Abstract
Bovine Respiratory Disease (BRD) is the leading cause of mortality in calves. The objective of this study was to examine the response of the host’s bronchial lymph node transcriptome to Bovine Respiratory Syncytial Virus (BRSV) in a controlled viral challenge. Holstein-Friesian calves were either inoculated with virus (103.5 TCID50/ml × 15 ml) (n = 12) or mock challenged with phosphate buffered saline (n = 6). Clinical signs were scored daily and blood was collected for haematology counts, until euthanasia at day 7 post-challenge. RNA was extracted and sequenced (75 bp paired-end) from bronchial lymph nodes. Sequence reads were aligned to the UMD3.1 bovine reference genome and differential gene expression analysis was performed using EdgeR. There was a clear separation between BRSV challenged and control calves based on gene expression changes, despite an observed mild clinical manifestation of the disease. Therefore, measuring host gene expression levels may be beneficial for the diagnosis of subclinical BRD. There were 934 differentially expressed genes (DEG) (p < 0.05, FDR <0.1, fold change >2) between the BRSV challenged and control calves. Over-represented gene ontology terms, pathways and molecular functions, among the DEG, were associated with immune responses. The top enriched pathways included interferon signaling, granzyme B signaling and pathogen pattern recognition receptors, which are responsible for the cytotoxic responses necessary to eliminate the virus.
Similar content being viewed by others
Introduction
Bovine Respiratory Disease (BRD) is the leading cause of morbidity and mortality in calves over one month of age in Ireland1,2,3,4 and internationally5,6,7,8. It is a multifactorial disease, involving infectious agents (both viral and bacterial), host factors, management practices, environmental stress factors and their interactions4,8,9,10,11. Viruses generally initiate BRD and the damage they cause to the respiratory tract predisposes the calves to a secondary infection caused by bacteria, many of which are normal flora of the bovine upper respiratory tract12.
Bovine Respiratory Syncytial Virus (BRSV) is one of the main viral infectious agents responsible for the onset of BRD13. BRSV is an enveloped, non-segmented, negative-stranded RNA virus and is a member of the Orthopneumovirus genus within the family Pneumoviridae14,15. The morbidity resulting from infection with BRSV is in the range of 60% to 80%, and mortality is reported to be 20%15. The disease resulting from infection with BRSV can vary from sub-clinical to severe manifestation of clinical signs including coughing, pyrexia, nasal discharge, anorexia and respiratory distress15. Lung pathology resulting from BRSV infection is primarily due to the host inflammatory response, particularly the induction of pro-inflammatory cytokines (IL12B, IFNG, TNF, IL6, IL18, CXCL8, CCL5, CCL2, CCL3, IFNA1 and IFNB1) and the influx of leukocytes, particularly neutrophils13,15. Establishment and maintenance of BRSV infection is facilitated through the virus’ ability to interfere with the host’s interferon (anti-viral) response and to induce immunomodulation by shifting the adaptive immune response away from a cell mediated response towards a Th2 dominated response15,16.
Despite the risk of BRD susceptibility being moderately heritable17, there is a paucity of literature describing the molecular immune response of the host to infection with agents of the bovine respiratory disease complex (BRDC), such as BRSV. Two RNA-Seq studies performed in crossbred Angus-Hereford beef calves that were artificially challenged with single pathogens of the BRDC at the University of California Davis have discovered multiple genes and pathways to be differentially expressed following a BRSV challenge in bronchial lymph node18 and in multiple lymphoid and lung tissues19. However, no work has been carried out to date to examine the transcriptional response to a BRSV challenge in artificially-reared Irish dairy bull calves. The aims of this study were: (1) to describe the clinical and haematological response to a BRSV challenge infection, (2) to elucidate the genes and pathways involved in the host’s bronchial lymph node transcriptome response to an experimental challenge with BRSV in Irish artificially-reared dairy calves, and (3) to compare the bronchial lymph node transcriptomic response of these dairy calves challenged with BRSV to that of the crossbred beef calves challenged with BRSV at the University of California Davis18. These differentially expressed genes (DEG), and in particular the genes which were commonly differentially expressed in both the present study and the US study18 may harbour variants which influence the host’s resistance to BRSV.
Materials and Methods
Preparation of the BRSV Inoculum
Foetal calf lung (FCL) primary cells were grown in T75 tissue culture flasks using G-MEM BHK-21 media (Gibco #21710-025) with 10% foetal calf serum. BRSV strain SVA 274/92 cell lysate20 was removed from the −80 °C freezer and defrosted. A 1:7 dilution was prepared using G-MEM BHK-21 media containing 2% foetal calf serum. Growth media was removed and the virus stock (2 ml) was added to each flask of FCL cells and incubated at 37oC in 5% CO2 for 1 hour. The flasks were removed from the incubator and G-MEM BHK-21 media (18 ml) containing 2% foetal calf serum was added. The flasks were transferred to the incubator and incubated until the cytopathic effect was greater than 50%. Cell sheets were freeze-thawed to release intracellular virus, centrifuged at 500 g for 5 minutes at 4 °C to remove cellular debris, aliquoted and stored at −80 °C.
Animal model
All animal studies were carried out in accordance with the UK Animals (Scientific Procedures) Act 1986 and with the approval of the Agri-Food and Biosciences Institute Northern Ireland Ethical Review Committee.
Animals were selected from a population of 30 Holstein-Friesian bull calves (mean age ± s.d. = 120.7 ± 14.15 days) and recruited into the study based on low BRSV specific maternally derived antibody (MDA) levels and negative BRSV PCR status two weeks prior to challenge. As the challenge and necropsy were staggered across three days, recruited animals were assigned to three groups (A, B and C) based on sire, age (A = 135 ± 14 days, B = 134 ± 16 days, C = 133 ± 22 days), weight (A = 154.7 ± 14.0 kg, B = 155.0 ± 14.00 kg, C = 155 ± 19.00 kg) and MDA levels (A = 11.9 ± 5.48 MDA, B = 11.8 ± 5.15 MDA, C = 11.3 ± 6.13 MDA). The study included two treatments; calves were either challenged with BRSV (n = 12; groups B and C) or were mock challenged with sterile phosphate buffered saline (PBS) (n = 6; group A) (Control). The difference between treatment groups B and C was that they were housed in separate locations.
Animal accommodation
The calves entered and were acclimatised to their houses on day −7 relative to the challenge (Fig. 1). Two of the houses (housing groups A and B) were class 3 animal houses which were identical in layout (10.03 m × 5.01 m) while the third house was an older, separate house (6.65 m × 3.70 m). The floors were covered with sawdust and a calf restraining chute was contained within each of the calf houses.
Animal diet
Prior to the trial, from arrival to Agri-Food Biosciences Institute (AFBI) Stormont at 3 weeks of age, the calves were reared indoors and received 2 feeds per day of 2 litres of 23% protein, 23% fat, calf milk replacer (Thompsons high fat; Trouw Nutrition Limited), ad libitum silage and approximately 200 g of calf weaner nuts (Calf Pride Weaner Mix; John Thompson and Sons, Limited) to encourage them to start eating concentrates. They were weaned from the calf milk replacer at 8–10 weeks of age and subsequently fed ad libitum silage and approximately 1.5 kg of Calf Pride Rearing Nuts which was increased to 2 kg per day as the calves grew. For the duration of the trial, the calves were offered water and silage ad libitum and were fed 2 kg concentrates (17% crude protein, 4% crude oil, 9.5% crude fibre, 7.5% crude ash, 0.28% magnesium, 0.28% sodium) (Calf Pride Rearing Nuts; John Thompson and Sons, Limited).
Animal sampling
On days −1, 0, 6 and 7, relative to the challenge (day 0), a 9 ml K3 EDTA blood tube was collected, gently inverted several times and placed on ice (Fig. 1). Whole blood was analysed for haematological variables (white blood cell count, neutrophil percentage, lymphocyte percentage, monocyte percentage and eosinophil percentage) on an ABAXIS H5 haematology analyser (ABRAXIS Model: H5 S/N: 364372) immediately following collection. Daily, from the challenge to the day of slaughter, clinical signs (nasal discharge, ocular discharge, general demeanour, size of mandibular lymph nodes, presence of a cough, respiratory rate, respiratory character, mouth breathing, dyspnoea, presence of an expiratory grunt and rectal temperature) were recorded and scored by a veterinarian, who was blinded to the calves’ treatment status (BRSV challenged or control), (Fig. 1) using a modified version of the clinical scoring system described by Gershwin et al.21 (Supplementary Table S1). Using this scoring system, points were accrued for each abnormal clinical symptom and the total number of points corresponded with the severity of disease, such that higher clinical scores were associated with more severe BRD21.
On the day of the challenge, calves were either administered with 103.5 TCID50/ml × 15 ml of BRSV inoculum (BRSV challenged calves in groups B and C) or mock challenged with 10 ml PBS (control calves in group A) by aerosol inhalation.
Animals were restrained in a cattle crush and the head was further restrained to prevent movement during nebulisation. Animals were nebulised with BRSV strain SVA 274/9220 or PBS using the Omron NE-U780 nebuliser (OMRON Model: NE-U780-E S/N: 20151200182AF) fitted with a veterinary face mask (GaleMed Model: VM-2 Animal Mask size 5 22mmlD Ref: 5635 Lot: 151019). Airflow and nebulisation volume settings were set to the maximum. BRSV inoculum (20 ml) was placed into the chamber and nebulised over 15 minutes. The remaining volume of inoculum was checked after 15 minutes and if less than 10 ml had been nebulised, nebulisation was continued for an additional 5–10 minutes.
On day 7 relative to the challenge, calves were euthanized by captive bolt. The lungs were scored for lesions by a veterinarian using an AFBI standard lung scoring system which assigns the percentages of lesions present on the total lung area and on component parts of the lungs (Supplementary Fig. S1).
The workspace and implements were thoroughly cleaned and disinfected with bleach and 75% ethanol and sprayed with RNaseZap for RNase inhibition, before tissue collection and between euthanisation of animals. Bronchial lymph node tissues were harvested immediately and flash frozen in liquid nitrogen, placed on dry ice and then transferred to a −80 °C freezer for storage.
Statistical analysis of clinical and haematological data
Clinical score and haematological variables (white blood cell number, neutrophil percentage, lymphocyte percentage, monocyte percentage and eosinophil percentage) were analysed using repeated measures mixed models (MIXED procedure of SAS v 9.4) where time-point defined the repeated measure. Data were first assessed for normality using PROC REG and PROC UNIVARIATE. All data were found to be normally distributed. Treatment (BRSV challenged or control), time-point or group (A, B or C) and their interactions were included as fixed effects. Calf was included as a random effect. A Tukey adjustment was used to correct for multiple testing. Means and their standard errors are presented in Figs 2 and S2 (graphs were generated in GraphPad Prism version 7.02). Lung scores were assessed for normality using PROC REG and PROC UNIVARIATE and analysed using a mixed model ANOVA (MIXED procedure of SAS v 9.4) with treatment (BRSV challenged or control) or group included as fixed effects. The presence or absence of lung lesions was analysed using a Fisher’s Exact test in SAS 9.4.
RNA extraction
Total RNA (including miRNAs) was extracted using the Qiagen RNeasy Plus Universal Mini Kit (Qiagen LTD, Manchester, UK), according to the manufacturer’s instructions including Appendix C of the manufacturer’s protocol. The quantity of the extracted RNA was determined by measuring the absorbance at 260 nm with a Nanodrop spectrophotometer (NanoDrop technologies; Wilmington, DE, USA). The quality of the extracted RNA was examined with the Agilent 2100 Bioanalyser (Agilent Technologies Ireland Ltd; Dublin, Ireland) using the RNA 6000 Nano LabChip kit (Agilent Technologies Ireland Ltd; Dublin, Ireland). Samples had a mean RNA Integrity Number of 8.6 ± 0.31.
RNA-Seq library preparation and sequencing
Extracted RNA was shipped frozen at −80 °C on dry ice to the University of Missouri’s DNA Core Facility for RNA-Seq library preparation using the TruSeq stranded mRNA Kit (Illumina, San Diego, California, USA) and high-throughput sequencing (75 bp paired-end) on an Illumina NextSeq 500. All sequence data produced in this study has been deposited to NCBI GEO repository and are available through the series accession number GSE131452.
Alignment of sequence reads to the bovine reference genome and differential gene expression analysis
Adapter trimmed sequence reads in FASTQ format were assessed for quality using FastQC (version 0.11.7) (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). All reads passed the basic quality statistics. Reads were aligned to the UMD3.1 bovine reference genome and read counts were generated by converting aligned reads into counts per gene using the Spliced Transcripts Alignment to a Reference (STAR) aligner (version 2.5.2b)22.
Differential gene expression was determined using the R (R version 3.4.2 2017-09-28) Bioconductor package EdgeR (version 3.20.9)23 which accounts for biological and technical variation by modelling data as a negative binomial distribution. To remove lowly expressed genes, any genes with less than one count per million in at least three of the samples, were removed from the analysis. Data were normalised across libraries using the trimmed mean of M-values normalisation method24. The quantile-adjusted conditional maximum likelihood (qCML) common dispersion and the qCML tagwise dispersions were used to estimate dispersion. Exact tests were used for the detection of DEG between BRSV challenged and control calves. Genes with a Benjamini-Hochberg false discovery rate (FDR) of 10% and a fold change of ≥2 were considered differentially expressed.
Pathway and gene ontology analysis
Statistically significant gene ontology terms, pathways and other gene function related terms were analysed from a ranked DEG list (DEG between BRSV challenged and control calves with an FDR of 10% and a fold change of >2, ranked by P-value) using g:Profiler version 2018-06-2125. Multiple testing correction was performed using the g:Profiler tailor-made algorithm g:SCS.
The DEG between BRSV challenged and control calves, with an FDR of 10% and a fold change of >2, were analysed for over-represented gene ontology terms/pathways and functional annotation clustering using the Database for Annotation, Visualization and Integrated Discovery (DAVID) (http://david.abcc.ncifcrf.gov/tools.jsp)26. The enrichment P-values for each annotation term were derived from a modified Fisher’s exact test called the EASE score. The group enrichment score for each functional annotation cluster was calculated from the geometric mean (in the -log scale) of all the enrichment P-values in each annotation term within each cluster and was used to determine the significance of results from the functional annotation clustering analysis. A group enrichment score threshold of 1.3 (EASE = 0.05) was applied.
To further examine over-represented pathways, cellular and molecular functions and predicted upstream regulators, the RNA-Seq data were analysed using the Ingenuity Pathway Analysis (IPA) (QIAGEN Inc., https://www.qiagenbioinformatics.com/products/ingenuitypathway-analysis), according to the manufacturer’s instructions27. Within IPA, Fisher’s exact test was used with the Benjamini-Hochberg correction for multiple testing for the identification of over-represented pathways and over-represented molecular and cellular functions with a FDR of 10%, from DEG between BRSV challenged and control calves, with an FDR of 10% and a fold change of >2. Additionally, IPA’s regulation Z-score algorithm, which predicts increases or decreases in functions based on directional changes in the DEG and expectations derived from the literature, was used to predict differences in the over-represented cellular and molecular functions. IPA software considered cellular and molecular functions with a regulation Z-score value of ≥2.0 to be significantly increased and cellular and molecular functions with a regulation Z-score value of ≤−2.0 to be significantly decreased.
Comparison to the BRSV-associated RNA-Seq Data from Tizioto et al. (2015)
FASTQ files were downloaded from the National Center for Biotechnology Information Sequence Read Archive under accession number SRP052314 (SRR1952354, SRR1952355, SRR1952356, SRR1952357, SRR1952358, SRR1952359, SRR1952360)18. Alignment, gene counts, differential gene expression analysis, pathway and gene ontology analyses were performed identically to the analyses described for the current study. Pathway and gene ontology analyses were additionally performed on genes which were commonly differentially expressed in this study and that of Tizioto et al.18 with an FDR of 10% and a fold change of >2.
Results
Clinical score
Clinical score was affected by treatment (BRSV challenged 42.0 ± = 4.02 versus control 27.3 ± 5.68; P = 0.03) (Fig. 2). Time-point of clinical assessment also affected clinical scores (P < 0.0001) (Fig. 2) with days 4 (52.7 ± 7.42) and 7 (45.2 ± 7.42) being significantly greater than day 0 (25.5 ± 7.42). There was a tendency towards an interaction between time-point and treatment affecting clinical scores (P = 0.1), with the BRSV challenged calves having numerically greater clinical scores on day 7 versus day 2 (P < 0.1) and the control calves having significantly greater clinical scores on days 4 and 6 compared to day 2 (P < 0.05). However, there were no significant differences or tendencies between the BRSV challenged calves and the control calves at any of the time-points (P > 0.05). Furthermore, there was no effect of group on clinical scores (P > 0.05).
Haematology variables
Control calves had greater (P < 0.0001) white blood cell counts (10.8 ± 0.47 × 109/L) than the BRSV challenged calves (7.5 ± 0.33 × 109/L) (Supplementary Fig. S2A). Time-point significantly influenced the white blood cell count (P < 0.006) which was lower on day 7 relative to day -1 (P < 0.05) (Supplementary Fig. S2A).
There were no effects of treatment (BRSV challenged versus control), time-point or an interaction between treatment and time-point for either neutrophil, monocyte or lymphocyte percentage (P > 0.05). While there were no effects of treatment, time-point or their interactions on eosinophil percentage (P > 0.05), there was an effect of group (A, B or C) (P = 0.02). BRSV challenged calves in group C had a greater percentage of eosinophils than the control calves in group A (P = 0.02) (BRSV challenged calves in group C; 7.3 ± 1.12%, BRSV challenged calves in group B; 4.3 ± 1.12%, control calves in group A; 2.3 ± 1.07%) (Supplementary Fig. S2B).
Lung pathology
Figure 3 shows the lung pathologies from a subset of the BRSV challenged calves. There were no differences in either the overall lung scores or the percentage of the right cranial lung lobe that was lesioned between the BRSV challenged calves and the controls (P > 0.05). This is likely due to the small percentages of lesioned tissues present on the lungs (BRSV challenged group average percentage of lesioned tissue overall was 1.34 ± 1.0% and the right cranial lobe 1.63 ± 0.8%, controls 0.08 ± 0.1% and 0.83 ± 0.8%) (Table 1).
However, overall, only one control calf exhibited a lesioned lung, while 5 out of the 6 calves in BRSV challenge group B and 4 out of the 6 calves in BRSV challenge group C had lung lesions (Table 1). The BRSV challenged calves had a higher probability of having a lesioned lung compared with the control calves (P = 0.04).
Sequence alignment
An average of 53,878,694 (2 × 75 bp) paired-end reads (i.e. 26,939,347 sequenced fragments) were generated for each sample (Supplementary Table S2). Overall, 7.2% of the sequence reads failed to map to the genome and 89.6% of sequence reads mapped uniquely to the genome. On average, 57.3% of the total number of sequence reads were assigned to a gene by STAR, 0.4% were ambiguous and 31.9% of the sequence reads aligned to no feature (Supplementary Table S2).
Differential gene expression and functional annotation
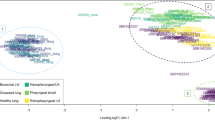
Using Edge-R, a multi-dimensional scaling (MDS) plot was produced which allowed an examination of the similarity of the samples based on global gene expression when samples were plotted using their first two principal components. The MDS plot indicated no differences between individuals in BRSV challenge groups B and C, but a clear separation between the BRSV challenged and the control calves (Fig. 4).
An MDS plot generated in EdgeR illustrating the similarity of the samples based on the first two principal components of gene expression covariance matrix among individuals. Samples from BRSV challenged calves are coloured in red and samples from control calves are coloured in blue. The numbers (1–18) refer to the calf ID and the letter S in front of the numbers refers to the word “sample”.
There were 934 DEG (p < 0.05, FDR < 0.1, fold change >2) between the BRSV challenged and control calves (Supplementary Table S3). There were two DEG between the calves in BRSV challenge group B and BRSV challenge group C (PPP1R1B and LYNX1).
From the g:Profiler analysis, there were 133 over-represented biological process Gene Ontology terms, 26 over-represented cellular component Gene Ontology terms, 23 over-represented molecular function Gene Ontology terms, 17 molecular function KEGG pathways and 20 over-represented Reactome pathways (Pcorrected <0.05) (Supplementary Table S4). The 12 most significant over-represented biological process Gene Ontology terms were related to the immune response (Table 2). The over-represented KEGG pathways were also associated with immune response and diseases and included KEGG:05164 Influenza A (Fig. 5)28. This pathway contained 19 up-regulated and two down-regulated genes (Supplementary Table S4, Fig. 5).
Genes up-regulated (red) in BRSV challenged calves (n = 12) in the KEGG:05164 Influenza A pathway28 (https://www.genome.jp/kegg/tool/map_pathway2.html).
There were 77 over-represented ontology terms in the DAVID analysis (p < 0.05, Benjamini-Hochberg FDR <0.1) (Supplementary Table S5). These were associated with the immune response, glycoproteins and secreted peptides. There were 28 enriched functional annotation clusters in the DAVID analysis (group enrichment score >1.3) (Supplementary Table S6). The enriched clusters contained terms relating to signal peptides, immunity, immunoglobulin, nucleotide-binding, thrombospondin, endoplasmic reticulum, double-stranded RNA binding, viral diseases, inflammatory responses, lectins, chemokine activity, extracellular matrix structure, cell division, calcium binding and the complement pathway.
Ingenuity pathway analysis showed that seventeen canonical pathways were enriched (P < 0.05, FDR <0.1) (Supplementary Table S7, Fig. 6). Four of these pathways were predicted to be upregulated (Interferon Signaling, Role of Pattern Recognition Receptors in Recognition of Bacteria and Viruses, Inhibition of Matrix Metalloproteases and Acute Phase Response Signaling) (Z-score >2) (Supplementary Table S7, Fig. 6).
The enriched canonical pathways found in the IPA analysis27 (P < 0.05, FDR <0.1). The pathways are shown on the x-axis and the –Log Benjamini-Hochberg adjusted p values are displayed on the y-axis. The threshold is set to 1 which equals a Benjamini-Hochberg adjusted p value of 0.1. The orange line representing the ratio corresponds to the ratio of the number of differentially expressed genes that map to the pathway divided by the total number of genes that map to the same pathway. Pathways with a positive z-score are predicted by IPA to have increased activity and pathways with a negative z-score are predicted to have decreased activity.
Thirty-one enriched biological functions were predicted to be increased in BRSV challenged animals compared to controls (Z-score >2) (Supplementary Table S8). Their functions were related to the recruitment, activation and death of leukocytes.
There were 256 up-stream regulators that were predicted by IPA to be activated (Z-score ≥2) (Supplementary Table S9). Nine of these had an observed increase in fold change (IFNG, IRF7, DDX58, CGAS, OSM, IL27, EIF2AK2, XBP1 and TBX21). There were 84 up-stream regulators that were predicted to be inhibited by IPA (Z-score ≤2) (Supplementary Table S9), but none of these were found to have a decreased expression in the BRSV challenged calves.
Differential gene expression and functional annotation of RNA-Seq data in comparison to Tizioto et al. (2015) study
An average of 106,159,694 paired-end reads (2 × 50 bp) (i.e. 53,079,847 sequenced fragments) were obtained from each BRSV challenged/control bronchial lymph node sample downloaded from the National Center for Biotechnology Information Sequence Read Archive under accession number SRP052314 (Supplementary Table S2)18. Overall, 11.7% of sequence reads failed to map to the genome and 82.7% of reads mapped uniquely to the genome, using the STAR aligner22. On average, 28.0% of the total number of sequence reads were assigned to a gene by STAR, 0.1% were ambiguous and 54.6% of reads aligned to no feature (Supplementary Table S2).
An MDS plot illustrating the similarity of the Tizioto et al.18 BRSV challenged and control bronchial lymph node samples based on log2 fold gene expression changes was produced in EdgeR (Fig. 7). For illustration of the similarities between the samples in the present study and those from the Tizioto et al.18 study, an MDS plot was produced in EdgeR based on log2 fold gene expression changes among all samples (Fig. 8).
An MDS plot generated in EdgeR illustrating the similarity of the Tizioto et al.18 BRSV challenged (n = 4) and control (n = 3) bronchial lymph node samples based on log2 fold gene expression changes. Samples from BRSV challenged calves are coloured in red and samples from control calves are coloured in blue. The sample names are the accession numbers of the samples.
An MDS plot (based on log2 fold gene expression changes) generated in EdgeR illustrating the similarity between the BRSV challenged (n = 12) and control (n = 6) bronchial lymph node samples from the present study and from the Tizioto et al.18 study.
One thousand and thirteen genes from the study of Tizioto et al.18 were differentially expressed between BRSV challenged and control calves (Supplementary Table S10). From the g:Profiler analysis, there were 198 over-represented biological process Gene Ontology terms, 19 over-represented cellular component Gene Ontology terms, 16 over-represented molecular function Gene Ontology terms, 18 over-represented KEGG pathways, 38 over-represented Human Phenotype Ontology terms, 1 over-represented CORUM term and 30 over-represented Reactome pathways (Pcorrected <0.05) (Supplementary Table S11). The over-represented biological process Gene Ontology terms were related to the immune response. The over-represented KEGG pathways were also associated with immune responses and diseases and included KEGG:05164 Influenza A and KEGG:04060 Cytokine-cytokine receptor interaction.
There were 354 DEG in common between the present study and the Tizioto et al.18 study (BRSV challenged and control bronchial lymph node samples) (Supplementary Table S12). From the g:Profiler analysis, there were 81 over-represented biological process Gene Ontology terms, 18 over-represented cellular component Gene Ontology terms, 13 over-represented molecular function Gene Ontology terms, 16 over-represented KEGG pathways, 12 over-represented Human Phenotype Ontology terms, 1 over-represented CORUM term and 20 over-represented Reactome pathways (Pcorrected <0.05) among the DEG in common between the two data sets (Supplementary Table S13).
Pathway analysis in IPA revealed that 15 pathways were over-represented among the DEG in common between the present study and the Tizioto et al.18 study (Fig. 9, Supplementary Table S14). The most significant of the over-represented pathways, interferon signaling and the role of pattern recognition receptors in recognition of bacteria and viruses, were also among the most significant over-represented pathways in the IPA pathway analysis of the DEG solely found in this study.
The enriched canonical pathways in the IPA analysis27 among DEG in common between the present study and the Tizioto et al.18 study (P < 0.05, FDR <0.1). The pathways are shown on the x-axis and the –Log Benjamini-Hochberg adjusted p values are displayed on the y-axis. The threshold is set to 1 which equals a Benjamini-Hochberg adjusted p value of 0.1. The orange line representing the ratio corresponds to the ratio of the number of differentially expressed genes that map to the pathway divided by the total number of genes that map to the same pathway. Pathways with a positive z-score are predicted by IPA to have increased activity and pathways with a negative z-score are predicted to have decreased activity.
Fourteen enriched biological functions, among DEG in common between the present study and the Tizioto et al.18 study, were predicted to be increased in BRSV challenged animals compared to controls (Z-score >2) (Supplementary Table S15). Functions were related to the antimicrobial and antiviral immune response. Twelve enriched biological functions, among DEG in common between the present study and the Tizioto et al.18 study, were predicted to be decreased in BRSV challenged animals compared to controls (Z-score >2) (Supplementary Table S15). These functions were associated with viral replication and morbidity.
Discussion
This is the first study to examine the host response at the transcriptional level in artificially-reared Holstein-Friesian calves to a challenge with BRSV. Previous studies have examined the bronchial lymph node pathological response to a BRSV challenge in Angus-Hereford crossbred beef calves18,19,21. However, immune responses, particularly at the level of transcription, have been demonstrated to be affected by breed29,30,31. This may be the reason that the majority of the variation observed in the MDS plot (Fig. 8) was due to study (the present study versus Tizioto et al.18), while treatment (BRSV challenged versus control) bronchial lymph node gene expression was the secondary source of variation. However, the different sequencing platforms used (NextSeq 500 versus HiSeq 2000), the varying length and depth of reads generated, the distinct BRSV viral strains used in the inoculum and the alternative husbandry and management practices carried out in the studies’ diverse geographical locations, may have also influenced the alterations in gene expression between the two studies. Despite the many sources of variation existing between the two studies, 38% of the DEG in this study were commonly differentially expressed in the RNA-Seq analysis of the BRSV challenged and control bronchial lymph node samples from the study of Tizioto et al.18. Furthermore, the majority of enriched pathways found in the two studies were in common. Therefore, BRSV appears to elicit many conserved alterations in gene expression.
In the present study, calves were challenged with a similar titre of BRSV that has consistently and successfully been used to establish infection in previous BRSV challenge studies18,21,32. Calves were euthanised 7 days post challenge as this is the time-point when the acute phase response has been observed to peak33 and when live BRSV and its antigen titres begin to decrease34. Bronchial lymph node tissues were harvested and RNA-Seq analysis was performed on these tissues to determine the gene expression changes induced by BRSV infection as these lymph nodes drain the lungs and are the site of antigen presentation and the initiation of the primary immune response35,36.
Infection with BRSV can be subclinical, present only in the upper respiratory tract or it can affect both the upper and lower parts of the respiratory tract15. BRSV mainly infects and produces clinical signs in calves less than one year of age but is usually more severe in young calves aged between one and three months32. It is common for paramyxoviruses, including BRSV, to be attenuated by passage through in vitro culture, and this can result in minimal clinical symptoms of respiratory disease37. Therefore, the age of the calves (approximately 5 months of age) and the effects of BRSV attenuation in culture may have influenced the primarily sub-clinical response to BRSV challenge observed in the present study. The calves in this study had substantially lower clinical scores than the calves challenged with BRSV in the Tizioto et al.18,21 study. This may have been due to the possible differing initial BRSV specific MDA levels of the calves, the diverse breeds of the calves or the distinct strains of BRSV inoculum used for the challenge. Furthermore, there were no changes in neutrophil, lymphocyte or monocyte percentages due to the BRSV challenge in this study. While control calves did have a higher white blood cell count compared with BRSV challenged calves throughout the study, the white blood cell numbers were within the normal range for healthy cattle38 and no increase in white blood cell count was observed following virus inoculation, in BRSV challenged calves.
Interestingly, despite these BRSV challenged calves only showing a modest clinical and haematological response to infection, and having only a small proportion of their lungs lesioned, the virus induced vast changes in bronchial lymph node gene expression levels. Therefore, measuring host gene expression levels may prove to be a more accurate way of diagnosing BRD than by the observation of clinical signs of the disease. This would be hugely beneficial to the global agricultural industry as sub-clinical BRD which remains undiagnosed is almost as prevalent in healthy cattle as BRD is among cattle which are diagnosed with respiratory disease and treated for BRD39. Although it would clearly not be possible to measure host gene expression in bronchial lymph node tissue in live calves if a small set of diagnostic genes are also perturbed in expression in white blood cells or nasal fluid, these more accessible media could be used as “liquid biopsies” for the future diagnosis of subclinical respiratory infections. Indeed increased expression of IFNG and IL6 have been observed along with clinical signs during an initial BRSV challenge and the increased expression of IFNG in the peripheral blood mononuclear cells of young calves with an absence of clinical symptoms has also been observed in a reinfection with BRSV34.
The majority of the alterations in bronchial lymph node gene expression due to BRSV challenge detected in the present study were associated with the immune response. The most highly upregulated gene was GZMB, which was upregulated sixty-seven fold. This gene was also the most up-regulated in response to a BRSV challenge in bronchial lymph node in US calves18 and the encoded protein has been shown to be significantly increased in CD8+ and CD4+ T cells and natural killer cells, in tracheal aspirates from children infected with respiratory syncytial virus (RSV)40. Furthermore, GZMB was found to be a member of several enriched ontologies and pathways including “defense response”, “immune response”, “granzyme-mediated apoptotic signaling pathway” and “granzyme B signaling”, in the present study. The serine protease protein encoded by this gene is one of the major components of the lytic granules which are released by cytotoxic T cells and natural killer cells to destroy virally infected cells41. It also plays a role in degradation of the extracellular matrix and induction of pro-inflammatory cytokine responses42. Furthermore, granzyme B has recently been found to be utilised by T regulatory cells to control effector B and T cell populations, thereby regulating the inflammatory response and reducing the severity of RSV disease in mice43. Consequently, it is likely to play an important role in defence to BRSV infection and alterations to this gene may be critically important to BRD resistance and susceptibility. Indeed, it has been shown that polymorphisms in murine GZMB can reduce the ability of CD8+ T cells to kill virally infected target cells44. It has also been postulated that that GZMB-expressing T regulatory cells could be used as a therapeutic strategy in human viral lung infections, due to their ability to dampen inflammation without elevating viral load43. However, a study of mice experimentally infected with Klebsiella pneumoniae found that while GZMB knockout mice had higher levels of lung inflammation at earlier stages in the disease, there were minimal differences in distant organ injury or survival between the knockout and wild type mice45. However, Klebsiella pneumoniae is caused by a bacterium rather than a virus and as GZMB is mainly involved in the cell mediated cytotoxic immune response, it may be more effective in the elimination of viral infections and intracellular bacteria.
Pathways and gene ontologies involved in the innate immune response, induction of cytokines and chemokines, humoral and cell mediated immunity were over-represented among the DEG. The KEGG pathway for Influenza A was enriched for DEG and this pathway contains upregulated cytokines responsible for the induction of the febrile response, mitochondrial genes involved in programmed cell death and interferon stimulated genes which induce cytotoxic T cell responses. Tizioto et al.18 also observed an enrichment of similar pathways and the DEG found in common in both studies were members of the most significantly enriched pathways from this study, including interferon signaling and role of pattern recognition receptors in recognition of bacteria and viruses. Furthermore, the induction of pro-inflammatory chemokines and cytokines in calves due to BRSV infection has been reported in pneumonic lung lesions46, bronchoalveolar lung fluid47 and bovine respiratory/turbinate epithelial cells48. Therefore, these immune response associated genes seem to play an important role in the bovine host response to BRSV infection. This is expected as pathogen recognition, induction of inflammatory cytokines and chemokines, and the initiation of the innate immune response are vital to control the virus upon entry. Subsequent elimination of the virus requires the development of CD8 cytotoxic T cells, which is facilitated by the production of IFNG and interferon stimulated genes such as DDX58, IFI44, IFIH1, IFIT1, IFIT2, IFIT3, IFIT5, ISG15, ISG20, MX1, MX2, OAS1, EIF2AK2, RTP4, RSAD2, OAS2 and OSM, which were upregulated in the present study49.
In agreement with Tizioto et al.18, we also predicted neurodegenerative diseases and functions including “progressive motor neuropathy”, “progressive neurological disorder”, “inflammatory demyelinating disease” and “multiple sclerosis” to be increased in the BRSV challenged calves. These findings are interesting as human RSV has been observed as being capable of infecting murine lung neuronal cells50, and also of infecting the human central nervous system and inducing neurological symptoms51.
BRSV modulates the immune response towards a T helper 2 (Th2) type response, with increased expression of Th2 promoting cytokines and increased concentrations of BRSV specific IgE antibodies in lymph fluid48. Despite enriched ontology terms including “B cell activation” and “immunoglobulin mediated immune response”, we found no evidence for an increase in the transcription of either IL4 or IL13 which initiate the Th2 bias52. This is consistent with the results of Grell et al.34, who did not detect an increase in IL4 gene expression following a BRSV challenge. However, although there was a 2 fold increase in the expression of IL12RB2, a transmembrane protein subunit of the interleukin 12 receptor complex, there was no increase in the expression of IL12, which is necessary to induce Th1 cell proliferation responses48, which is consistent with the expected Th2 bias for BRSV.
Lipopolysaccharide (LPS) was a predicted upstream regulator in this study and that of Tizioto et al.18. Furthermore, LBP was the second greatest up-regulated gene in response to the BRSV challenge infection in this study (fold change = 52). This is likely because the fusion protein from BRSV interacts with TLR4 to initiate the innate immune response in a manner that is similar to LPS48. Tizioto et al.18 suggested that LPS has the potential to be used in new treatments aiming to prevent the occurrence of BRD as it induces a cascade of both anti-viral and anti-bacterial genes. Other predicted upstream regulators in the present study include IFNG and IRF7, which play roles in IFN antiviral innate immune responses53.
BRSV is closely related to human RSV, and induces similar pathologies15,37 and alterations in host gene expression. Genes involved in the type 1 interferon antiviral response, including BP1, RSAD2, MX2, MX1, ISG15, IRF7, IFITM3, IFIT3, IFIT2, IFIT1, IFIH1, IFI44, which were upregulated in the lungs of C57BL/6 mice, in response to infection with RSV54, were also up-regulated in the calves challenged with BRSV in the present study. Therefore, the gene expression changes induced by BRSV infection detected in this study may provide an insight into the human bronchial lymph node transcriptional response to RSV. Future studies will also elucidate the bovine host micro-RNA gene expression responses and determine the regulatory regions controlling these transcriptional alterations due to infection with BRSV, in bronchial lymph node, and in other important respiratory tissues including lesioned and healthy lung tissue, mediastinal and retropharyngeal lymph nodes, and the pharyngeal tonsil. The DEG identified in this study, particularly those also observed to be DEG in the study of Tizioto et al.18, and therefore involved in the bovine host response to BRSV infection along with the regulatory regions controlling transcriptional changes in these genes may be interrogated in future large scale studies for the identification of variants which may be associated with resistance to BRSV infection. These variants could be included on genotyping assays routinely used in the international beef and dairy cattle industries for use in breeding programmes targeted on the generation of healthier, more robust cattle with a greater potential to resist BRSV infection and subsequent BRD.
Data Availability
All sequence data produced in this study has been deposited to NCBI GEO repository and are available through series Accession Number GSE131452.
References
Johnston, D. et al. Electronic feeding behavioural data as indicators of health status in dairy calves. Irish J. Agr. Food Res. 55, 159–168, https://doi.org/10.1515/ijafr-2016-0016 (2016).
AFBI/DAFM. All-Island Disease Surveillance Report (2016).
Murray, G. M. et al. Risk factors associated with exposure to bovine respiratory disease pathogens during the peri-weaning period in dairy bull calves. BMC Vet. Res. 14, 53, https://doi.org/10.1186/s12917-018-1372-9 (2018).
Murray, G. M. et al. Pathogens, patterns of pneumonia, and epidemiologic risk factors associated with respiratory disease in recently weaned cattle in Ireland. J. Vet. Diagn. Investig. 29, https://doi.org/10.1177/1040638716674757 (2017).
Lindholm-Perry, A. K. et al. Complete blood count data and leukocyte expression of cytokine genes and cytokine receptor genes associated with bovine respiratory disease in calves. BMC Res. Notes 11, 786–786, https://doi.org/10.1186/s13104-018-3900-x (2018).
Svensson, C., Hultgren, J. & Oltenacu, P. A. Morbidity in 3–7-month-old dairy calves in south-western Sweden, and risk factors for diarrhoea and respiratory disease. Prev. Vet. Med. 74, 162–179, https://doi.org/10.1016/j.prevetmed.2005.11.008 (2006).
Windeyer, M. C. et al. Factors associated with morbidity, mortality, and growth of dairy heifer calves up to 3 months of age. Prev. Vet. Med. 113, 231–240, https://doi.org/10.1016/j.prevetmed.2013.10.019 (2014).
Hay, K. E., Barnes, T. S., Morton, J. M., Clements, A. C. A. & Mahony, T. J. Risk factors for bovine respiratory disease in Australian feedlot cattle: Use of a causal diagram-informed approach to estimate effects of animal mixing and movements before feedlot entry. Prev. Vet. Med. 117, 160–169, https://doi.org/10.1016/j.prevetmed.2014.07.001 (2014).
Edwards, T. A. Control methods for bovine respiratory disease for feedlot cattle. Vet Clin N Am Food Anim Pract 26, 273–284, https://doi.org/10.1016/j.cvfa.2010.03.005 (2010).
Taylor, J. D., Fulton, R. W., Lehenbauer, T. W., Step, D. L. & Confer, A. W. The epidemiology of bovine respiratory disease: What is the evidence for preventive measures? Can. Vet. J 51, 1351–1359 (2010).
Johnston, D. et al. Illumina MiSeq. 16S amplicon sequence analysis of bovine respiratory disease associated bacteria in lung and mediastinal lymph node tissue. BMC Vet. Res. 13, 118, https://doi.org/10.1186/s12917-017-1035-2 (2017).
Griffin, D., Chengappa, M. M., Kuszak, J. & McVey, D. S. Bacterial pathogens of the bovine respiratory disease complex. Vet. Clin. North Am. Food Anim. Pract. 26, 381–394, https://doi.org/10.1016/j.cvfa.2010.04.004 (2010).
Sudaryatma, P. E. et al. Bovine respiratory syncytial virus infection enhances Pasteurella multocida adherence on respiratory epithelial cells. Vet. Microbiol. 220, 33–38, https://doi.org/10.1016/j.vetmic.2018.04.031 (2018).
Rima, B. et al. ICTV Virus Taxonomy Profile: Pneumoviridae. J Gen Virol. 98, 2912–2913, https://doi.org/10.1099/jgv.0.000959 (2017).
Valarcher, J.-F. & Taylor, G. Bovine respiratory syncytial virus infection. Vet. Res. 38, 153–180 (2007).
Gershwin, L. Bovine respiratory syncytial virus infection: immunopathogenic mechanisms. Anim. Health Res. Rev. 8, 207–213 (2007).
Neibergs, H. L. et al. Susceptibility loci revealed for bovine respiratory disease complex in pre-weaned Holstein calves. BMC Genomics 15, 1164, https://doi.org/10.1186/1471-2164-15-1164 (2014).
Tizioto, P. C. et al. Immunological response to single pathogen challenge with agents of the bovine respiratory disease complex: an RNA-sequence analysis of the bronchial lymph node transcriptome. PLoS ONE 10, e0131459, https://doi.org/10.1371/journal.pone.0131459 (2015).
Behura, S. K. et al. Tissue tropism in host transcriptional response to members of the bovine respiratory disease complex. Sci. Rep. 7, 17938, https://doi.org/10.1038/s41598-017-18205-0 (2017).
Graham, D. A. et al. Detection of IgM responses to bovine respiratory syncytial virus by indirect ELISA following experimental infection and reinfection of calves: abolition of false positive and false negative results by pre-treatment of sera with protein-G agarose. Vet. Immunol. Immunopathol. 71, 41–51, https://doi.org/10.1016/S0165-2427(99)00086-0 (1999).
Gershwin, L. J. et al. Single pathogen challenge with agents of the bovine respiratory disease complex. PLoS One 10, e0142479, https://doi.org/10.1371/journal.pone.0142479 (2015).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 10, 15–21 (2013).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Robinson, M. & Oshlack, A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 11, R25 (2010).
Reimand, J. et al. g:Profiler—a web server for functional interpretation of gene lists (2016 update). Nucleic Acids Res. 44(W1), W83–9, https://doi.org/10.1093/nar/gkw199 (2016).
Huang, D. W., Sherman, B. T. & Lempicki, R. A. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 37, 1–13, https://doi.org/10.1093/nar/gkn923 (2009).
Krämer, A., Green, J., Pollard, J. & Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 30, 523–530, https://doi.org/10.1093/bioinformatics/btt703 (2014).
Kanehisa, M. & Goto, S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30, https://doi.org/10.1093/nar/28.1.27 (2000).
Johnston, D. et al. Characterisation of haematological profiles and whole blood relative gene expression levels in Holstein-Friesian and Jersey bull calves undergoing gradual weaning. Animal 10, 1547–1556, https://doi.org/10.1017/S1751731115002438 (2016).
Johnston, D. et al. Characterisation of the whole blood mRNA transcriptome in Holstein-Friesian and Jersey calves in response to gradual weaning. PLoS ONE 11, e0159707, https://doi.org/10.1371/journal.pone.0159707 (2016).
Surlis, C. et al. Blood immune transcriptome analysis of artificially fed dairy calves and naturally suckled beef calves from birth to 7 days of age. Sci. Rep. 8, 15461, https://doi.org/10.1038/s41598-018-33627-0 (2018).
Blodörn, K. et al. A bovine respiratory syncytial virus model with high clinical expression in calves with specific passive immunity. BMC Vet. Res. 11, 76, https://doi.org/10.1186/s12917-015-0389-6 (2015).
Heegaard, P. M. H. et al. The acute phase response of haptoglobin and serum amyloid A (SAA) in cattle undergoing experimental infection with bovine respiratory syncytial virus. Vet. Immunol. Immunopathol. 77, 151–159, https://doi.org/10.1016/S0165-2427(00)00226-9 (2000).
Grell, S. N., Kirsten, T., Larsen, L. E. & Heegaard, P. M. H. Marked induction of IL-6, haptoglobin and IFNγ following experimental BRSV infection in young calves. Vet. Immunol. Immunopathol. 103, 235–245, https://doi.org/10.1016/j.vetimm.2004.09.025 (2005).
Lehmann, C. et al. Lymphocytes in the bronchoalveolar space reenter the lung tissue by means of the alveolar epithelium, migrate to regional lymph nodes, and subsequently rejoin the systemic immune system. Anat. Rec. 264, 229–236, https://doi.org/10.1002/ar.1163 (2001).
Lambrecht, B. N., Prins, J.-B. & Hoogsteden, H. C. Lung dendritic cells and host immunity to infection. Eur. Respir. J. 18, 692–704 (2001).
Ellis, J. A. How efficacious are vaccines against bovine respiratory syncytial virus in cattle? Vet. Microbiol. 206, 59–68, https://doi.org/10.1016/j.vetmic.2016.11.030 (2017).
Jones, M. & Allison, R. Evaluation of the ruminant complete blood cell count. Vet. Clin. North Am. Food Anim. Pract. 23, 377–402 (2007).
Kiser, J. N. et al. Rapid Communication: Subclinical bovine respiratory disease – loci and pathogens associated with lung lesions in feedlot cattle. J. Anim. Sci. 95, 2726–2731, https://doi.org/10.2527/jas.2017.1548 (2017).
Bem, R. A. et al. Activation of the granzyme pathway in children with severe respiratory syncytial virus infection. Pediatr. Res. 63, 650, https://doi.org/10.1203/PDR.0b013e31816fdc32 (2008).
Prager, I. & Watzl, C. Mechanisms of natural killer cell-mediated cellular cytotoxicity. J. Leukoc. Biol. 105, 1319–1329, https://doi.org/10.1002/jlb.mr0718-269r (2019).
Hirota, J. A. et al. Granzyme B Deficiency Exacerbates Lung Inflammation in Mice after Acute Lung Injury. Am. J. Respir. Cell Mol. Biol. 49, 453–462, https://doi.org/10.1165/rcmb.2012-0512OC (2013).
Loebbermann, J. et al. Regulatory T cells expressing granzyme B play a critical role in controlling lung inflammation during acute viral infection. Mucosal Immunol. 5, 161–72, https://doi.org/10.1038/mi.2011.62 (2012).
Andoniou, C. E. et al. A natural genetic variant of granzyme B confers lethality to a common viral infection. PLoS Pathog. 10, e1004526, https://doi.org/10.1371/journal.ppat.1004526 (2014).
García-Laorden, M. I. et al. Granzymes A and B regulate the local inflammatory response during Klebsiella pneumoniae pneumoniae pneumonia. J. Innate Immun. 8, 258–268 (2016).
Sacco, R. E. et al. Differential expression of cytokines in response to respiratory syncytial virus infection of calves with high or low circulating 25-hydroxyvitamin D3. PLoS ONE 7, e33074, https://doi.org/10.1371/journal.pone.0033074 (2012).
Antonis, A. F. G. et al. Age-dependent differences in the pathogenesis of bovine respiratory syncytial virus infections related to the development of natural immunocompetence. J. Gen. Virol. 91, 2497–2506, https://doi.org/10.1099/vir.0.020842-0 (2010).
Gershwin, L. J. Immunology of bovine respiratory syncytial virus infection of cattle. Comp. Immunol. Microbiol. Infect. Dis. 35, 253–257, https://doi.org/10.1016/j.cimid.2012.01.005 (2012).
Schoggins, J. W. & Rice, C. M. Interferon-stimulated genes and their antiviral effector functions. Curr. Opin. Virol. 1, 519–525, https://doi.org/10.1016/j.coviro.2011.10.008 (2011).
Li, X. Q., Fu, Z. F., Alvarez, R., Henderson, C. & Tripp, R. A. Respiratory syncytial virus (RSV) infects neuronal cells and processes that innervate the lung by a process involving RSV G protein. J. Virol. 80, 537–540, https://doi.org/10.1128/JVI.80.1.537-540.2006 (2006).
Yuan, X. et al. Respiratory syncytial virus prolifically infects N2a neuronal cells, leading to TLR4 and nucleolin protein modulations and RSV F protein co-localization with TLR4 and nucleolin. J. Biomed. Sci. 25, 13, https://doi.org/10.1186/s12929-018-0416-6 (2018).
Bao, K. & Reinhardt, R. L. The differential expression of IL-4 and IL-13 and its impact on type-2 immunity. Cytokine 75, 25–37, https://doi.org/10.1016/j.cyto.2015.05.008 (2015).
Ning, S., Pagano, J. S. & Barber, G. N. IRF7: activation, regulation, modification and function. Genes Immun. 12, 399–414, https://doi.org/10.1038/gene.2011.21 (2011).
Pennings, J. L. A. et al. Transcriptomics in lung tissue upon respiratory syncytial virus infection reveals aging as important modulator of immune activation and matrix maintenance. Sci. Rep. 8, 16653, https://doi.org/10.1038/s41598-018-35180-2 (2018).
Acknowledgements
This project was funded by the Irish Department of Agriculture and the Department of Agriculture, Environment and Rural Affairs, Northern Ireland, as part of the US-Ireland R&D partnership call (RMIS_0033 Project 16/RD/US-ROI/11). JFT and JWK were supported by Grant Number 2017-67015-26760 from the United States Department for Agriculture National Institute for Food and Agriculture.
Author information
Authors and Affiliations
Contributions
S.M.W., J.F.T., J.W.K., B.E. and S.L.C. conceived and designed the experiments. K.L., C.D., M.M. and S.L.C. developed and executed the animal challenge model. D.J., K.L., C.D., M.M., J.W.K., S.M.W., B.E. and M.S.M. performed the experiments. D.J. and G.B. performed the bioinformatics. D.J. analysed the data. D.J. wrote the paper. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Johnston, D., Earley, B., McCabe, M.S. et al. Experimental challenge with bovine respiratory syncytial virus in dairy calves: bronchial lymph node transcriptome response. Sci Rep 9, 14736 (2019). https://doi.org/10.1038/s41598-019-51094-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-51094-z
This article is cited by
-
Use of nCounter mRNA profiling to identify at-arrival gene expression patterns for predicting bovine respiratory disease in beef cattle
BMC Veterinary Research (2022)
-
ATAC-Seq identifies regions of open chromatin in the bronchial lymph nodes of dairy calves experimentally challenged with bovine respiratory syncytial virus
BMC Genomics (2021)
-
Review on bovine respiratory syncytial virus and bovine parainfluenza – usual suspects in bovine respiratory disease – a narrative review
BMC Veterinary Research (2021)
-
Genes and regulatory mechanisms associated with experimentally-induced bovine respiratory disease identified using supervised machine learning methodology
Scientific Reports (2021)
-
Messenger RNA biomarkers of Bovine Respiratory Syncytial Virus infection in the whole blood of dairy calves
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.