Abstract
Hyperlipidemia, a very common disease throughout the world, usually gives rise to severe liver damages. The current experiment was to investigate the antihyperlipidemic and hepatoprotective properties of alkali- and enzyme-extractable Dictyophora indusiata polysaccharides (Al-DPS and En-DPS) on the hyperlipidemic mice. The results of animal experiment in vivo showed that treatment with Al-DPS or En-DPS could improve the excessive level of lipid profiles in serum and liver, as well as strengthen antioxidant status. In addition, the histopathological observations of liver testified that polysaccharides were capable of attenuating hepatic cell injury. The primary structural features of Al-DPS and En-DPS were demonstrated by HPGPC, HPLC, FT-IR and NMR. Glucose tolerance test manifested that polysaccharides were able to restrain the rise of blood sugar. The results indicated that Al-DPS and En-DPS may be considered as novel compounds to treat hyperlipidemia and also act as hepatoprotective agents.
Similar content being viewed by others
Introduction
Hyperlipidemia is a systemic disease, which is characterized by elevated lipid levels in blood including total cholesterol (TC), total glyceride (TG), and low-density lipoprotein cholesterol (LDL-C) and so on1. In addition, it is an important risk factor leading to fatty liver, cardiovascular disease and atherosclerosis2, and becomes the first killer of human health. It has been reported that nearly 23.6 million people will die from cardiovascular diseases originating from hyperlipidemia by 20303. Furthermore, the incidence of hyperlipidemia is increasing gradually, and may aggravate with aged tendency of population4. Hence, the prevention and management of hyperlipidemia is important. Previous studies have indicated that oxidative stress from reactive oxygen species (ROS) could accelerate the pathogenic progress of hyperlipidemia and its complications5,6. Increasing evidences have suggested that the hepatoprotective effects of substance may be related to their known antioxidant and pre-oxidant properties7. To date, many hypolipidemic agents were commonly used to treat hyperlipidemia including atorvastatin, fibrates, statins, nicotinic acid, probucol and other compound preparations clinically8. However, the application of these drugs is limited as a result of their serious side effects, such as diarrhea, nausea, myositis, and abnormal liver functions of statins9. Thus, it is eager to exploit natural alternative components possessing antioxidant and hepatoprotection effects aiming to reduce damages linked to hyperlipidemia.
Polysaccharide, an important natural bio-macromolecule, is widely found in nature10. In recent years, numerous studies have shown that many natural polysaccharides from mushrooms have been proved to possess potential antihyperlipidemic activities11,12,13. Dictyophora indusiata, a kind of famous edible mushroom, is widely distributed and eaten around the world. It is designated as the queen of the mushrooms rich in various nutrients including polysaccharide, protein, mineral, vitamin, amino acids, riboflavin and nicotinic acid14,15. For the past few years, polysaccharide extracted from D. indusiata has attracted great attention because of a number of pharmacological functions. For instance, Deng et al. separated a polysaccharide from D. indusiata and investigated its molecular mechanism involved in the immunostimulatory activity on RAW264.7 cells. Results indicated that TLR4 is the major receptor responsible for the interaction of polysaccharide and macrophages14. A regenerated triple helical polysaccharide isolated from D. indusiata was reported to have effects on inhibiting tumor growth in vivo and this antitumor activity maybe associated to immunostimulation16. However, few reports focused on the relationships between polysaccharides from D. indusiata and anti-hyperlipidemia activities. Many evidences demonstrated that high-fat emulsion could resoundingly induce hyperlipidemia in researches7.
In the experiment, we adopted aqueous alkali and enzyme solution to separate two kinds of polysaccharides from D. indusiata fruiting bodies. Meanwhile, the structures of alkali- and enzyme-extractable Dictyophora indusiata polysaccharides (Al-DPS and En-DPS) were preliminarily explored, and the animal experiment was performed to investigate the hypolipidemic and hepatoprotective effect of polysaccharides subsequently.
Results
Acute toxicity analysis
The mice treated with Al-DPS or En-DPS did not exhibit any gross behavioral changes, toxic responses or deaths even at a dose of 1200 mg/kg during the feeding period in comparison with control group. Besides, there was no significant difference in the body weights, biochemical analysis (ALT and AST), hepatosomatic index and histopathological observation of liver between dosage group and control group (Fig. 1). In addition, general morphological of liver was normal. The above results manifested that Al-DPS and En-DPS were practically non-toxic substances.
Effects on body weight and HI
In present work, the yield of Al-DPS and En-DPS were 4.85 ± 0.18% and 4.01 ± 0.22%, respectively. As shown in Table 1, there was no significant difference in initial body weight among all the groups. After 33 days, the body weight in MC group was notably higher than that in NC group due to high-fat emulsion. However, all the polysaccharide groups and PC group showed lower weight compared with MC group, indicating Al-DPS, En-DPS and simvastatin had potential contributions in reducing the gain of weight. As for HI, it was higher in MC group than all the other groups, whereas obvious decline was observed after the supplement of two polysaccharides, especially En-DPS.
Effects on serum lipid levels
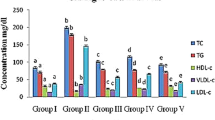
According to Fig. 2, TC, TG, LDL-C and AI levels were higher and the HDL-C level was lower in MC group than those in the NC group, which showed that the hyperlipidemic model of mice was successful. After administration of Al-DPS or En-DPS at a high dose of 400 mg/kg/d, the levels of TC, TG, LDL-C and AI were decreased to 2.67 ± 0.07 mmol/L, 1.65 ± 0.04 mmol/L, 1.09 ± 0.04 mmol/L and 0.46 ± 0.02 as well as 2.44 ± 0.05 mmol/L, 1.41 ± 0.06 mmol/L, 0.89 ± 0.02 mmol/L and 0.36 ± 0.03, which were all lower than that in the MC group. At the same time, the HDL-C in serum was increased by 81.48 ± 0.26% or 103.70 ± 0.14% after treatment with Al-DPS or En-DPS at high dosage compared with the model control group.
Effects on hepatic lipid levels
Hepatic lipid levels including TC, TG and NEFA increased visibly in the MC group when compared with that in the NC group, which were the indications of hepatocyte damages (Fig. 3). Significant decreases of hepatic lipid levels in the dosage groups whether 400 or 200 mg/kg/d appeared in comparison with those of the model group, testifying Al-DPS and En-DPS could fight against liver injury.
Effects on blood glucose level
As demonstrated in Fig. 4A, the blood sugar rose rapidly and peaked at 30 min. As time went on, it declined gradually. The blood glucose level of MC group was higher than NC group, which made clear that the ability to sense and inhibit the rise of blood sugar was reduced in hyperlipidemic mice. Meanwhile, the blood glucose of polysaccharide groups was lower when compared with MC group, suggesting that Al-DPS and En-DPS were able to inhibit the increase of blood glucose, especially in the high dose group.
Effects on the contents of INS, LEP and ADPN
In this study, the levels of INS, LEP and ADPN were also examined (Fig. 4B–D). High-fat emulsion resulted in the decrease in ADPN level and the increases in INS and LEP in serum. It was remarkable that the addition of Al-DPS and En-DPS with two dosages alleviated the situation effectively. In addition, the mice treated with En-DPS at 400 mg/kg/d worked best when compared with the mice in MC group.
Effects on serum enzyme activities and TBIL level
In order to analyze the liver protection effect of Al-DPS and En-DPS on hyperlipidemia, we measured the serum enzyme activities (ALT, AST, ALP, LDH and CK) and TBIL level, and the results were displayed in Fig. 5. When compared with NC group, the activities of serum enzymes and the content of TBIL were enhancive observably in hyperlipidemic mice (MC group) and this was a sign of liver damage. Thankfully, significant reductions of above indices were observed. Especially in the high-dose group of En-DPS, the values of ALT, AST, ALP, LDH, CK and TBIL were decreased by 45.87 ± 1.02%, 36.46 ± 1.99%, 44.25 ± 2.11%, 43.70 ± 3.24%, 53.66 ± 3.12% and 39.43 ± 1.04% compared with those of the MC group, implying that En-DPS had prominent protection effects on the hepatic damages induced by hyperlipidemia.
Effects on hepatic antioxidant activities
Antioxidant activities of liver in different groups were showed in Fig. 6. From the figure, decreased antioxidant enzyme activities (SOD, GSH-Px and CAT), reduced non-enzymatic antioxidant capacity (T-AOC), as well as increased lipid product contents (MDA and LPO) were observed in hyperlipidemic mice in comparison with that in the NC group, indicating that serious oxidative stress occurred. Interestingly, these pathological changes could be attenuated by supplementation of Al-DPS or En-DPS.
After En-DPS administration (400 mg/kg/d), the activities of SOD, GSH-Px, CAT and T-AOC (Fig. 6A–D) reached 122.37 ± 4.56 U/mg prot, 106.76 ± 3.67 U/mg prot, 79.63 ± 2.94 U/mg prot and 11.43 ± 0.45 U/mg prot, which were 49.85 ± 2.12%, 68.36 ± 3.23%, 93.23 ± 2.22% and 55.72 ± 3.19% higher than those of MC group, while these activities were increased by 34.33 ± 3.05%, 56.52 ± 4.32%, 68.62 ± 2.36% and 36.78 ± 4.23%, respectively, when compared with the MC group after Al-DPS administration (400 mg/kg/d). The contents of lipid peroxides including MDA and LPO (Fig. 6E,F) were reduced by 44.06 ± 0.31% and 39.55 ± 0.41% in En-DPS-treated mice at 400 mg/kg/d as well as 39.16 ± 0.35% and 26.76 ± 0.22% in Al-DPS-treated mice at 400 mg/kg/d compared with MC group. The results indicated that both Al-DPS and En-DPS successfully suppressed hepatic oxidative damage.
Liver histopathological observation
In the current work, the histopathological observations of liver were performed by H&E staining (Fig. 7). The hyperlipidemic mice in MC group showed serious liver damages characterized by loss of cell borders, cell swelling and accumulations of fat differing from typical hepatic cells in NC group with regular hepatocyte morphology, well-defined cell borders and distinct hepatic nucleus. Nevertheless, Al-DPS and En-DPS showed potential effects on preventing liver damages on the basis of Fig. 7D–G. Moreover, the En-DPS recovered morphological structure and degeneration of hepatocytes better than Al-DPS at same dosage.
Chemical characterizations
The molecular weights of Al-DPS and En-DPS were analyzed by HPGPC and the results were showed in Table 2. It can be seen that the average molecular weights (Mw) of Al-DPS and En-DPS were 5.69 × 105 and 3.82 × 105 Da, respectively. Furthermore, the results of HPLC revealed that Al-DPS was made up of nine kinds of monosaccharides and glucose was the most abundant. En-DPS which contained ten kinds of monosaccharides was mainly composed of galactose. Compared with Al-DPS, a little of galacturonic acid was found in the En-DPS. The detailed percentage compositions of monosaccharides were shown in Table 2.
FT-IR spectroscopy is typically used for the qualitative measurement of organic functional groups and investigating the vibrations of molecules and polar bonds between the different atoms. The FT-IR spectrum of Al-DPS and En-DPS were presented in Fig. 8A,B. A strong and broad absorption peak at approximately 3400 cm−1 for O-H stretching vibrations was observed17. The peak at about 2930 cm−1 was ascribed to C-H stretching vibrations18. Moreover, the strong extensive absorption in the region of 900–1200 cm−1 for coupled C-O and C-C stretching and C-OH bending vibrations in the Al-DPS and En-DPS indicated the characteristic absorptions of polysaccharides19. There was a band at 1400 cm−1 due to C=O (-COOH) stretching vibration20. The presence of absorption band at 880 cm−1 in Al-DPS and 857 cm−1 in En-DPS manifested that Al-DPS and En-DPS were respectively typical polysaccharides linked by β- and α-type glycosidic bonds21.
In the 1H and 13C NMR spectrum of Al-DPS (Fig. 8C,D), dense signals distributed in the range of 3.0–5.0 ppm and 60–110 ppm indicating the representative peaks of carbohydrate22. The chemical shifts from 160 to 180 ppm in the 13C NMR spectrum of Al-DPS and En-DPS were attributed to uronic acids, which identified with the results of HPLC23. The signals found in the anomeric carbon region at δ 97.86 and 98.21 in the 13C NMR spectrum of En-DPS (Fig. 8F) confirmed the glucosyl linkage was α form. Furthermore, the anomeric signals appeared at 5.01, 5.02, 5.05 and 5.28 ppm (Fig. 8E) further indicated that the existence of α-glycosidic residues in En-DPS, which was consistent with those of the previous FT-IR analysis23,24. There was no signals at 80–90 ppm in the 1H NMR spectrum of Al-DPS, indicating that Al-DPS did not contain a 1 → 3 glucosidic bond. In the 1H NMR spectrum of Al-DPS and En-DPS, the signals observed at 1.22 ppm and 1.25 ppm respectively were assigned to the methyl protons of fucose25.
Discussion
For a long time, mushrooms have always been treasured and appreciated owing to their unique taste and flavor. Besides, many researchers have documented that polysaccharides isolated from edible mushrooms were corroborated to possess various physiological activities that are beneficial to people’s health. Hence, the usage of mushrooms has expanded up to a wider extent not only as food but also as pharmaceuticals and nutraceuticals26. Polysaccharides extracted from D. indusiata, which is a precious edible mushroom well-accepted by consumers in China and other Asian countries, exhibited many bioactivities, including antioxidant, hepatoprotective, immunomodulatory, anti-tumor and hypolipidemic effects16,27. However, there were few reports on the antihyperlipidemic and hepatoprotective properties of alkali and enzyme extractable polysaccharides by D. indusiata until now and this was the reason why we carried on the research.
It was known that hyperlipidemia is a systemic disease, which is mainly characterized by dyslipidemia in serum1,28. An elevated level of TC, TG, LDL-C, AI and reduced level of HDL-C are major factors for the development of many lipid-related diseases such as atherosclerosis, cardiovascular disease and obesity29. Excess LDL-C, the major transporter of TC, can gather in the blood and is easily oxidized, thus induces the occurrence of oxidative damage. Conversely, HDL-C can prevent from coronary heart disease and atherosclerosis30. In addition, AI is also an important criterion to examine dyslipidemia. In the study, after the replenishment of high-fat emulsion for 33-days, the levels of TC, TG and LDL-C in the serum and AI of MC group were higher than NC group. Simultaneously, HDL-C level was decreased in mice of model control group as compared to normal control group as mentioned. The results proved that hyperlipidemic mice model induced by high-fat emulsion was successful. Nevertheless, polysaccharide administration almost returned the serum lipids to normal status, suggesting that Al-DPS and En-DPS had the potential to improve dyslipidaemia against hyperlipidemia by entering the blood circulation. Reports have shown that the decreases of LDL-C were possibly in connection with the enhancement of LDL-C catabolism through hepatic receptors. And the restoration of the catabolic metabolism of TG may be due to an increased stimulation of the lipolytic activity of plasma lipoprotein lipase (LPL)31. Similar conclusions were acquired in many previous reports7,32.
Reactive oxygen species (ROS), one kind of prooxidants, are highly reactive O2 metabolites. Documented literature indicated that oxidative stress which is defined as an imbalance between prooxidants and antioxidants, plays a critical role in the pathogenesis of more than 100 human diseases. Under normal physiological conditions, the elimination of ROS and the balance between generation and removal of ROS are performed by intracellular antioxidants to resist the oxidative damages. Whereas when the balance between generation and removal of ROS is broken, excessive accumulation of ROS will cause oxidative stress and injuries of tissues and organs19,33. MDA and LPO, as superfluous lipid intermediates, are capable to disturb the antioxidant defence and provide demonstrations for the pathogenic roles of oxidative stress20. The antioxidant enzymes such as SOD, GSH-Px and CAT, could reflect the production of free radicals and defense against the formation of ROS under the oxidative stress34. At the same time, T-AOC activity could reflect and represent the capacity of the non-enzymatic antioxidant defense system in the organs11. In the present work, increased activities of SOD, CAT, GSH-Px and T-AOC as well as decreased contents of MDA and LPO in the mice treated with polysaccharides compared with that in the MC groups showed that either Al-DPS or En-DPS as novel bioactive compounds could tend antioxidative system in liver to be normalized, thus treating hyperlipidaemia.
It was well known that hyperlipidaemia, obesity and hyperglycemia have indispensable relationships. Glucose tolerance test indicated that the hyperlipidemic mice exhibited impaired glucose tolerance. The abnormal changes of INS content which is a hormone known to stable blood glucose levels also verified the results of glucose tolerance test. LEP produced by white adipocytes could regulate energy homeostasis and appetite35, and ADPN is known related to glucose metabolism, fatty acid oxidation as well as insulin sensitivity36. In the experiment, we found that hyperlipidemia also had an impact on the concentrations of LEP and ADPN.
It was well known that some enzymatic activities in serum have long been considered as biochemical and sensitive indicators to assess hepatic injury37. Increased activities of ALT, AST, ALP, LDH and CK in serum after the gavage of high-fat emulsion (MC group) compared with those of normal mice indicated hepatocytes were damaged and their transport function and membrane permeability were also altered, leading to the leakage of enzymes from the cells consequently38. However, a noteworthy inhibition of the activities of serum enzyme was observed after the addition of polysaccharides, suggesting that Al-DPS and En-DPS had the ability to stabilize plasma membrane, thereby recovering the impaired hepatic cells.
It was reported that high-fat diet could accelerate the formation of radicals in vivo and destroy the intrinsic antioxidant defense, thus induces oxidative stress. Excess free radicals react with unsaturated fatty acids, leading to the generation of lipid intermediates and the alteration of cellular membranes integrity20. Subsequently, lipid intermediates can further disturb the antioxidant defense in liver. The damaged permeability of cell membrane results in that ALT, AST and ALP could be leached out from hepatocytes into blood circulation11. Polysaccharides, as a natural antioxidant supplementation, could recover the balance between generation and removal of ROS and reduce the level of oxidative stress. Therefore, the elevated levels of ALT, AST, ALP and so on in serum could be reduced. On the other hand, the intake of high-fat emulsion increases the levels of total cholesterol and causes the disordered circulation of lipid metabolism in the blood11. Documented literatures have shown that the lipid-lowering effects may be related to the inhibition of cholesterol biosynthesis and increased fecal bile acid excretion31.
Moreover, haematoxylin-eosin staining was implemented to assess the injury of liver visually38. In comparison with hepatic cellular architecture of mice from the NC group, severe liver damages had occurred after injection of high-fat emulsion. As expected, the hepatic lesions were markedly ameliorated by pretreatment with polysaccharides. The results were in good agreement with the results of biochemical analysis in serum. It was well-known that the bioactive properties of polysaccharides were closely related to their structural characterizations. The En-DPS exhibited the stronger biological effect than Al-DPS may be associated with its molecular weight and configurations20.
Conclusions
According to the current work, Al-DPS and En-DPS exhibited protective action against hyperlipidemia induced by high-fat emulsion as evidenced by reducing lipid profile and mitigating oxidative stress in liver, which was confirmed by histopathologic observation. The results demonstrated that Al-DPS and En-DPS played momentous roles in the prevention and treatment of hyperlipidemia related liver impairments.
Materials and Methods
Materials and reagents
The dried fruiting bodies of D. indusiata used in this experiment were provided by Taian Academy of Agricultural Sciences (Taian, China). The diagnostic kits for analyzing the activities of glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), catalase (CAT) and total antioxidant capacity (T-AOC), the levels of total cholesterol (TC), triglycerides (TG) and nonestesterified fatty acid (NEFA) as well as the contents of lipid peroxide (LPO), malondialdehyde (MDA) were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Besides, the insulin (INS), leptin (LEP) and adiponectin (ADPN) contents were measured using commercial enzyme-linked immunosorbent assay (ELISA) kits from Jiangsu Meibiao Biological Technology Company Limited (Jiangsu, China). All the other reagents used in the work were of analytical grade and supplied by local chemical suppliers.
Preparation of Al-DPS and En-DPS
Two kinds of polysaccharide samples were prepared on the basis of methods reported previously with slight modifications39. To obtain Al-DPS or En-DPS, the powders of D. indusiata fruiting bodies pulverized by a disintegrator were extracted with NaOH solution (0.5 mol/L, 1:10, w/v) at 85 °C for 5 h or snailase solution (4%, 1:4, w/v) at 38 °C for 4 h, respectively. The extraction steps above were repeated three times and supernatants centrifuged at 3000 r/min for 10 min were mixed with four-fold volumes of 95% ethanol (v/v) and kept overnight at 4 °C. The precipitate separated by centrifugation was deproteinated by employing the Sevag method40. Finally, after being dialyzed and freeze-dried (Labconco, USA), Al-DPS and En-DPS were prepared successfully and used for further experiments. The percentage of polysaccharides yield was calculated according to the following formula41.
where W1 was the weights of Al-DPS or En-DPS (g) and W0 was powders sample weights (g).
Acute toxicity study
According to a modified method previously described by reports, acute toxicity experiment was performed42,43. Forty-two male Kunming strain mice were divided into seven groups at random including one control group and six dosage groups. After fasted overnight, the mice in dosage groups were gavaged with Al-DPS or En-DPS at the dose of 800, 1000 and 1200 mg/kg respectively and control group received isometric normal saline solution. Normal feed and free access to drinking water were given to all the mice. After dosing, the mice were kept continuously observations for gross behavioral changes, toxic symptoms and mortality for 14 days (with special attention given during the first 24 h). At the end of the experiment, all the mice were weighted and sacrificed by euthanasia. The alamine aminotransferase (ALT) and aspertate aminotransferase (AST) of serum were measured. The liver was quickly excised, weighed and macroscopically examined. Moreover, haematoxylin-eosin (H&E) staining of liver was performed.
Animal experiments
The high-fat emulsion was prepared according to a reported method34. Lard oil (25 g) heated to 100 °C was mixed thoroughly with 10 g cholesterol, 1 g methylthiouracil and 25 mL of Tween-80, which was oil phase. Simultaneously, the water phase contained 30 mL distilled water, 20 mL propylene glycol and 2 g sodium deoxycholate. In the end, the high-fat emulsion was finished after the mix of oil phase and water phase before animal administration.
Seventy Kunming strain mice (20 ± 2 g, male) were purchased from Taibang Biological Products Ltd. Co. (Taian, China) and maintained at an animal room with controlled temperature (25 ± 2 °C), humidity (55 ± 5%) and a 12 h light/dark cycle with free access to water and standard food. All experiments were performed in accordance with the Regulations of Experimental Animal Administration issued by the State Committee of Science and Technology of the People’s Republic of China.
After one-week acclimatization period, all mice were randomly distributed into seven groups with ten mice in each group containing two Al-DPS groups (400, 200 mg/kg/d), two En-DPS groups (400, 200 mg/kg/d), as well as three control groups including normal control group (NC), model control group (MC), and positive control group (PC). To induce hyperlipidemia, all the mice were administered with gastric injection of high-fat emulsion every day except NC group which received isometric saline solution. Subsequently, the mice were gavaged with different dosage polysaccharides, NC and MC groups with isometric saline solution and PC group with simvastatin (200 mg/kg/d).
After a 33-consecutive gavage, all mice were weighed and sacrificed under anaesthetic treatment after overnight fasting following the last administration. Then serum sample was separated from the blood through centrifugation (6000 r/min, 10 min). The levels of TC, TG, low density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C) as well as the activities of ALT, AST, alkaline phosphatase (ALP), lactate dehydrogenase (LDH), creatine kinase (CK) were analyzed using an automated biochemical analyzer (Shenzhen, China).
The tissue of liver was immediately excised, washed with ice-cold saline, weighed and homogenized (1:9, g/mL) in phosphate buffer solutions (PBS, 0.2 mol/L, pH 7.4). The homogenates were centrifuged (4000 r/min) at 4 °C for 10 min and the supernatants were collected for further biochemical analysis. Hepatosomatic index (HI) and atherogenic index (AI) were calculated according to the following formulas44.
In addition, the fresh liver was washed with normal saline instantaneously, fixed in 4% formalin and embedded in paraffin. Subsequently, the tissue was cut into 5 μm slices for H&E staining45. In the end, all the sections were observed under a microscope (400 × magnification) for histological analyses.
Glucose tolerance test
Before sacrifice, we tested blood glucose level from mice tail vein after fasting for 12 h by a glucometer and set it as the blood sugar at time 0 min. Subsequently, the mice were given glucose solution of 1.5 g/kg through intraperitoneal injection and blood glucose was detected at 30, 60, 90, and 120 min, respectively. Ultimately, we drew the curve of blood glucose with time according to the records.
Structural characterization of Al-DPS and En-DPS
Molecular weight determination
Briefly, the molecular weight of Al-DPS and En-DPS were measured by high performance gel permeation chromatography (HPGPC) that was operated with a HPLC system (Agilent 1260, Agilent Technologies, CA, USA) equipped with a Shodex SB-806HQ column (8 mm × 300 mm, Showa Denko K.K., Tokyo, Japan) and a differential refractive index detector. A series of standard dextrans (Sigma) with different molecular weights were used to establish the calibration curve20.
Monosaccharide composition analysis
The monosaccharide compositions were determined by high performance liquid chromatography (HPLC, Ultimate 3000) equipped with Xtimate C18 column (4.6 × 200 mm, 5 um) at 30 °C with a flow rate of 1.0 mL/min. By the comparisons with standard sugars, the monosaccharide compositions of samples were assayed.
Fourier transform infrared spectroscopy (FT-IR) analysis
Two kinds of polysaccharides were mixed with potassium bromide (KBr) powder severally and pressed into pellets for the FT-IR spectral measurement with a wavenumber range of 4000–400 cm−1, which was performed through an infrared spectrometer (Nicolet 6700, Thermo Fisher Scientific, USA)46.
Nuclear magnetic resonance (NMR) spectroscopy analysis
After dissolved in D2O, the 1H and 13C NMR spectra of Al-DPS and En-DPS were recorded via a Bruker DRX-500 spectrometer at 25 °C.
Statistical analysis
The data were expressed as the mean ± standard deviations (S.D.) and significant differences between the experimental groups were determined by the one-way ANOVA followed by Duncan-test (SPSS 19.0 software package, USA). Meanwhile, P < 0.05 was considered to be statistically significant.
Compliance with ethical standards
The experiments were performed as approved by the Institutional Animal Care and Use Committee of Shandong Agricultural University, and in accordance with the Animals (Scientific Procedures) Act 1986 (amended 2013).
References
Nie, C. H. et al. Determination of quality markers of Xuezhiling tablet for hyperlipidemia treatment. Phytomedicine. 44, 231–238 (2018).
Hirsch, R. L., McKay, D. G., Travers, R. I. & Skraly, R. K. Hyperlipidemia, fatty liver, and bromsulfophthalein retention in rabbits injected intravenously with bacterial endotoxins. J Lipid Res. 5, 563–568 (1964).
Kong, X. J. et al. Effect of lipid lowering tablet on blood lipid in hyperlipidemia model rats. Saudi J Biol Sci. 25, 715–718 (2018).
Dixon, D. L., Donohoe, K. L., Ogbonna, K. C. & Barden, S. M. Current drug treatment of hyperlipidemia in older adults. Drug Aging. 32, 127–138 (2015).
Wang, X. M. et al. In vivo antihyperlipidemic and antioxidant activity of porphyran in hyperlipidemic mice. Carbohydr Polym. 174, 417–420 (2017).
Zhang, Q. et al. Novel functional polysaccharides from Radix Polygoni Multiflori water extracted residue: preliminary characterization and immunomodulatory activity. Carbohydr Polym. 137, 625–631 (2016).
Yang, Z. W. et al. Antihyperlipidemic and hepatoprotective activities of polysaccharide fraction from Cyclocarya paliurus in high-fat emulsion-induced hyperlipidaemic mice. Carbohydr Polym. 183, 11–20 (2018).
Yao, H. Q. et al. Efficacy and safety of Yinchenwuling powder for hyperlipidemia: a systematic review and Meta-analysis. J Tradit Chin Med. 36, 135–143 (2016).
Demyen, M., Alkhalloufi, K. & Pyrsopoulos, N. T. Lipid-lowering agents and hepatotoxicity. Clin Liver Dis. 17, 699–714 (2013).
Chen, L. & Huang, G. L. The antiviral activity of polysaccharides and their derivatives. Int J Biol Macromol. 115, 77–82 (2018).
Zhao, H. J. et al. The antihyperlipidemic activities of enzymatic and acidic intracellular polysaccharides by Termitomyces albuminosus. Carbohydr Polym. 151, 1227–1234 (2016).
Zhu, K. X. et al. A newly identified polysaccharide from Ganoderma atrum attenuates hyperglycemia and hyperlipidemia. Int J Biol Macromol. 57, 142–150 (2013).
Zhang, Y., Wang, Z. W., Jin, G., Yang, X. D. & Zhou, H. L. Regulating dyslipidemia effect of polysaccharides from Pleurotus ostreatus on fat-emulsion-induced hyperlipidemia rats. Int J Biol Macromol. 101, 107–116 (2017).
Deng, C. et al. Mechanism of the immunostimulatory activity by a polysaccharide from Dictyophora indusiata. Int J Biol Macromol. 91, 752–759 (2016).
Zhang, J. et al. Antioxidant and neuroprotective effects of Dictyophora indusiata polysaccharide in Caenorhabditis elegans. J Ethnopharmacol. 192, 413–422 (2016).
Deng, C. et al. Anti-tumor activity of the regenerated triple-helical polysaccharide from Dictyophora indusiata. Int J Biol Macromol. 61, 453–458 (2013).
Fan, J. L. et al. Characterization, antioxidant and hepatoprotective activities of polysaccharides from Ilex latifolia Thunb. Carbohydr Polym. 101, 990–997 (2014).
Li, X., Zhao, L., Zhang, Q. H., Xiong, Q. P. & Jiang, C. X. Purification, characterization and bioactivity of polysaccharides from Glossaulax didyma. Carbohydr Polym. 102, 912–919 (2014).
Jiang, C. X. et al. Preliminary characterization and potential hepatoprotective effect of polysaccharides from Cipangopaludina chinensis. Food Chem Toxicol. 59, 18–25 (2013).
Song, X. L. et al. Antioxidative and hepatoprotective effects of enzymatic and acidic-hydrolysis of Pleurotus geesteranus mycelium polysaccharides on alcoholic liver diseases. Carbohyd Polym. 201, 75–86 (2018).
Jiang, C. X. et al. Antioxidant activity and potential hepatoprotective effect of polysaccharides from Cyclina sinensis. Carbohydr Polym. 91, 262–268 (2013).
Wu, Y., Cui, W., Eskin, N. A. M., Goff, H. D. & Nikiforuk, J. NMR analysis of a methylated non-pectic polysaccharide from water soluble yellow mustard mucilage. Carbohyd Polym. 84, 69–75 (2011).
Gao, Z. et al. Characteristic anti-inflammatory and antioxidative effects of enzymatic- and acidic- hydrolysed mycelium polysaccharides by Oudemansiella radicata on LPS-induced lung injury. Carbohyd Polym. 204, 142–151 (2019).
Li, B. et al. The core structure characterization and of ginseng neutral polysaccharide with the immune-enhancing activity. Int J Biol Macromol. 123, 713–722 (2019).
Wang, H. X. et al. Structural investigation of a uronic acid-containing polysaccharide from abalone by graded acid hydrolysis followed by PMP-HPLC–MSn and NMR analysis. Carbohyd Res. 402, 95–101 (2015).
Singdevsachan, S. K. et al. Mushroom polysaccharides as potential prebiotics with their antitumor and immunomodulating properties: A review. Bioact Carbohydr Diet Fib. 7, 1–14 (2016).
Wang, Y. X., Shi, X. D., Yin, J. Y. & Nie, S. P. Bioactive polysaccharide from edible Dictyophora spp.: Extraction, purification, structural features and bioactivities. Bioact Carbohydr Diet Fib. 14, 25–32 (2018).
Irie, K. et al. Hyperlipidemia is involved in apoptosis in rat submandibular glands. Arch Oral Biol. 81, 136–140 (2017).
Hassan, S., El-Twab, S. A., Hetta, M. & Mahmoud, B. Improvement of lipid profile and antioxidant of hypercholesterolemic albino rats by polysaccharides extracted from the green alga Ulva lactuca Linnaeus. Saudi J Biol Sci. 18, 333–340 (2011).
Surhio, M. M. et al. Antihyperlipidemic and hepatoprotective properties of selenium modified polysaccharide from Lachnum sp. Int J Biol Macromol. 99, 88–95 (2017).
Irudayaraj, S. S., Sunil, C., Duraipandiyan, V. & Ignacimuthu, S. In vitro antioxidant and antihyperlipidemic activities of Toddalia asiatica (L) Lam. Leaves in Triton WR-1339 and high fat diet induced hyperlipidemic rats. Food Chem Toxicol. 60, 135–140 (2013).
Hu, Y. B. et al. Hypolipidemic study of xylanase-modified corn bran fibre in rats. Food Chem. 123, 563–567 (2010).
Liang, D. et al. Studies on the antioxidant and hepatoprotective activities of polysaccharides from Talinum triangulare. J Ethnopharmacol. 136, 316–321 (2011).
Zhang, C. et al. Antihyperlipidaemic and hepatoprotective activities of acidic and enzymatic hydrolysis exopolysaccharides from Pleurotus eryngii SI-04. BMC Complem Altern Med. 17, 403 (2017).
Sayed, S. E. et al. Evaluation of leptin and MMP2 genes methylation in childhood obesity. Gene Rep. 11, 79–86 (2018).
Hsueh, Y. M. et al. Adiponectin gene polymorphisms and obesity increase the susceptibility to arsenic-related renal cell carcinoma. Toxicol Appl Pharmacol. 350, 11–20 (2018).
Lu, X. S., Zhao, Y., Sun, Y. F., Yang, S. & Yang, X. B. Characterisation of polysaccharides from green tea of Huangshan Maofeng with antioxidant and hepatoprotective effects. Food Chem. 141, 3415–3423 (2013).
Yang, X. B., Yang, S., Guo, Y. R., Jiao, Y. D. & Zhao, Y. Compositional characterisation of soluble apple polysaccharides, and their antioxidant and hepatoprotective effects on acute CCl4 -caused liver damage in mice. Food Chem. 138, 1256–1264 (2013).
Lin, L. et al. Antioxidative and renoprotective effects of residue polysaccharides from Flammulina velutipes. Carbohydr Polym. 146, 388–395 (2016).
Miao, S. S. et al. Antitumor activity of polysaccharides from Lepista sordida against laryngocarcinoma in vitro and in vivo. Int J Biol Macromol. 60, 235–240 (2013).
Deng, Q. F., Zhou, X. & Chen, H. G. Optimization of enzyme assisted extraction of Fructus Mori polysaccharides and its activities on antioxidant and alcohol dehydrogenase. Carbohydr Polym. 111, 775–782 (2014).
Hor, S. Y. et al. Acute and subchronic oral toxicity of Coriolus versicolor standardized water extract in Sprague-Dawley rats. J Ethnopharmacol. 137, 1067–1076 (2011).
Cui, J. F. et al. Characterization and hypoglycemic activity of a rhamnan-type sulfated polysaccharide derivative. Mar Drugs. 17, 21 (2019).
Qiu, T., Ma, X. J., Ye, M., Yuan, R. Y. & Wu, Y. N. Purification, structure, lipid lowering and liver protecting effects of polysaccharide from Lachnum YM281. Carbohydr Polym. 98, 922–930 (2013).
Chang, Y. Y. et al. Hepatoprotection of noni juice against chronic alcohol consumption: lipid homeostasis, antioxidation, alcohol clearance, and anti-inflammation. J Agr Food Chem. 61, 11016–11024 (2013).
Xu, Y. Q., Zhang, L., Yang, Y., Song, X. M. & Yu, Z. Y. Optimization of ultrasound-assisted compound enzymatic extraction and characterization of polysaccharides from blackcurrant. Carbohydr Polym. 117, 895–902 (2015).
Acknowledgements
This work was supported by grants from Mushroom Technology System of Shandong Province (SDAIT-07-05).
Author information
Authors and Affiliations
Contributions
Wenshuai Wang, Le Jia and Zhen Song designed the research. Wenshuai Wang and Jianjun Zhang analyzed data. Wenshuai Wang, Jianjun Zhang and Yiwen Zhang performed research. Wenshuai Wang, Yiwen Zhang, Yanbo Feng and Fangfang Yuan prepared figures and tables. Wenshuai Wang wrote the manuscript. Yiwen Zhang, Xinling Song, Honghong Liu and Zheng Gao contributed to the improvements of the English language. Honghong Liu, Zhen Song and Le Jia provided the funds for the whole experiments. All authors were involved in checked the paper and contributed to the preparation of the final manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, W., Liu, H., Zhang, Y. et al. Antihyperlipidemic and hepatoprotective properties of alkali- and enzyme-extractable polysaccharides by Dictyophora indusiata. Sci Rep 9, 14266 (2019). https://doi.org/10.1038/s41598-019-50717-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-50717-9
This article is cited by
-
Increasing the production of the bioactive compounds in medicinal mushrooms: an omics perspective
Microbial Cell Factories (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.