14, triazole15,16,17,18, pyrazole19,20, tetrazole21,22,23, thiazole24,25,26 and thiadiazole10,27,28, significant reductions in the corrosion of copper have been achieved. However, several of these compounds are toxic to the environment. Therefore, research has been directed towards finding environmentally acceptable corrosion inhibitors. There are numerous studies indicating that amino acids29,30,31, purine and its derivatives32,33,34,35,36,37, plant extracts38,39,40 and pharmaceutical compounds41,42,43,44 are potentially environmentally friendly corrosion inhibitors for copper in a variety of media such as chloride, nitrate and sulfate solutions.
Researchers are working to find an efficient, low cost and non-toxic corrosion inhibitor. According to the investigations45,46, traditional inhibitors could be replaced by pharmaceuticals. By comparing the price of the pharmaceutical active compounds and other organic compounds, active compounds of drugs are more costly. Among drugs, natural compounds like plant extracts are attractive as green corrosion inhibitors47,48. In order to obtain a plant extract, various techniques such as extraction are involved which increase the coast while expired pharmaceuticals are available49,50. It is worth noting that after the expiration date, higher than 90% of active component of drug remains stable for a long time51. Further, an expired drug can be realized in the environment due to inappropriate disposal. Drugs are brought in the environment through household waste or toilet52,53,54. The pharmaceuticals that are unsuitable for further human usage should be degraded by adequate technique. In accordance with these, using of expired drugs as possible corrosion inhibitors can decrease environmental pollution and also reduce the degradation costs.
Various research groups52,53,54,55,56 concluded that pharmaceutical industries are also responsible for disposing expired or unused drugs into the environment. Having this in mind, unused drugs should be degraded by using an adequate technique like photochemical process57,58, biodegradation process59,60, adsorption61 and nanofiltration process62. According to the obtained results by Kanama63, and Paxeus64 the carrousel-type activated sludge system decreased the influent concentrations of all target PPCPs by 40–98% before their eventual discharge. Taking into account the numerous investigations42,43,44,50 about using drugs as possible corrosion inhibitors, it may be assumed that these compounds have ability to adsorb on metal surface and form complexes with metals. On that way, the reuse of drugs limits the environmental pollution and reduces the disposal costs, so they have prompted interest for use in corrosion testing.
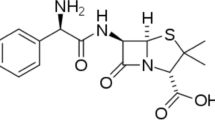
Ibuprofen (2-(p-isobuthylphenyl) propionic acid) is an anti-inflammatory, analgesic and antipyretic drug largely used in the treatment of muscle and head pain, inflammation in rheumatic disease and for the treatment of fever65. With this in mind, it is interesting to examine the ability of expired ibuprofen to protect copper from corrosion in synthetic acid rain solution.
Materials and Methods
Electrochemical measurements
Electrochemical measurements were performed using potentiostat (IVIUM XRE, IVIUM Technologies) with the appropriate software in a three electrode configuration. Copper electrode with exposed surface area of 0.49 cm2 was used as the working electrode, while a standard calomel electrode (SCE) and a platinum wire were used as the reference and auxiliary electrodes, respectively. Prior to each measurement, the copper electrode was polished with alumina (0.3μm Al2O3, Buehler USA), washed with distilled water and then dried.
The following electrochemical methods were used in the research: open circuit potential (OCP) measurements, potentiodynamic polarization, cyclic voltammetry, electrochemical impedance spectroscopy and beside these weight loss measurements. The open circuit potential was measured for 30 minutes and before the potentiodynamic polarization measurements were performed. The polarization measurement was recorded in the anodic direction from the open circuit potential to 0.25 V (vs. SCE) as well as in the cathodic direction from the open circuit potential to −0.25 V (vs. SCE). Cyclic voltammetry was performed over potential range of −1 V (vs. SCE) to 1 V (vs. SCE). The scan rate was 1 mV/s for the potentiodynamic polarization measurements and 10 mV/s for the cyclic voltammetry measurements. Electrochemical impedance spectroscopy measurements were conducted at open circuit potential over a frequency range of 10 kHz – 0.01 Hz, with a single amplitude perturbation of 10 mV using IVIUM soft.
The synthetic acid rain solution (SAR) was prepared using the following compounds: Na2SO4 (0.2 g/l) (Zorka Pharmacy, Serbia), NaHCO3 (0.2 g/l) (Zorka Pharmacy, Serbia) and NaNO3 (0.2 g/l) (Zorka Pharmacy, Serbia)66. A pH value of 2.42 for the synthetic acid rain was achieved by the addition of an H2SO4 solution. Inhibitor solutions of ibuprofen were prepared by dissolving the required amount of ibuprofen in the synthetic acid rain solution in order to obtain concentration of 1·10–2M. The solution with the highest concentration was diluted in order to obtain solutions with lower concentrations (5·10−3M, 1·10−3M and 5·10−4M). In our investigation expired ibuprofen syrup is used (purchased at a local pharmacy). Based on the drug specification where the content of the active substance (ibuprofen) is 100 mg in 5 ml of syrup, the calculation for the concentration of 1·10−2M was made. Further, the appropriate volume of ibuprofen syrup was dissolved in synthetic acid rain solution.
Weight loss measurements
Copper specimens 30 × 30 × 0.5 mm in dimension were used in the weight loss experiments. These samples were immersed in synthetic acid rain solutions in the absence and presence of various concentrations of ibuprofen for five days at room temperature. Before immersion, each sample was polished with emery paper, washed with ethanol and distilled water and then weighed (analytical balance OHAUS PA214CM; accuracy of weighing process 0.0001 g). After treatment in the test solutions, the copper samples are withdrawn, washed, dried and then reweighed. The weight loss measurements are triplicated.
Analysis of copper surfaces by scanning electron microscopy with energy dispersive spectroscopy
The surface characterization of the copper samples treated in different acid solutions was carried out to confirm the protective ability of the ibuprofen. For this purpose a Tescan VEGA 3 LM scanning electron microscope with Oxford EDS X-act Inca 350 system was used. The samples were prepared using the same methods used for the weight loss measurements and after being immersed for five days in the test solutions the surface characterization of the samples was performed.
Results and Discussion
Open circuit potential and potentiodynamic polarization measurements
Determination of the open circuit potential (OCP) values for copper in synthetic acid rain solution without and with the addition of an inhibitor was performed for 30 minutes, and obtained curves are shown in Fig. 1. At the beginning of measurement in the blank solution, the OCP is less significantly shifted to more negative values compared with the trends in the presence of ibuprofen. This is the result of the deposition of corrosion products on the copper surface. This shift is more obvious with increase in the inhibitor concentration and could be explained by the adsorption of inhibitors on the active corrosion sites of the copper surface67. By comparing the OCP values obtained at the end of the experiments in uninhibited and in inhibited solutions, the ibuprofen could be classified as a mixed-type inhibitor with a more pronounced effect on the cathodic process68,69,70.
After determining the OCP values, potentiodynamic polarization curves were recorded in both the anodic and cathodic directions. The obtained potentiodynamic polarization curves for copper in synthetic acid rain solutions without and with the addition of inhibitor are shown in Fig. 2. On the basis of the presented curves, it is obvious that the corrosion current density (jcorr) is reduced in the presence of the expired drug. The corrosion potential (Ecorr), is shifted in the negative direction in the inhibited solution in comparison to the Ecorr of the blank solution. This parameter becomes more negative with increase in the inhibitor concentration. However, the change of Ecorr in inhibited solutions is lower than 85 mV in regard to Ecorr value in blank solution. Based on the literature71,72 if the displacement of Ecorr in inhibited solution is higher than 85 mV compared to Ecorr value in uninhibited solution, the tested compound is classified as an anodic or cathodic type. However, if this change in Ecorr values is less than 85 mV, it is about mixed type. Thus, ibuprofen can be classified as mixed type inhibitor.
A similar conclusion was observed for the open circuit potential measurements. The obtained data for the corrosion potentials as well as the corrosion current densities, anodic (ba) and cathodic (bc) Tafel slopes, polarization resistance (Rp) and inhibition efficiencies (IE) are presented in Table 1.
The parallel cathodic Tafel lines in SAR containing ibuprofen indicated that the presence of this compound does not modify the cathodic reaction73. Additionally, the values of ba and bc changed with the addition of the inhibitor because of the adsorption of inhibitor molecules on the metal surface to form protective layer74. By analyzing the polarization resistance in Table 1, it can be said that this parameter increases upon the addition of inhibitors. Rp also rises with increased inhibitor concentration. The addition of ibuprofen in SAR leads to decreased current density, which becomes more pronounced with increases in the ibuprofen concentration (Fig. 2). This shows that ibuprofen can to protect copper under these conditions. The calculated values of inhibition efficiency and polarization resistance using Eqs. (1) and (2) confirm the inhibitory properties of the expired ibuprofen75,76. The inhibition action is related to the adsorption of inhibitor molecules on the copper surface and is dependents on the inhibitor concentration.
Where jcorr and jcorr(inh) are corrosion current densities in the absence and presence of the inhibitor, respectively.
Polarization resistance values were calculated according to Stern-Geary equation:77,78
Cyclic voltammetry
Another electrochemical method used to examine the inhibitory ability of ibuprofen in synthetic acid rain is cyclic voltammetry. This method is performed over a wider potential range than potentiodynamic polarization, and obtained curves are shown in Fig. 3. The curves obtained in the inhibitor-free solution indicate the dissolution of copper and the formation of Cu+ ions (reaction 3). Furthermore, the current density increases with the potential due to the formation of Cu2+ ions (reaction 4)79. A similar mechanism of copper dissolution in SAR has been proposed by Magaino80. Additionally, formed Cu+ ions can react with anionic species (Xn-) present in the SAR solution by reaction (5). In the reverse scan two cathodic peaks are observed corresponding to the reduction of the formed copper species.
In the presence of the lowest concentration of inhibitor, the copper surface is not adequately covered so the dissolution continues. However, the addition of higher concentrations of inhibitor (5·10−3M and 1·10−2M) leads to higher copper surface coverage and the current density is significantly reduced in comparison to the blank solution. Additionally, the decrease of cathodic peak intensity relative to the inhibitor-free solution points to the protective effect of the ibuprofen81. Furthermore, the second cathodic peak is not evident in the presence of ibuprofen which indicates the irreversibility of the process.
Electrochemical impedance spectroscopy
In order to investigate in more detail the influence of ibuprofen on the corrosion behavior of copper in SAR, electrochemical impedance spectroscopy experiments were carried out. The obtained results are shown in Fig. 4(a–c). According to these figures, EIS parameters obtained by fitting are summarized in Table 2. By analyzing the Nyquist diagram (Fig. 4c), it can be seen that semicircle diameter increases as increases the concentration of inhibitor. Thus, the corrosion rate is reduced82. Additionally, in the low frequency area, Warburg impedance is observed indicating the diffusion processes, i.e. diffusion of dissolved oxygen or other corrosive species to the surface of copper83 or the diffusion of soluble copper species84.
In addition to the Nyquist diagram, Bode plots are shown in Fig. 4(a,b). In accordance with these figures, it is obvious that impedance values have increasing trend in the whole frequency area with the addition of ibuprofen. The increasing trend of impedance is related with ibuprofen inhibitory ability83,84. Furthermore, Bode phase plots show that phase angle is higher in the presence of inhibitor in comparison to the phase angle in SAR that implies the inhibition of copper dissolution.
The IVIUM soft program and the equivalent circuit shown in Fig. 5 were used for fitting experimental data where Rs is the solution resistance, Rf is the resistance of protective inhibitor film formed on copper surface, Rct is the charge transfer resistance, Qf and Qdl represent CPE – constant phase elements, Cf represents film capacitance and Cdl is double layer capacitance, W is the Warburg impedance and n represents deviation parameter42,85. Cf and Cdl parameters are calculated according to the Eqs. (6) and (7):
According to the results shown in Table 2, n values increase in the presence of ibuprofen which indicates the increase of the surface homogeneity due to the adsorption of inhibitor84. Furthermore, as the concentration of inhibitor increased, values of Cf and Cdl decreased, while values of W increased. This is related with the adsorption of inhibitor molecules on the copper surface leading to decrease exposed copper surface to aggressive ions. According to the Iroh and Su86 and Ameer et al.87 and also according to the obtained results, it is assumed that the copper surface is uniformly coated.
Inhibition efficiency is calculated according to the following equation:
where Rp0 is the total polarization resistance of the copper electrode in SAR solution and Rp is the total polarization resistance of the in the presence of the inhibitor. The values of Rp are calculated following the equation \(\,{R}_{p}={R}_{f}+{R}_{ct}\).
The calculated IE values are in agreement with the values obtained from potentiodynamic polarization and weight loss measurements.
Weight loss measurements
In addition to the electrochemical measurements, the inhibitory ability of ibuprofen was also tested by using the weight loss method. The copper specimens were immersed for five days in the SAR and inhibited solutions, at room temperature. The effect of the different concentrations of ibuprofen on the corrosion rate is examined. From the results of the weight loss test, the values of the corrosion rate (CR) and inhibition efficiency (IE) were calculated using Eqs. (9) and (10) and the average values are summarized in Table 3:
Where CR and CR1 (g/m2h) are the corrosion rates of copper in synthetic acid rain in the absence and presence of inhibitor, respectively. W0 and W (g) are the weights of the copper samples before and after treatment in the appropriate solutions, respectively, while A (m2) is the surface area of the samples and t (h) is the immersion period.
By analyzing these parameters, it can be seen that the corrosion rate decreases as the concentration of inhibitor increases. Additionally, the inhibition efficiency increases with increased ibuprofen concentration which agrees with the results obtained by the electrochemical techniques. It is assumed that a higher degree of copper surface is covered with a protective layer as the concentration of inhibitor increases. This leads to a decrease in corrosion rate in the SAR solutions. Additionally, the highest inhibition efficiency is achieved in the presence of 1·10−2 M ibuprofen, which is consistent with the results obtained in the potentiodynamic polarization experiments.
Adsorption isotherm
To obtain information about the type and degree of interaction between the copper surface and inhibitor molecules, adsorption isotherm studies are necessary. In this study, the obtained data are best fitted using Langmuir adsorption isotherm which is shown in Fig. 6:
The straight line of Cinh/θ vs. Cinh as well as the values of the regression coefficient (R2) and the slope (Table 4) confirms the adsorption of the ibuprofen molecules fits a Langmuir isotherm. This isotherm shows that the adsorbed molecule occupies only one active site on the electrode surface88. The Gibbs free energy of adsorption is calculated using the following equation:
Where R stands for universal gas constant, T is the thermodynamic temperature, 55.5 stands for molar concentration of water and Kads represents the equilibrium constant of adsorption. In general, a high value of Kads is associated with high adsorption efficiency indicating that ibuprofen under experimental conditions can be adsorbed. Thus, this is in good agreement with results obtained by electrochemical and weight loss measurements. The calculated value of the Gibbs free energy implies strong and spontaneous adsorption of ibuprofen molecules on the copper surface in the synthetic acid rain solutions.
Considering the pH value of the tested SAR solution (pH 2.42) and the pKa value for ibuprofen (4.91)89, it is assumed that this compound is in a protonated form during the tests. The mechanism of inhibitor action could be due to adsorption of anionic species presented in SAR on the copper surface which further facilitates the adsorption of the protonated inhibitor.
Surface characterization by scanning electron microscopy with energy dispersive spectroscopy
The surface characterization of copper coupons treated in synthetic acid rain in the absence and presence of the highest concentration of ibuprofen is carried out by scanning electron microscopy with energy dispersive spectroscopy (SEM – EDS). The coupons were immersed for five days at room temperature in different solutions, and the obtained SEM micrographs are shown in Figs 7 and 8. By analyzing these figures it is seen that the copper surface is smoother in the presence of the inhibitor as opposed to the pits and cracks obtained in the inhibitor-free solution. This can be a result of the formation of a compact layer of ibuprofen on the metal surface.
According to the EDS results (Fig. 7) it is assumed that copper corrosion products are formed on the sample surface when inhibitor is not added to the SAR which agrees with the previously proposed corrosion mechanism. The copper coupon treated in the solution containing ibuprofen is also subjected to EDS analysis (Fig. 8). The absence of O atomic peak and also the presence of C atomic peak which is derived from inhibitor molecule leads to the conclusion that formed film hinders the formation of corrosion products. Hence, the presence of ibuprofen diminishes the corrosion rate of copper in SAR solution which is consistent with the experimental results. According to the EDS analysis (Figs 7 and 8) and CV curves (Fig. 3) obtained in inhibited solutions, it can be assumed that inhibitor molecules form complex with cuprous ions, thus formed complex is adsorbed on the copper surface and leads to decreasing the corrosion rate. Similar results are observed by Quartarone et al.90 and Tan et al.91.
Quantum chemical calculations
To determine the relationship between the inhibition efficiency of the ibuprofen and its molecular structure, quantum chemical calculations have been performed. The molecular structure of ibuprofen has been geometrically optimized using DFT calculations performed with method using ArgusLab 4.0 software92 and that the following parameters have been calculated: the energy of the highest occupied molecular orbital (EHOMO), the energy of the lowest unoccupied molecular orbital (ELUMO), the energy gap barrier (ΔE) and the dipole moment (μ). Furthermore, ionization potential (I), electron affinity (A), electronegativity (χ), global hardness (η) and number of transferred electrons (ΔN) are calculated according to Eqs. (13) – (17). All the mentioned parameters are presented in Table 5. The spatial distributions of the HOMO (highest occupied molecular orbital) and the LUMO (lowest unoccupied molecular orbital) of ibuprofen are illustrated in Figs 9 and 10. The lower value of ΔE is associated with the higher affinity of the inhibitor molecules to be adsorbed on the metal surface93. According to this parameter, it is assumed that ibuprofen has high tendency to be adsorbed on the copper surface, which is consistent with the inhibition efficiency obtained in the experimental measurements. The lower electronegativity of ibuprofen also confirms high inhibition efficiency94.
where χCu and χinh are the absolute electronegativity of copper (4.48 eV/mol) and the inhibitor molecule respectively, and ηCu and ηinh are the absolute hardness of copper (0 eV/mol) and the ibuprofen molecule95. The higher value of the dipole moment of ibuprofen (4.29 D) than water (1.85 D) could be associated with a higher tendency of the ibuprofen to interact with the copper surface94. Due to the high value of dipole moment of ibuprofen, a high IE of this compound is expected96, which agrees with the results obtained by the electrochemical and weight loss measurements. On the bases of the quantum chemical parameters, the ibuprofen molecules have the ability to be adsorbed on the copper surface by replacing previously adsorbed water molecules94.
Conclusion
Expired ibuprofen has the ability to protect copper from corrosion in synthetic acid rain solution. According to results obtained by electrochemical and weight loss measurements, the inhibition efficiency raises with increase in ibuprofen concentration. Potentiodynamic polarization results classify ibuprofen as a mixed-type corrosion inhibitor. SEM and EDS analysis of copper coupons treated in SAR containing ibuprofen revealed the formation of a protective layer on the metal surface that reduced copper dissolution. The protective layer is formed by the adsorption of ibuprofen molecules on the copper surface according to a Langmuir adsorption isotherm. Quantum chemical parameters agree with the results obtained experimentally.
Data Availability
In accordance with the institution’s policy, data are not available.
References
Qiang, Y., Zhang, S., Xu, S. & Yin, L. The effect of 5-nitroindazole as an inhibitor for the corrosion of copper in a 3.0% NaCl solution. RSC Adv. 5, 63866–63873 (2015).
Qiang, Y., Zhang, S., Xu, S. & Li, W. Experimental and theoretical studies on the corrosion inhibition of copper by two indazole derivatives in 3.0% NaCl solution. J. Colloid Interf. Sci. 472, 52–59 (2016).
Jing, Z., Liu, Z., Han, G. C., Chen, S. L. & Chen, Z. Inhibition of copper corrosion by the formation of Schiff base self-assembled monolayers. Appl. Surf. Sci. 389, 601–608 (2016).
Vastag, G. et al. Influence of the N-3 alkyl chain length on improving inhibition properties of imidazolium-based ionic liquids on copper corrosion. J. Mol. Liq. 264, 526–533 (2018).
Tian, H., Cheng, Y. F., Li, W. & Hou, B. Triazolyl-acylhydrazone derivatives as novel inhibitors for copper corrosion in chloride solutions. Corros. Sci. 100, 341–352 (2015).
Nakomcic, J., Vastag, G., Shaban, A. & Nyikos, L. Effect of Thiazole Derivatives on Copper Corrosion in Acidic Sulphate Solution. Int. J. Electrochem. Sci. 10, 5365–5381 (2015).
Li, X., Li, W., Yang, S. & Hou, L. Using methionine as an environment-friendly corrosion inhibitor for copper–nickel alloy in a chloride solution. Mater. Express 7(6), 480–490 (2017).
Edwards, M., Hidmi, L. & Gladwell, D. Phosphate inhibition of soluble copper corrosion by-product release. Corros. Sci. 44, 1057–1071 (2002).
Munoz, A. I., Antón, J. G., Guiñón, J. L. & Herranz, V. P. Comparison of inorganic inhibitors of copper, nickel and copper–nickels in aqueous lithium bromide solution. Electrochim. Acta 50, 957–966 (2004).
Qin, T. T., Li, J., Luo, H. Q., Li, M. & Li, N. B. Corrosion inhibition of copper by 2,5-dimercapto-1,3,4-thiadiazole monolayer in acidic solution. Corros. Sci. 53, 1072–1078 (2011).
Tasic, Z. & Antonijevic, M. Copper corrosion behaviour in acidic sulphate media in the presence of 5-methyl-1H-benzotriazole and 5-chloro-1H-benzotriazole. Chem. Pap. 70, 620–634 (2016).
Petrović Mihajlović, M. B., Radovanović, M. B., Tasić, Ž. Z. & Antonijević, M. M. Imidazole based compounds as copper corrosion inhibitors in seawater. J. Mol. Liq. 225, 127–136 (2017).
Kovačević, N., Milošev, I. & Kokalj, A. The roles of mercapto, benzene, and methyl groups in the corrosion inhibition of imidazoles on copper: II. Inhibitor–copper bonding. Corros. Sci. 98, 457–470 (2015).
Durainatarajan, P., Prabakaran, M., Ramesh, S. & Periasamy, V. Self-assembly on copper surface by using imidazole derivative for corrosion protection. J. Adhes. Sci. Technol. 32(16), 1733–1749 (2018).
Ofoegbu, S. U. et al. Corrosion inhibition of copper in aqueous chloride solution by 1H-1,2,3-triazole and 1,2,4-triazole and their combinations: electrochemical, Raman and theoretical studies. Phys. Chem. Chem. Phys. 19, 6113–6129 (2017).
Sudheer, M. A. Quraishi, Electrochemical and theoretical investigation of triazole derivatives on corrosion inhibition behavior of copper in hydrochloric acid medium. Corros. Sci. 70, 161–169 (2013).
Kovačević, N., Milošev, I. & Kokalj, A. How relevant is the adsorption bonding of imidazoles and triazoles for their corrosion inhibition of copper? Corros. Sci. 124, 25–34 (2017).
El Ibrahimi, B. et al. Computational study of some triazole derivatives (un- and protonated forms) and their copper complexes in corrosion inhibition process. J. Mol. Liq. 1125, 93–102 (2016).
Jhuang, J.-Y. et al. Adsorption and Reaction Pathways of 1H-Pyrazole on Cu(100) and O/Cu(100). J. Phys. Chem. C 122(11), 6195–6208 (2018).
Goswami, A., Koskey, S., Mukherjee, T. & Chyan, O. Study of Pyrazole as Copper Corrosion Inhibitor in Alkaline Post Chemical Mechanical Polishing Cleaning Solution. ECS J. Solid State Sci. Technol. 3(10), P293–P297 (2014).
Mihit, M. et al. A study of tetrazoles derivatives as corrosion inhibitors of copper in nitric acid. Pigm. Resin. Technol. 35, 151–157 (2006).
Al Kharafi, F. M., Ghayad, I. M. & Abdullah, R. M. Corrosion Inhibition of Copper in Non-polluted and Polluted Sea Water Using 5-phenyl-1-H-tetrazole. Int. J. Electrochem. Sci. 7, 3289–3298 (2012).
Sherif, E.-S. M., Erasmus, R. M. & Comins, J. D. Inhibition of corrosion processes on copper in aerated sodium chloride solutions by 5-(3-aminophenyl)-tetrazole. J. Appl. Electrochem. 39, 83–91 (2009).
Shaban, A., Vastag, G., Pilbath, A., Kek, I. & Nyikos, L. Electrochemical study of copper corrosion inhibition in acidic environment by 5-(4’-isopropylbenzylidene)−2,4-dioxotetrahydro-1,3-thiazole. J. Mater. Environ. Sci. 7, 2572–2582 (2016).
Ramezanzadeh, B., Arman, S. Y., Mehdipour, M. & Markhali, B. P. Analysis of electrochemical noise (ECN) data in time and frequency domain for comparison corrosion inhibition of some azole compounds on Cu in 1.0 M H2SO4 solution. Appl. Surf. Sci. 289, 129–140 (2014).
Qiang, Y. et al. Effective Protection for Copper Corrosion by Two Thiazole Derivatives in Neutral Chloride Media: Experimental and Computational Study. Int. J. Electrochem. Sci. 11, 3147–3163 (2016).
Sherif, E.-S. M. & Park, S.-M. Effects of 2-amino-5-ethylthio-1,3,4-thiadiazole on copper corrosion as a corrosion inhibitor in aerated acidic pickling solutions. Electrochim. Acta 51, 6556–6562 (2006).
Sherif, E.-S. M. Effects of 2-amino-5-(ethylthio)−1,3,4-thiadiazole on copper corrosion as a corrosion inhibitor in 3% NaCl solutions. Appl. Surf. Sci. 252(24), 8615–8623 (2006).
Petrović, M. B., Radovanović, M. B., Simonović, A. T., Milić, S. M. & Antonijević, M. M. The effect of cysteine on the behaviour of copper in neutral and alkaline sulphate solutions. Int. J. Electrochem. Sci. 7, 9043–9057 (2012).
Radovanović, M. B., Petrović, M. B., Simonović, A. T., Milić, S. M. & Antonijević, M. M. Cysteine as a green corrosion inhibitor for Cu37Zn brass in neutral and weakly alkaline sulphate solutions. Environ. Sci. Pollut. Res. 20, 4370–4381 (2013).
Simonović, A. T., Petrović, M. B., Radovanović, M. B., Milić, S. M. & Antonijević, M. M. Inhibition of copper corrosion in acidic sulphate media by eco-friendly amino acid compound. Chem. Pap. 68, 362–371 (2014).
Scendo, M. Inhibitive action of the purine and adenine for copper corrosion in sulphate solutions. Corros. Sci. 49, 373–390 (2007).
Scendo, M. Inhibition of copper corrosion in sodium nitrate solutions with nontoxic inhibitors. Corros. Sci. 50, 1584–1592 (2008).
Petrović, M. B., Simonović, A. T., Radovanović, M. B., Milić, S. M. & Antonijević, M. M. Influence of purine on copper behavior in neutral and alkaline sulfate solutions. Chem. Pap. 66, 664–676 (2012).
Radovanović, M. B., Simonović, A. T., Petrović, M. B., Milić, S. M. & Antonijević, M. M. Influence of purine on brass behavior in neutral and alkaline sulphate solutions. Int. J. Electrochem. Sci. 7, 11796–11810 (2012).
Alonso, C., Casero, E., Román, E., Campos, S. F. P. & De Mele, M. F. L. Effective inhibition of the early copper ion burst release by purine adsorption in simulated uterine fluids. Electrochim. Acta 189, 54–63 (2016).
Alvarez, F. et al. Decrease in cytotoxicity of copper-based intrauterine devices (IUD) pretreated with 6-mercaptopurine and pterin as biocompatible corrosion inhibitors. ACS Appl. Mater. Inter. 5, 249–255 (2013).
Krishnaveni, K. & Ravichandran, J. A Study on the Inhibition of Copper Corrosion in Sulphuric Acid by Aqueous Extract of Leaves of Morinda tinctoria. Journal of Failure Analysis and Prevention 15, 711–721 (2015).
Abd-El-Nabey, B. A., Abdel-Gaber, A. M., Said Ali, M. E., Khamis, E. & El-Housseiny, S. Inhibitive Action of Cannabis Plant Extract on the Corrosion of Copper in 0.5 M H2SO4. Int. J. Electrochem. Sci. 8, 7124–7137 (2013).
Deepa Rani, P. & Selvaraj, S. Emblica Officinalis (AMLA) leaves extract as corrosion inhibitor for copper and its alloy (Cu-27Zn) in natural sea water. Archives of Applied Science Research 2, 140–150 (2010).
Tasić, Ž. Z., Petrović Mihajlović, M. B., Radovanović, M. B., Simonović, A. T. & Antonijević, M. M. Cephradine as corrosion inhibitor for copper in 0.9% NaCl solution. J. Mol. Struct. 1159, 46–54 (2018).
Tasić, Ž. Z., Petrović Mihajlović, M. B., Radovanović, M. B. & Antonijević, M. M. Electrochemical investigations of copper corrosion inhibition by azithromycin in 0.9% NaCl. J. Mol. Liq. 265, 687–692 (2018).
Samide, A. et al. Electrochemical and Theoretical Study of Metronidazole Drug as Inhibitor for Copper Corrosion in Hydrochloric Acid Solution. Int. J. Electrochem. Sci. 11, 5520–5534 (2016).
Wang, D., Xiang, B., Liang, Y., Song, S. & Liu, C. Corrosion control of copper in 3.5 wt.% NaCl Solution by Domperidone: Experimental and Theoretical Study. Corros. Sci. 85, 77–86 (2014).
Dandia, A., Gupta, S. L., Singh, P. & Quraishi, M. A. Ultrasound assisted synthesis of pyrazolo[3,4-b]pyridines as potential corrosion inhibitors for mild steel in 1.0 M HCl. ACS Sustain. Chem Eng 1, 1303–1310 (2013).
Amin, A. M., Abd El Rehim, S. S. & Abdel-Fatah, H. T. M. Electrochemical frequency modulation and inductively coupled plasma atomic emission spectroscopy methods for monitoring corrosion rates and inhibition of low alloy steel corrosion in HCl solutions and a test for validity of the Tafel extrapolation method. Corros. Sci. 51, 882–894 (2009).
Rahal, C. et al. Olive leaf extract as natural corrosion inhibitor for pure copper in 0.5 M NaCl solution: A study by voltammetry around OCP. J. Electroanal. Chem. 769, 53–61 (2016).
Vrsalovic, L. et al. Corrosion protection of copper in sodium chloride solution using propolis. Int. J. Electrochem. Sci. 13, 2102–2117 (2018).
Ofoegbu, S. U. & Ofoegbu, P. U. Corrosion Inhibition of Mild Steel in 0.1 M Hydrochloric Acid Media by Chloroquine Diphosphate. ARPN. J. Eng. Appl. Sci. 7(3), 272–276 (2012).
Aziz, M. A., Khan, МdZ. H., Khatun, M. S. & Hasan, M. R. Corrosion Inhibition Study of Mild Steel in Acidic Medium by Antibiotic. Drugs: A Comparative Study, Aceh Int. J. Sci. Technol. 3(1), 19–26 (2014).
Singh, P., Chauhan, D. S., Srivastava, K., Srivastava, V. & Quraishi, M. A. Expired atorvastatin drug as corrosion inhibitor for mild steelin hydrochloric acid solution, Int. J. Ind. Chem. 8, 363–372 (2017).
Bound, J. P. & Voulvoulis, N. Household disposal of pharmaceuticals as a pathway for aquatic contamination in the United Kingdom. Environ. Health Perspect. 113(12), 1705–1711 (2005).
Tong, A. Y. C., Peake, B. M. & Braund, R. Disposal practices for unused medications in New Zealand community pharmacies, J. Prim. Health Care 3(3), 197–203 (2011).
Seehusen, D. A. & Edwards, J. Patient Practices and Beliefs Concerning Disposal of Medications. J. Am. Board Fam. Med. 19(6), 542–547 (2006).
Ngwuluka, N. C., Ochekpe, N. A. & Odumosu, P. A. An assessment of pharmaceutical waste management in some Nigerian pharmaceutical industries, African. J. Biotechnol. 10(54), 11259–11268 (2011).
Heberer, T. Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: a review of recent research data. Toxicol. Lett. 131, 5–17 (2002).
Packer, J. L., Werner, J. J., Latch, D. E., McNeill, K. & Arnold, W. A. Photochemical fate of pharmaceuticals in the environment: Naproxen, diclofenac, clofibric acid, and ibuprofen. Aquat. Sci. 65, 342–351 (2003).
Felis, E. & Miksch, K. Removal of analgesic drugs from the aquatic environment using photochemical methods. Water Sci. Technol 60(9), 2253–2259 (2009).
Ferrando-Climent, L. et al. Comprehensive study of ibuprofen and its metabolites in activated sludge batch experiments and aquatic environment. Sci. Total Environ. 438, 404–413 (2012).
Girardi, C. et al. Microbial degradation of the pharmaceutical ibuprofen and the herbicide 2,4-D in water and soil — Use and limits of data obtained from aqueous systems for predicting their fate in soil. Sci. Total Environ. 444, 32–42 (2013).
González-Naranjo, V., Boltes, K. & Biel, M. Mobility of ibuprofen, a persistent active drug, in soils irrigated with reclaimed water. Plant Soil Environ. 59(2), 68–73 (2013).
Vergili, I. Application of nanofiltration for the removal of carbamazepine, diclofenac and ibuprofen from drinking water sources, J. Environ. Manag. 127, 177–187 (2013).
Kanama, K.M., Daso, A.P., Mpenyana-Monyatsi, L. & Coetzee, M.A.A., Assessment of Pharmaceuticals, Personal Care Products, and Hormones in Wastewater Treatment Plants Receiving Inflows from Health Facilities in North West Province, South Africa. Journal of Toxicologyo, https://doi.org/10.1155/2018/3751930 (2018).
Paxéus, N. Removal of selected non-steroidal anti-inflammatory drugs (NSAIDs), gemfibrozil, carbamazepine, b-blockers, trimethoprim and triclosan in conventional wastewater treatment plants in five EU countries and their discharge to the aquatic environment. Water Sci. Technol. 50(5), 253–260 (2004).
Wudarska, E., Chrzescijanska, E., Kusmierek, E. & Rynkowski, J. Electrochemical Behavior of 2-(p-isobutylphenyl)propionic Acid at Platinum Electrode. Int. J. Electrochem. Sci. 10, 9433–9442 (2015).
Fabjan, E. Š., Kosec, T., Kuhar, V. & Legat, A. Corrosion stability of different bronzes in simulated urban rain. Mater. Technol. 45(6), 585–591 (2011).
Jmiai, A. et al. Chitozan as an eco-friendly inhibitor for copper corrosion in acidic medium: protocol and characterization. Cellulose 24, 3843–3867 (2017).
Ismail, K. M. Evaluation of cysteine as environmentally friendly corrosion inhibitor for copper in neutral and acidic chloride solutions. Electrochim. Acta 52, 7811–7819 (2007).
Sherif, E. M., Erasmus, R. M. & Comins, J. D. In situ Raman spectroscopy and electrochemical techniques for studying corrosion and corrosion inhibition of iron in sodium chloride solutions. Electrochim. Acta 55, 3657–3663 (2010).
Abd El-Lateef, H., Soliman, K. A. & Tantawy, A. H. Novel synthesized Schiff Base-based cationic Gemini surfactants: Electrochemical investigation, theoretical modeling and applicability as biodegradable inhibitors for mild steel against acidic corrosion. J. Mol. Liq. 232, 478–498 (2017).
Ferreira, E., Giacomelli, C., Giacomelli, F. & Spinelli, A. Evaluation of the inhibitor effect of L-ascorbic acid on the corrosion of mild steel. Mater. Chem. Phys. 83, 129–134 (2004).
Riggs, O. L., Jr. In C. C. Nathan (Ed.), Corrosion Inhibitors, NACE, Houston, TX, 1973.
Karthik, G. & Sundaravadivelu, M. Studies on the inhibition of mild steel corrosion in hydrochloric acid solution by atenolol drug. Egyptian Journal of Petroleum 25, 183–191 (2016).
Yan, Y., Li, W., Cai, L. & Hou, B. Electrochemical and quantum chemical study of purines as corrosion inhibitors for mild steel in 1 M HCl solution. Electrochim. Acta 53, 5953–5960 (2008).
Zhang, D. Q., Cai, Q. R., Gao, L. X. & Li, K. Y. Effect of serine, threonine and glutamic acid on the corrosion of copper in aerated hydrochloric acid solution. Corros. Sci. 50, 3615–3621 (2008).
Li, D., Chen, S., Zhao, S. & Ma, H. The corrosion inhibition of the self-assembled Au, and Ag nanoparticles films on the surface of copper. Colloid Surface A. 273, 16–23 (2006).
Migahed, M. A. & Nassar, I. F. Corrosion inhibition of Tubing steel during acidization of oil and gas wells. Electrochim. Acta 53, 2877–2882 (2008).
Zadeh, A. R. H., Danaee, I. & Maddahy, M. H. Thermodynamic and adsorption behavior of medicinal nitramine as a corrosion inhibitor for AISI steel alloy in HCl solution. J. Mater. Sci. Technol. 29, 884–892 (2013).
Badawy, W. A., Ismail, K. M. & Fathi, A. M. The influence of the copper/nickel ratio on the electrochemical behavior of Cu –Ni alloys in acidic sulfate solutions. J. Alloys Compd. 484, 365–370 (2009).
Magaino, S. Corrosion rate of copper rotating – disk – electrode in simulated acid rain. Electrochim. Acta 42, 377–382 (1997).
Szocs, E., Vastag, G., Shaban, A. & Kalman, E. Electrochemical behaviour of an inhibitor film formed on copper surface. Corros. Sci. 47, 893–908 (2005).
Amin, M. A. & Khaled, K. F. Copper corrosion inhibition in O2-saturated H2SO4 solutions. Corros. Sci. 52, 1194–1204 (2010).
Li, C.-C. et al. Adsorption and corrosion inhibition of phytic acid calcium on the copper surface in 3 wt% NaCl solution. Corros. Sci. 83, 147–154 (2014).
Wei, N. et al. 4-Phenylpyrimidine monolayer protection of a copper surface from salt corrosion. RSC Adv. 8, 7340–7349 (2018).
Qiang, Y., Zhang, S., Yan, S., Zou, X. & Chen, S. Three indazole derivatives as corrosion inhibitors of copper in a neutral chloride solution. Corros. Sci. 126, 295–304 (2017).
Iroh, J. O. & Su, W. Corrosion performance of polypyrrole coating applied to low carbon steel by an electrochemical process. Electrochim. Acta 46, 15–24 (2000).
Ameer, M. A., Fekry, A. M. & Othman, A. Electrochemical investigation of green inhibitor adsorption on low-carbon steel in produced water. Int. J. Electrochem. Sci. 9, 1964–1985 (2014).
Cano, E., Polo, J. L., La Iglesia, A. & Bastidas, J. M. A study on the adsorption of benzotriazole on copper in hydrochloric acid using the inflection point of the isotherm. Adsorption 10, 219–225 (2004).
PubChem, Open Chemistry database [Internet], Bethesda, USA [cited 2018, November 20]. Available from, https://pubchem.ncbi.nlm.nih.gov/compound/ibuprofen#section=Vapor-Pressure.
Quartarone, G., Battilana, M., Bonaldo, L. & Tortato, T. Investigation of the inhibition effect of indole-3-carboxylic acid on the copper corrosion in 0.5 M H2SO4. Corros. Sci. 50, 3467–3474 (2008).
Tan, B. et al. Insight into the corrosion inhibition of copper in sulfuric acid via two environmentally friendly food spices: Combining experimental and theoretical methods. J. Mol. Liq. 286, 110891 (2019).
Thompson, M. A., Planaria Software LLC, Seattle, W. A., http://www.arguslab.com.
Millan-Ocampo, D. E. et al. Experimental and theoretical study of ketoconazole as corrosion inhibitor for bronze in NaCl+Na2SO4 solution. Int. J. Electrochem. Sci. 12, 11428–11445 (2017).
Verma, C. et al. Corrosion inhibition of mild steel in 1 M HCl by D-glucose derivatives of dihydropyrido [2,3-d:6,5-d’] dipyrimidine-2,4,6,8(1 H, 3 H, 5 H, 7 H)-tetraone. Sci. Rep. 7, https://doi.org/10.1038/srep44432 (2017).
Zarrouk, A. et al. A theoretical investigation on the corrosion inhibition of copper by quinoxaline derivatives in nitric acid. Int. J. Electrochem. Sci. 7, 6353–6364 (2012).
Ramya, K., Mohan, R., Anupama, K. K. & Joseph, A. Electrochemical and theoretical studies on the synergistic interaction and corrosion inhibition of alkyl benzimidazoles and thiosemicarbazide pair on mild steel in hydrochloric acid. Mater. Chem. Phys. 149–150, 632–647 (2015).
Acknowledgements
The authors gratefully acknowledge the financial support of the Ministry of Education, Science and Technological Development of Republic of Serbia through Project No 172031.
Author information
Authors and Affiliations
Contributions
Zaklina Z. Tasić, Marija B. Petrović Mihajlović, Ana T. Simonović, Milan B. Radovanović and Milan M. Antonijević planned and carried out the experiments. All authors contributed to the interpretation of the results, provided critical feedback and helped shape the research, analysis and the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tasić, Z.Z., Mihajlović, M.B.P., Simonović, A.T. et al. Ibuprofen as a corrosion inhibitor for copper in synthetic acid rain solution. Sci Rep 9, 14710 (2019). https://doi.org/10.1038/s41598-019-51299-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-51299-2
This article is cited by
-
Recycling of expired ciprofloxacin in synthetic acid rain (SAR) solution as a green corrosion inhibitor for copper: a theoretical and experimental evaluation
Journal of Applied Electrochemistry (2024)
-
Corrosion inhibition of mild steel in hydrochloric acid solution by the expired Ampicillin drug
Scientific Reports (2023)
-
Drugs: On Sustainable and Green Solution for the Prevention of Metallic Corrosion
Journal of Bio- and Tribo-Corrosion (2023)
-
Theoretical, chemical, and electrochemical studies of Equisetum arvense extract as an impactful inhibitor of steel corrosion in 2 M HCl electrolyte
Scientific Reports (2022)
-
Investigation of Solupred as a pharmaceutical drug as a corrosion inhibitor for copper corrosion in 1.0 M sulfamic acid solution
Chemical Papers (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.