Abstract
Mycoplasma hominis is an opportunistic human pathogen associated with genital and neonatal infections. Until this study, the lack of a reliable transformation method for the genetic manipulation of M. hominis hindered the investigation of the pathogenicity and the peculiar arginine-based metabolism of this bacterium. A genomic analysis of 20 different M. hominis strains revealed a number of putative restriction-modification systems in this species. Despite the presence of these systems, a reproducible polyethylene glycol (PEG)-mediated transformation protocol was successfully developed in this study for three different strains: two clinical isolates and the M132 reference strain. Transformants were generated by transposon mutagenesis with an efficiency of approximately 10−9 transformants/cell/µg plasmid and were shown to carry single or multiple mini-transposons randomly inserted within their genomes. One M132-mutant was observed to carry a single-copy transposon inserted within the gene encoding P75, a protein potentially involved in adhesion. However, no difference in adhesion was observed in cell-assays between this mutant and the M132 parent strain. Whole genome sequencing of mutants carrying multiple copies of the transposon further revealed the occurrence of genomic rearrangements. Overall, this is the first time that genetically modified strains of M. hominis have been obtained by random mutagenesis using a mini-transposon conferring resistance to tetracycline.
Similar content being viewed by others
Introduction
Mycoplasma hominis is an opportunistic pathogen that is a common commensal bacterium of the human female genital tract with the ability to cause genital and neo-natal infections and systemic infections in immunocompromised patients1. This pathogen is frequently associated with Trichomonas vaginalis and can influence gene expression in this parasite2. The genome of the reference strain M. hominis PG21 has been sequenced (665 kb, 27.1% GC)3 and is the second smallest genome known to be capable of sustaining life after that of M. genitalium4. An analysis of the genome sequence of this member of the class Mollicutes revealed that its ability to produce energy depends on the hydrolysis of arginine, which is in contrast to the glucose or urea used by most bacteria colonizing the same urogenital niche.
Our understanding of the metabolism and mechanisms of infection of this minimal organism remains severely limited, primarily because of the lack of genetic manipulation tools. A transformation method for this bacterium based on electroporation was described in 20005. However, until the current study, the procedure could not be repeated. Another study describing the transfer of the Tn916 transposon from Streptococcus faecalis to M. hominis has been reported6, but these results could not be reproduced either. More recently, a technique combining random chemical mutagenesis and high-throughput screening for nucleotide polymorphisms has been used in M. hominis PG217. This reverse genetic method, called TILLING, for Targeting-Induced Local Lesions IN Genomes, allowed the first M. hominis PG21 mutants to be produced. Although this achievement paved the way for the development of functional genomics in M. hominis, the process remains rather fastidious for routine application and may generate multiple undesired mutations across the genome7.
A synthetic biology approach is now commonly used for mycoplasma species belonging to the mycoides cluster8. In this procedure, the mycoplasma genome is first isolated from a bacterial cell and is subsequently cloned into the yeast Saccharomyces cerevisiae, where it can be modified at large scale using the genetic tools available for this host, such as the tandem repeat coupled with endonuclease cleavage (TREC) or the CRISPR/Cas9 systems9,10. Next, the highly modified genome is transplanted into a recipient cell of the same species or a phylogenetically proximate species11 to generate the cognate mutants. This technique is currently under development for M. hominis, but the last transplantation step is yet to be successfully achieved12. As with any transformation procedure13, the restriction-modification (R-M) barrier must be taken into consideration in the genome transplantation process14. Indeed, incorrectly methylated genomes could be immediately hydrolyzed by the restriction enzymes present in the recipient cells15.
Over the past decades, efforts have been made to develop protocols for DNA transfer in M. hominis with no conclusive results. However, such procedures were successfully generated for both M. arthritidis, the closest relative of M. hominis16, and for Ureaplasma parvum, a human pathogen known to be difficult to transform, that shares the same ecological niche as M. hominis13. These results encouraged us to pursue the development of a transformation procedure for M. hominis. In this study, we report a reproducible polyethylene-glycol (PEG)-based transformation protocol for M. hominis using plasmids carrying a transposon.
Results
Restriction-modification system analyses
Many restriction-modification systems (R-M systems) have been described in the genomes of mollicutes species (Supplementary Fig. S1), with several having been functionally characterized8,16,17,18. These systems provide immunity against foreign DNA and may therefore constitute an important barrier to both natural and artificial bacterial transformations14,19. Thus, to facilitate the development of a transformation procedure for M. hominis, we sought a strain containing the fewest of these defense mechanisms.
Twenty sequenced M. hominis genomes (13 from clinical strains described in this study, six already published3,20,21,22, and the M132 reference strain) were analyzed and compared using Molligen and Rebase databases23,24. Many putative R-M systems were identified (Table 1), with each strain studied containing between two and six complete R-M systems. Interestingly, two putative R-M systems were shared by all strains, the type I EcoR124II-like system and a type III system. The type II Sau96I-like system is present in 19 out 20 strains, but 7 seem to harbor an incomplete system. Other predicted systems were rather diverse in terms of their type and specificity (three different type I and seven different type II systems), and/or copy numbers (four copies of an incomplete type II DpnII-like system were predicted in the strain 3364) (Table 1). R-M systems were considered to be incomplete if one or more subunits were missing.
This analysis suggested a great diversity of R-M systems in the M. hominis lineage. Since no strain had a very low number or no R-M systems, we decided to pursue further investigations with the strains available in the laboratory: two reference strains (PG21 and M132) and the 13 clinical isolates (referred to as 35, 331, 2674, 3299, 3364, 3631, 4016, 4235, 4788, 4796, 5012, 5060, and 5096).
Development of the M. hominis PEG-mediated transformation protocol
The PEG-mediated transformation protocol for M. hominis was developed based on the previously developed transformation protocols for M. arthritidis16 and U. parvum13.
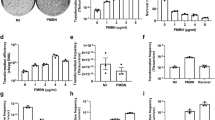
Many different transformation conditions were tested using the plasmid pMT85-Tet25 to generate genetically modified M. hominis mutants. The first assays were performed using the reference strain PG21, but no transformants were obtained. Subsequently, we attempted a series of experiments using the M. hominis reference strain M132. Two transformants were produced using a preliminary protocol, after which efforts were made to improve the reproducibility and efficiency of the protocol. In particular, we optimized conditions for M. hominis cell growth, the wash buffer composition, the methylation of the plasmid to be introduced and the PEG concentration, molecular weight and time of contact with the cells. Close to 150 variations of the protocols were tested, and the primary parameters that were adjusted are summarized in Fig. 1.
The impact of M. hominis M132 growth phase on the transformation protocol was evaluated with respect to its growth over time. Three culture phases (early, mid-log and late) were defined and used separately or in combination for transformation assays (Supplementary Fig. S2). The best efficiencies were obtained using a mix of the three growth phases compared to a particular growth phase.
Different wash buffers, which were also used to prepare the PEG solution, were tested during the protocol optimization. We tested PBS and Tris-sucrose (pH 6.5), two buffers commonly used for mycoplasma transformations, the eukaryotic cell culture buffer HBSS, and the T-Buffer used to transform M. arthritidis. While no transformants were generated using the Tris-sucrose (pH 6.5) and HBSS buffers, some transformants were obtained using PBS and T Buffer (Tris 10 mM, pH 6.5). However, the results obtained using PBS buffer were not reproducible.
Another important parameter considered was the methylation of the plasmid pMT85-Tet, because, as previously mentioned, R-M systems may have significant impact on transformation efficiency. The attempted transformation of M. hominis using unmethylated plasmid yielded no colonies. To protect the plasmid against restriction endonucleases and develop a protocol that could be broadly applied, we attempted to identify enzymes that could methylate the plasmid DNA at multiple sites. We tested two different commercial methyltransferases, the methyltransferase SssI, which methylates CpG islets, and the methyltransferase CviPI, which methylates GpC islets. In our experiments, transformants were only obtained using plasmid that had been methylated with the CpG methyltransferase. The methylation of pMT85-Tet with both the SssI and CviPI methyltransferases did not increase the transformation efficiency. Here, we did not address the adenine methylation issue mainly because we noticed that (1) most of these systems are either incomplete or absent in the strains of interest (Tables 1 and S1) and (2) some systems (as the GATC restriction-modification system) are present in the DH5α E. coli strain used to propagate the plasmid. This means that methyl groups are added to the adenine of the sequence 5′-GATC-3′ by the E. coli dam methylase prior transformation. Fully protected before the entry of the plasmid in M. hominis cells, the plasmid cannot be cleaved at those specific sites by the M. hominis cognate restriction enzyme.
With respect to PEG-mediated protocol, the initial transformation assays were performed using 40 to 50% PEG 8000, a concentration range that is commonly used for M. arthritidis and U. parvum. Assays using PEG of different molecular weights and concentrations were also tested. We observed that the concentration of PEG used was crucial to the success of the transformation. Transformations using low concentrations of PEG (10, 20, and 30%) were unsuccessful, while those using high concentrations (40, 50, and 60%) yielded a total of 24 transformants on selective media. The best efficiency was observed using 60% PEG, but the results were not reproducible. Only 40% PEG based protocol gave reproducible results (Table 2). Finally, different times of contact between cells and PEG were investigated (1, 2, 5, 10, 30, and 60 min). The most transformants were obtained when cells were incubated for 30 min with the methylated plasmid in presence of 40% PEG 8000 (Table 2). This table focuses only on the conditions that yielded transformants. The transformation efficiency average from at least three independent experiments is shown as well as the minimal and maximal transformation efficiency obtained in each condition. Several other parameters were investigated, including the culture volume (3 to 10 mL), the number of washes (1 to 3) and the quantity of plasmid (1 to 20 µg) (Fig. 1), but their impact on transformation efficiency was unclear.
The results of these experiments led to the development of a reproducible PEG-mediated transformation protocol (see Material and Methods) that yielded M132 transformants at an efficiency of about 10−10 transformants/cell/µg of plasmid, with the highest score reaching 2.3 × 10−9 transformants/cell/µg of plasmid (Table 2).
The protocol was tested using 13 other clinical strains available in the laboratory that were susceptible to tetracycline, with only transformants obtained for strains 4016 (three mutants) and 5012 (one mutant).
Genotypic analyses of M. hominis transformants
Colonies generally appeared on selective plates after 3 to 14 days of incubation, with most colonies exhibiting a typical “fried-egg” morphology that is characteristic of the wild-type M. hominis strain.
All of the transformants tested were positive by PCR for the tet(M) gene, suggesting that the transposon in the pMT85-Tet plasmid was present into the M. hominis cells. Moreover, the transformants were confirmed as M. hominis via sequencing of the 16S rDNA PCR products.
Further analyses were performed to precisely identify the site of transposon insertion. Single-primer PCRs using the gDNA of 24 transformants was followed by Sanger sequencing of the PCR products, with results obtained for 16 of the 24 mutants. For these 16 mutants, the sequencing data showed that only one copy of the transposon was present in the bacterial genome and that its insertion seemed to occur at random position in the genome (Table 3). Transposons were found inserted into intergenic regions, a hypothetical protein, lipoprotein-encoding genes, or at the 3′ end of essential genes (Table 3). For the remaining eight isolates, insertion sites for two could not be determined because of technical problems, while six had sequencing profiles showing several superimposed peaks, suggesting that several copies of the transposon were present in the genome (see below).
Phenotypic analyses of M. hominis mutant 28-2
The M. hominis mutant 28-2 was of particular interest, as the tet(M) gene was integrated in the middle of a gene encoding a precursor of the P75 lipoprotein that is potentially involved in the pathogenicity of M. hominis (Fig. 2). The expression of the gene encoding P75 was investigated by semi-quantitative RT-PCR. A small transcript corresponding to the beginning of the gene (before the tet(M) insertion) could be detected, whereas no transcript was produced around the insertion site of the transposon, suggesting that only a short transcript was produced for the gene (Supplementary Fig. S3).
Insertion site of the tet(M) gene in the genome of the M. hominis M132 transformant 28-2. (A) Scheme of the transposon inserted into the M. hominis M132 genome. The region contains inverted repeats (point rectangles), the sequence of the pMT85 plasmid (white rectangles) and the tet(M) gene (dark gray rectangle). (B) Scheme of insertion site for transformant 28-2. Hatched rectangles represent the M. hominis M132 gene where the tet(M) gene inserted. Double thin lines represent the position of the insertion inside the M. hominis M132 gene. Clear gray rectangles correspond to sequence of the bacterial genome. Numbers above single thin lines indicate the position around the genome. Black arrows indicate the position of the PCR primers P75-F1 and P75-R1, and gray arrows indicate the position of the PCR primers P75-F2 and P75-R2.
The ability of this mutant to adhere to HeLa cells was tested together with the M. hominis M132 wild-type strain. No difference in adhesion was observed between the M132 wild-type strain and the 28-2 mutant, suggesting the mutant fully retained its ability to adhere to eukaryotic cells. For both samples, the quantity of adhered M. hominis increased linearly as a function of the inoculum (Fig. 3). As expected, the correlation disappeared at high concentrations of M. hominis cells.
Test of adhesion to HeLa cells. The graph shows the quantity of adhered M. hominis (copies/µL) as a function of the M. hominis inoculum (in CFU/mL). Blue points correspond to the M132 wild-type strain and gray points correspond to the 28-2 mutant. This experiment was performed in triplicate, and average values are represented. Standard deviation represents the above points by vertical lines.
Detection of large DNA rearrangements by WGS
To confirm that the genomes of some mutants carried multiple copies of the transposon, the genomes of two mutants (28-1 and 29-1) were completely sequenced. The WGS data revealed the presence of variable copy numbers of the transposon throughout the genomes M. hominis of the two assayed clones. Indeed, five copies of the transposon were identified in the genome of transformant 28-1, while three copies were identified in the genome of transformant 29-1. Among the five transposon copies present in transformant 28-1, four were part of tandem repeats, three of which included copies of the entire pMT85-Tet plasmid (Fig. 4), while the remaining copy of the transposon was located 300 kb away. Surprisingly, this 300-kb region flanked by transposons was inverted compared to the wild-type strain. This inversion resulted in the disruption of the tyrosine recombinase-encoding xerC gene into two sections. For clone 29-1, a perfect duplication of approximately 9 kb flanked the double insertion of tet(M) at position 256,474 of the genome (Fig. 4). The third copy was observed to be inserted within a conserved hypothetical protein-encoding gene at position 208,266 of the genome. Altogether, these results suggested that large DNA rearrangement events in the M. hominis genome occurred adjacent to insertions of multiple copies of the transposon.
Analyses of the antibiotic resistance of clones carrying several copies of the tet(M) gene
Minimal inhibitory concentrations (MICs) to tetracycline were determined to assess whether the copy number of the tet(M) gene influenced the level of tetracycline resistance. Compared to M132, whose MIC for tetracycline was 0.125 µg/mL, the 28-2 mutant, which carries only one copy of tet(M) gene, had an MIC of 8 µg/mL. This value was similar to that of the 29-1 mutant (16 µg/mL), which had three copies of the transposon. Interestingly, the clone 28-1, harboring five insertions of the tet(M) gene, had an MIC for tetracycline that was two to four times higher than that of the other mutants (32 µg/mL).
Discussion
In this study, we developed a reproducible PEG-based transformation protocol for M. hominis. We showed that the procedure can be used for random mutagenesis using a plasmid carrying a mini-transposon. In the future, we believe that the use of this procedure could lead to a protocol for the directed mutagenesis of M. hominis, which has been accomplished for other mycoplasma species26.
To achieve this goal, we first sought to identify an M. hominis strain containing a low number of R-M systems. These systems are composed of a restriction endonuclease and a DNA methyltransferase, the latter of which prevents DNA cleavage by the cognate endonuclease (except for the type IV R-M system), and are well known to be a barrier to DNA transformation14,27. The genomes of 20 M. hominis strains were scrutinized for the presence of such systems, with the in silico analysis predicting the presence of numerous R-M systems encoded within the assayed strains (Table 1 and Supplementary Table S1). All of the studied genomes harbored a minimum of four systems total (complete plus incomplete), with up to nine systems identified for some clinical isolates (331 and 3364). Although no information is available regarding their activity, the high prevalence of R-M systems in the assayed M. hominis genomes combined with their high diversity (types I, II, III and potentially IV) may explain the difficulties encountered by the research community in developing strategies to genetically modify M. hominis.
Regarding the transformation protocol, several parameters appeared to be crucial to be successful (Fig. 1). First, we observed that the success of transformation depended on the selected strains. The PG21 strain was initially selected for our experiments as the most widely studied M. hominis representative7,12. Unfortunately, because no satisfying results were obtained, our efforts were redirected toward other strains. Among the 15 other strains tested, only three (M132, 4016 and 5012) were successfully transformed, although the reason for the observed success using these strains could not be ascertained. Indeed, the genomes of those three strains contained 5, 4 (7 including incomplete copies) and 3 (8 including incomplete copies) different complete R-M systems respectively; that is to say, a similar number of R-M systems as most of the other strains assayed (Table 1 and Supplementary Table S1). Moreover, all three strains contained different types of R-M systems (types I, II, III), sometimes in duplicate or triplicate (clinical strains 4016 and 5012). Although some R-M systems are perhaps not active, this is certainly not all of the systems detected, mainly because, bacteria that are subjected to degenerative evolution, such as mycoplasmas, have a tendency to lose genetic material that is not essential for their survival. Thus, may all these observations suggest that barriers other than R-M systems, not yet identified, may exist and prevent artificial DNA transfer in M. hominis?
It should be pointed out that a potential type IV R-M system (members of which are only composed of a restriction enzyme that cuts methylated DNA) was predicted in the M. hominis M132 genome. This system shares similarities with the E. coli McrBC system, which cleaves DNA containing methylcytosine on one or both strands28. In our assays, successful transformation uniquely occurred when the plasmid DNA was treated with the CpG methyltransferase SssI, suggesting that the McrBC-like system is present but either not functional under our experimental conditions or not active in the provided substrate. In any case, all assays performed with unmethylated plasmid or plasmid methylated with the GpC methyltransferase CviPI did not yield any transformants.
Another important parameter to consider was the growth phase of the bacteria used in transformation assays. Indeed, transformants were only obtained when an equal volume of the early, mid and late log phases were combined (Supplementary Fig. S2). Although this result was reported for U. parvum13, early or mid-log phase bacterial cultures are generally preferred in many other well-established transformation protocols29,30,31,32. Together with the bacterial growth phase, we noticed that some wash buffers were more suited to the transformation of M. hominis species than others. The T-Buffer used in the M. arthritidis transformation16 was successful, whereas other buffers commonly used for mycoplasmas (PBS or 10 mM Tris-HCl and 0.5 M sucrose, pH 6.5) were not.
The last crucial parameters to optimize for the M. hominis transformation procedure were the molecular weight and the concentration of the fusogenic chemical agent (PEG) used for transformation. The time of contact between the bacterial cells and the plasmid mixture was also of high importance. The optimal procedure developed required the use of PEG with an average molecular weight of 8,000 g/mol (PEG 8000) at high concentrations (40%, 50%, or 60% in T-Buffer), where the time of contact with bacterial cells/plasmid mixture lasted for 10 min or more. This need for the latter adjustment was unexpected, as most of the previously published PEG-mediated transformation protocols recommended that the incubation time of contact not be allowed to proceed for more than 2 min due to the presumed toxicity of the PEG toward the cells. In our experiments, we did not observe a higher mortality of M. hominis cells when their membranes were permeabilized by PEG for 10 to 30 min compared to 2 min (data not shown). Finally, for the transformation experiment to succeed, we noticed that more than 10 µg of plasmidic DNA are required since lower quantity did not yield any transformants.
Two antibiotic resistance-encoding genes were tested for selection during the transformation experiments. In contrast to the tetracycline determinant, the gentamicin resistance gene only allowed for the recovery of false-positive clones corresponding to spontaneous resistance mutants (data not shown). The development of a replicative plasmid containing the origin of replication of M. hominis was attempted, although convincing results were not obtained (data not shown).
By combining all of these parameters, we generated a reliable transformation protocol for M. hominis. Relatively low efficiencies were obtained for each experiment (up to 2.3.10−9 transformants/cell/µg of plasmid (corresponding to 1 to 3 transformants per experiment), indicating that the protocol should be further optimized. However, the results obtained using this protocol are reproducible and resulted in the generation of genetically modified M. hominis cells by transposon mutagenesis. The protocol may be further refined by counteracting of R-M systems. For example, the transformed DNA could be protected by in vitro methylation before its entry into the cells. In the current protocol, plasmids are methylated with the commercial methyltransferase SssI, which only provides protection against some restriction endonucleases (those recognizing CpG sites), but not all. DNA protection with other types of cytosine methyltransferases, but also with adenine methyltransferases should be considered, both type of R-M systems being found in M. hominis genomes (Tables 1 and S1). The best way to tackle the problem would be to prepare M. hominis cellular crude extracts (or M. hominis recombinant methyltransferases) in order to be able to methylate the exogenous DNA with M. hominis endogenous methyltransferases just before transformation8. However, it could be laborious and time-consuming to obtain crude extracts with fully functional methyltransferases or determine the laboratory conditions in which recombinant methyltransferases are active. The incoming DNA can be degrading by R-M systems once in the cell, but can also be degraded by surface nucleases before its entry into the cell33. Most nucleases require a divalent cation as a cofactor to be fully active (usually Mg2+ or Ca2+). Washing the cells with the chelators EDTA or EGTA may help neutralizing their activity and increasing the number of transformants. Finally, reaching better transformation efficiencies may also rely on the construction of a plasmid that is more adapted to M. hominis. Different promotors for driving the tet(M) expression gene could be tested, and a codon optimized version of the tet(M) gene could also be designed.
Mutants of interest were isolated over the course of our transformation experiments, such as mutant 28-2, which harbors the tet(M)-carrying transposon within the P75 lipoprotein gene that is potentially involved in the cytoadherence of M. hominis34,35. After verifying that the gene was knocked-out by PCR and RT-PCR, the adhesion ability of the mutant strain was tested using HeLa cells (Fig. 3). The results showed no difference in adhesion between the M132 wild-type strain and the derivative mutant 28-2. A few hypotheses should be considered with respect to this result: (i) the truncated protein may still be expressed at the surface with a conformation allowing adhesion to HeLa cells, antibodies against the P75 protein could be helpful to confirm this hypothesis by Western Blot; (ii) the adhesion test to HeLa cells was not sensitive enough, a possibility for which a negative control using a nonadherent strain (which is not currently available) would allow us to confirm or rule out this hypothesis; and, (iii) the absence of one protein is not sufficient to observe an altered adhesion, as other surface proteins have been shown to be important in M. hominis adhesion36,37. Additionally, adhesion experiments using other cell lines could certainly be done, but those tests would require some optimizations.
The use of next generation sequencing for deciphering the genome sequence of two transformants (mutants 28-1 and 29-1), confirmed the presence of multiple copies of the transposon throughout their genomes. A large 300-kb genomic inversion was observed in mutant 28-1, which caused the inactivation of a gene annotated as putative tyrosine recombinase XerC. This type of recombinases is known for its role as a mediator in site-specific recombination events and inversion events in bacteria38,39,40,41,42,43. Interestingly, the transposon was shown to be integrated into this gene in three mutants (two with a single insertion and one with a multiple insertion) out of the 24 obtained during this study. We concluded that xerC was certainly a “hotspot” of integration of this transposon in M. hominis M132 species, similarly to the four genes MG339 (recA), MG414 (Hypothetical protein), MG415 (hypothetical protein), and MG428 (putative regulatory protein) in M. genitalium. which constituted about 31% of the total transposon insertions during gene essentiality study44. We could also hypothesize that mutations in this locus facilitate fitness and growth. Multiple insertions of transposons as well as whole plasmid integration have been previously observed in mycoplasmas using the transposons Tn4001 and Tn91613,45,46,47,48,49,50. In the pMT85-Tet plasmid, the transposase-encoding gene was moved from the transposon to limit multiple insertions and full plasmid integration25,45. To the best of our knowledge, we showed for the first time the presence of multiple transposon integrations, whole plasmid recombination and highlighted large DNA rearrangements in the genomes of M. hominis cells that were certainly induced by the presence of the plasmid. It would be interesting to analyze more mutants with suspected multiple insertions (i) to look for the presence of small or large genomic rearrangements, (ii) study their nature (insertions, duplications, inversions) and finally (iii) to understand the underlying mechanisms.
Another interesting outcome of this work was that the tet(M) copy number may influence the level resistance of a strain to an antibiotic. In our experiments, clone 28-1, which had the highest copy number of the tet(M) gene, has a tetracycline MIC that is two to four times higher than that observed of the other mutants (32 µg/mL).
Altogether, these data demonstrate that we are now capable of obtaining some genetically modified M. hominis mutants by random mutagenesis. Even though the transformation efficiency obtained using this protocol is currently low, these results are encouraging and lay the foundation for the function studies of genes in this bacterium. These results may also pave the way toward the development of genome transplantation to permit targeted mutagenesis and direct inactivation of genes of interest in M. hominis.
Materials and Methods
Mycoplasmas strains, culturing and numeration
The M. hominis reference strains PG21 (ATCC 23114) and M132 (ATCC 43521) and the clinical isolates 2674, 3299, 331, 3364, 35, 3631, 4016, 4235, 4788, 4796, 5012, 5060, and 5096 (SRA accession number: PRJNA493181) were grown at 37 °C in Hayflick modified medium supplemented with arginine for 24 to 48 hours51. Transformants were cultured in the same medium containing 2 µg/mL of tetracycline. Bacterial titers were evaluated both by determining color changing units (CCU) and colony-forming units (CFUs) as previously described51. MICs were determined according to the Clinical & Laboratory Standards Institute guidelines52.
Plasmid and in vitro methylation
The vector used for M. hominis transformation was the plasmid pMT85-Tet, which was derived from the Tn4001-based mini transposon plasmid pMT8525. The pMT85-Tet plasmid carried the tetracycline resistance gene tet(M), the expression of which was driven by the spiralin promotor (PS) instead of the gentamicin resistance gene53,54. Before transformation, the plasmid was methylated by the CpG methyltransferase from Spiroplasma sp. strain MQ1 (M. SssI, New England Biolabs, Ipswich, Ma, United States) according to manufacturer’s recommendations.
M. hominis transformation protocol
After determining the optimal conditions, the transformation protocol was performed as follows: a 108 CFU/mL pre-culture was diluted from 10−1 to 10−10 and incubated for 24 h at 37 °C to obtain cultures at three different growth phases (e.g., early, mid-log and late log phase). The pool of these three different growth stages (which corresponded to the 10−3, 10−4 and 10−5 dilutions in three milliliters of culture) was centrifuged at 10,000 g, 4 °C for 20 min. The pellet was washed twice with cold T-Buffer (Tris 10 mM, pH 6.5) and centrifuged at 10,000 g, 4 °C for 10 min. After centrifugation, the cells were resuspended in 400 µL of cold 0.1 M CaCl2 and incubated at 4 °C for 30 min. Cold CaCl2-incubated cells (100 µL) were gently mixed with 10 µg of yeast tRNA (Life technologies, Carlsbad, CA, United States) and 10 µg of plasmid methylated with the methyltransferase SssI (New England Biolabs, Ipswich, Ma, United States). This mixture was aliquoted onto the surface of 1.5 mL of 40% PEG 8000 (Sigma-Aldrich, Saint-Louis, MO, United States) for 30 min. The contact was stopped by the addition of 7.5 mL of Hayflick arginine liquid medium and the cells were incubated for 3 hours at 37 °C. The transformation reaction was centrifuged at 8,000 g, room temperature for 10 min. The pellet was suspended in 1 mL of Hayflick arginine liquid medium and 200 µL was plated onto selective solid medium supplemented with 2 µg/mL of tetracycline (Sigma-Aldrich, Saint-Louis, MO, United States) and incubated at 37 °C with 5% CO2. Transformed M. hominis colonies appeared 3 to 7 days after transformation.
Colonies obtained on selective plates were picked and transferred into 1 mL of Hayflick arginine plus tetracycline (2 µg/mL) medium and incubated at 37 °C for 24 to 96 hours. Cultures were stocked at −80 °C. PCRs were performed with DNA extracts obtained using a NucleoSpin® Tissue kit (Macherey-Nagel, Düren, Germany). After checking for the presence of the tet(M) gene and confirmation of the isolates as M. hominis by PCR (see below), the positive transformants were subcloned three times by successive passages on selective solid medium.
Screening of M. hominis transformants by PCR
The tet(M) PCR mixture (50 µL final volume) contained 1X PCR buffer (Promega), 3 mM MgCl2, 0.2 mM dNTPs, 1 μM of each primers (IntMtet1 and IntMtet2), 1 U of HotStart G2 DNA polymerase (Promega), and 5 μL of DNA template. Amplification was performed as described by Dordet-Frisoni et al.53 Species determination was performed by amplification and sequencing (Eurofins genomics) of the 16S rRNA gene, according to55.
Determination of the tet(M) gene insertion site by Single-Primer PCR
M. hominis total genomic DNA extractions were performed using 10 mL cultures with NucleoBond® AXG20 columns and the NucleoBond® Buffer Set III from Macherey Nagel according to the manufacturer’s instructions. Transposon insertion sites were then determined by single-primer PCR. The 25 µL final reaction volume contained 1X PCR Buffer (Promega), 3 mM MgCl2, 1 µM of SG9 primer (5′-TTTGGTTCAGAAACTGGTGCT-3′), 0.2 mM dNTPs, 0.1 µL of HotStart G2 DNA polymerase (Promega), and 2.5 µL of transformant DNA. The PCR amplification cycle was performed as previously described53. The insertion positions were determined by Sanger sequencing (Eurofins genomics) of the PCR products with the nested primer MT85-1 (5′-ACAGTAATTGCGGGTGGATC-3′).
Analyses of mutant 28-2
The expression of the P75 gene (Mhom132_02390) in the M. hominis mutant 28-2 was assessed by semi-quantitative RT-PCR. Total RNA was extracted using a NucleoSpin®RNA plus kit (Macherey Nagel, Düren, Germany) according to manufacturer’s instructions. Reverse transcription was performed on total extracts with Avian Myeloblastosis Virus (AMV) Reverse Transcriptase following the recommendations provided by New England Biolabs. The presence of transcripts was finally verified by PCR amplification of cDNA. The 50 µL final reaction volume contained 1X PCR Buffer (Promega), 3 mM MgCl2, 1 µM of each primer, 0.2 mM dNTPs, 0.2 µL of HotStart G2 DNA polymerase (Promega), and 5 µL of cDNA. Two primer pairs were used, P75-F1 5′-GGCTTTTGGACTTTTAGCGC-3′ and P75-R1 5′-GGCTATTGTTTTCAGGGCTTG-3′, allowing the 5′ region of the gene to be amplified; and P75-F2 5′-GCAGCGCATGACGAATTAAG-3′ and P75-R2 5′-GCGCTTCATTTGGCTTGACT-3′, allowing the region around the transposon insertion site to be amplified. Amplification was performed as follows: 95 °C for 15 min, followed by 35 cycles of 1 min at 95 °C, 1 min at 58 °C and 1 min at 72 °C, with a final incubation for 5 min at 72 °C. The adhesion of the M. hominis mutant 28-2 to immobilized HeLa cells was assessed as previously reported7. A control with no HeLa cells was used to exclude the fact that M. hominis adheres to the plate (data not shown).
Whole genome sequencing (WGS)
WGS with 1D Native barcoding genomic DNA (with EXP-NBD103 and SQK-LSK108) was performed using an Oxford Nanopore Technologies (ONT) GridION device by the Genome Transcriptome Facility of Bordeaux, France. Each step was performed according to the ONT recommendations. Each purified barcoded DNA sample was quantified using a Qubit fluorometer, and DNA sizes were checked using a Tapestation instrument. Sequencing was performed with a R9.4.1 Flowcell on the GridION device for 42 hours using live basecalling through the GridION dedicated basecaller Guppy. Whole genomes of the transformants 28-1 and 29-1 were analyzed to determine if multiple insertion of the tet(M) gene occurred. Genomes were assembled using a 500X coverage depth with reads having an average length of 8800 bp with Canu 1.756 and was circularized with apc57.
Data Availability
All data analysed during this study are included in this published article. Additional datasets generated are available from the corresponding author upon request.
References
Waites, K. B., Schelonka, R. L., Xiao, L., Grigsby, P. L. & Novy, M. J. Congenital and opportunistic infections: Ureaplasma species and Mycoplasma hominis. Seminars in fetal & neonatal medicine 14, 190–199, https://doi.org/10.1016/j.siny.2008.11.009 (2009).
Furnkranz, U., Henrich, B. & Walochnik, J. Mycoplasma hominis impacts gene expression in Trichomonas vaginalis. Parasitology research, https://doi.org/10.1007/s00436-018-5761-6 (2018).
Pereyre, S. et al. Life on arginine for Mycoplasma hominis: clues from its minimal genome and comparison with other human urogenital mycoplasmas. PLoS genetics 5, e1000677, https://doi.org/10.1371/journal.pgen.1000677 (2009).
Fraser, C. M. et al. The minimal gene complement of Mycoplasma genitalium. Science 270, 397–403, https://doi.org/10.1126/science.270.5235.397 (1995).
Aleksandrova, N. M., Bevova, M. R. & Govorun, V. M. Transformation of Mycoplasma hominis by plasmid pAM120 using electroporation. Genetika 36, 309–313 (2000).
Roberts, M. C. & Kenny, G. E. Conjugal transfer of transposon Tn916 from Streptococcus faecalis to Mycoplasma hominis. Journal of bacteriology 169, 3836–3839, https://doi.org/10.1128/jb.169.8.3836-3839.1987 (1987).
Pereyre, S. et al. Generation of Mycoplasma hominis gene-targeted mutants by targeting-induced local lesions in genomes (TILLING). BMC genomics 19, 525, https://doi.org/10.1186/s12864-018-4917-1 (2018).
Lartigue, C. et al. Creating bacterial strains from genomes that have been cloned and engineered in yeast. Science 325, 1693–1696, https://doi.org/10.1126/science.1173759 (2009).
Tsarmpopoulos, I. et al. In-Yeast Engineering of a Bacterial Genome Using CRISPR/Cas9. ACS synthetic biology 5, 104–109, https://doi.org/10.1021/acssynbio.5b00196 (2016).
Chandran, S. et al. TREC-IN: gene knock-in genetic tool for genomes cloned in yeast. BMC genomics 15, 1180, https://doi.org/10.1186/1471-2164-15-1180 (2014).
Labroussaa, F. et al. Impact of donor-recipient phylogenetic distance on bacterial genome transplantation. Nucleic acids research, https://doi.org/10.1093/nar/gkw688 (2016).
Rideau, F. et al. Cloning, stability, and modification of Mycoplasma hominis genome in yeast. ACS synthetic biology 6, 891–901, https://doi.org/10.1021/acssynbio.6b00379 (2017).
Aboklaish, A. F. et al. Random insertion and gene disruption via transposon mutagenesis of Ureaplasma parvum using a mini-transposon plasmid. International journal of medical microbiology: IJMM 304, 1218–1225, https://doi.org/10.1016/j.ijmm.2014.09.003 (2014).
Vasu, K. & Nagaraja, V. Diverse functions of restriction-modification systems in addition to cellular defense. Microbiology and molecular biology reviews: MMBR 77, 53–72, https://doi.org/10.1128/MMBR.00044-12 (2013).
Lartigue, C. et al. Genome transplantation in bacteria: changing one species to another. Science 317, 632–638, https://doi.org/10.1126/science.1144622 (2007).
Voelker, L. L. & Dybvig, K. Gene transfer in Mycoplasma arthritidis: transformation, conjugal transfer of Tn916, and evidence for a restriction system recognizing AGCT. Journal of bacteriology 178, 6078–6081, https://doi.org/10.1128/jb.178.20.6078-6081.1996 (1996).
Algire, M. A., Montague, M. G., Vashee, S., Lartigue, C. & Merryman, C. A Type III restriction-modification system in Mycoplasma mycoides subsp. capri. Open biology 2, 120115, https://doi.org/10.1098/rsob.120115 (2012).
Luo, W., Tu, A. H., Cao, Z., Yu, H. & Dybvig, K. Identification of an isoschizomer of the HhaI DNA methyltransferase in Mycoplasma arthritidis. FEMS microbiology letters 290, 195–198, https://doi.org/10.1111/j.1574-6968.2008.01428.x (2009).
Dupuis, M. E., Villion, M., Magadan, A. H. & Moineau, S. CRISPR-Cas and restriction-modification systems are compatible and increase phage resistance. Nature communications 4, 2087, https://doi.org/10.1038/ncomms3087 (2013).
Allen-Daniels, M. J. et al. Identification of a gene in Mycoplasma hominis associated with preterm birth and microbial burden in intraamniotic infection. American journal of obstetrics and gynecology 212, 779 e771–779 e713, https://doi.org/10.1016/j.ajog.2015.01.032 (2015).
Calcutt, M. J. & Foecking, M. F. Analysis of the Complete Mycoplasma hominis LBD-4 Genome Sequence Reveals Strain-Variable Prophage Insertion and Distinctive Repeat-Containing Surface Protein Arrangements. Genome announcements 3, https://doi.org/10.1128/genomeA.01582-14 (2015).
Calcutt, M. J. & Foecking, M. F. Complete Genome Sequence of Mycoplasma hominis Strain Sprott (ATCC 33131), Isolated from a Patient with Nongonococcal Urethritis. Genome announcements 3, https://doi.org/10.1128/genomeA.00771-15 (2015).
Roberts, R. J., Vincze, T., Posfai, J. & Macelis, D. REBASE–a database for DNA restriction and modification: enzymes, genes and genomes. Nucleic acids research 43, D298–299, https://doi.org/10.1093/nar/gku1046 (2015).
Barre, A., de Daruvar, A. & Blanchard, A. MolliGen, a database dedicated to the comparative genomics of Mollicutes. Nucleic acids research 32, D307–310, https://doi.org/10.1093/nar/gkh114 (2004).
Zimmerman, C. U. & Herrmann, R. Synthesis of a small, cysteine-rich, 29 amino acids long peptide in Mycoplasma pneumoniae. FEMS microbiology letters 253, 315–321, https://doi.org/10.1016/j.femsle.2005.09.054 (2005).
Renaudin, J., Breton, M. & Citti, C. Molecular genetic tools for mollicutes. In: Browning G, Citti C, editors. Mollicutes: molecular biology and pathogenesis. Norwich UK: Horizon scientific press; 2014. p. 55–76 (2014).
Brocchi, M., Vasconcelos, A. T. R. D. & Zaha, A. Restriction-modification systems in Mycoplasma spp. Genetics and Molecular Biology 30, 236–244 (2007).
Gowher, H., Leismann, O. & Jeltsch, A. DNA of Drosophila melanogaster contains 5-methylcytosine. The EMBO journal 19, 6918–6923, https://doi.org/10.1093/emboj/19.24.6918 (2000).
Whetzel, P. L., Hnatow, L. L., Keeler, C. L. Jr. & Dohms, J. E. Transposon mutagenesis of Mycoplasma gallisepticum. Plasmid 49, 34–43 (2003).
Sharma, S. et al. Development and host compatibility of plasmids for two important ruminant pathogens, Mycoplasma bovis and Mycoplasma agalactiae. PloS one 10, e0119000, https://doi.org/10.1371/journal.pone.0119000 (2015).
Washburn, L. R., Bird, D. W. & Dybvig, K. Restoration of cytoadherence to an adherence-deficient mutant of Mycoplasma arthritidis by genetic complementation. Infection and immunity 71, 671–675, https://doi.org/10.1128/iai.71.2.671-675.2003 (2003).
Lartigue, C., Blanchard, A., Renaudin, J., Thiaucourt, F. & Sirand-Pugnet, P. Host specificity of mollicutes oriC plasmids: functional analysis of replication origin. Nucleic acids research 31, 6610–6618, https://doi.org/10.1093/nar/gkg848 (2003).
Minion, F. C. & Goguen, J. D. Identification and preliminary characterization of external membrane-bound nuclease activities in Mycoplasma pulmonis. Infection and immunity 51, 352–354 (1986).
Henrich, B., Kretzmer, F., Deenen, R. & Kohrer, K. Validation of a novel Mho microarray for a comprehensive characterisation of the Mycoplasma hominis action in HeLa cell infection. PloS one 12, e0181383, https://doi.org/10.1371/journal.pone.0181383 (2017).
Roachford, O. S. E., Nelson, K. E. & Mohapatra, B. R. Comparative genomics of four Mycoplasma species of the human urogenital tract: Analysis of their core genomes and virulence genes. International journal of medical microbiology: IJMM 307, 508–520, https://doi.org/10.1016/j.ijmm.2017.09.006 (2017).
Goret, J. et al. Surface lipoproteome of Mycoplasma hominis PG21 and differential expression after contact with human dendritic cells. Future microbiology 11, 179–194, https://doi.org/10.2217/fmb.15.130 (2016).
Henrich, B., Feldmann, R. C. & Hadding, U. Cytoadhesins of Mycoplasma hominis. Infection and immunity 61, 2945–2951 (1993).
Chopra-Dewasthaly, R. et al. Phase-locked mutants of Mycoplasma agalactiae: defining the molecular switch of high-frequency Vpma antigenic variation. Molecular microbiology 67, 1196–1210, https://doi.org/10.1111/j.1365-2958.2007.06103.x (2008).
Castillo, F., Benmohamed, A. & Szatmari, G. Xer Site Specific Recombination: Double and Single Recombinase Systems. Frontiers in microbiology 8, 453, https://doi.org/10.3389/fmicb.2017.00453 (2017).
Das, B., Martinez, E., Midonet, C. & Barre, F. X. Integrative mobile elements exploiting Xer recombination. Trends in microbiology 21, 23–30, https://doi.org/10.1016/j.tim.2012.10.003 (2013).
Midonet, C. & Barre, F. X. Xer site-specific recombination: promoting vertical and horizontal transmission of genetic information. Microbiology spectrum 2, https://doi.org/10.1128/microbiolspec.MDNA3-0056-2014 (2014).
Zimmerman, C.-U. R., Rosengarten, R. & Spergser, J. Interaction of the putative tyrosine recombinases RipX (UU145), XerC (UU222), and CodV (UU529) of Ureaplasma parvum serovar 3 with specific DNA. FEMS microbiology letters 340, 55–64, https://doi.org/10.1111/1574-6968.12077 (2013).
Zimmerman, C.-U. R., Herrmann, R. & Rosengarten, R. XerC-mediated DNA inversion at the inverted repeats of the UU172-phase-variable element of Ureaplasma parvum serovar 3. Microbiological Research 170, 263–269, https://doi.org/10.1016/j.micres.2014.09.002 (2015).
Glass, J. I. et al. Essential genes of a minimal bacterium. Proceedings of the National Academy of Sciences of the United States of America 103, 425–430, https://doi.org/10.1073/pnas.0510013103 (2006).
Pour-El, I., Adams, C. & Minion, F. C. Construction of mini-Tn4001tet and its use in Mycoplasma gallisepticum. Plasmid 47, 129–137, https://doi.org/10.1006/plas.2001.1558 (2002).
Foissac, X., Saillard, C. & Bove, J. M. Random insertion of transposon Tn4001 in the genome of Spiroplasma citri strain GII3. Plasmid 37, 80–86, https://doi.org/10.1006/plas.1996.1271 (1997).
Mahairas, G. G. & Minion, F. C. Random insertion of the gentamicin resistance transposon Tn4001 in Mycoplasma pulmonis. Plasmid 21, 43–47 (1989).
Dybvig, K. & Alderete, J. Transformation of Mycoplasma pulmonis and Mycoplasma hyorhinis: transposition of Tn916 and formation of cointegrate structures. Plasmid 20, 33–41 (1988).
Chopra-Dewasthaly, R., Zimmermann, M., Rosengarten, R. & Citti, C. First steps towards the genetic manipulation of Mycoplasma agalactiae and Mycoplasma bovis using the transposon Tn4001mod. International journal of medical microbiology: IJMM 294, 447–453, https://doi.org/10.1016/j.ijmm.2004.09.010 (2005).
Ruffin, D. C. et al. Transposon mutagenesis of Mycoplasma gallisepticum by conjugation with Enterococcus faecalis and determination of insertion site by direct genomic sequencing. Plasmid 44, 191–195, https://doi.org/10.1006/plas.2000.1485 (2000).
Waites, K. B., Bébéar, C. M., Robertson, J. A., Talkington, D. F. & GE, K. Laboratory Diagnosis of Mycoplasmal Infections. (Eds Cumitech 34, Washington D. C.: American Society for Microbiology Press, 2001).
Waites, K. B. et al. Methods for antimicrobial susceptibility testing for human mycoplasmas: Approved guideline. Report No. Document M43-A, (Clinical and Laboratory Standards Institute, Wayne (PA), 2011).
Dordet Frisoni, E. et al. ICEA of Mycoplasma agalactiae: a new family of self-transmissible integrative elements that confers conjugative properties to the recipient strain. Molecular microbiology 89, 1226–1239, https://doi.org/10.1111/mmi.12341 (2013).
Dordet-Frisoni, E. et al. Chromosomal transfers in mycoplasmas: when minimal genomes go mobile. mBio 5, e01958, https://doi.org/10.1128/mBio.01958-14 (2014).
Teyssou, R. et al. Detection of mollicutes contamination in cell cultures by 16S rDNA amplification. Molecular and cellular probes 7, 209–216, https://doi.org/10.1006/mcpr.1993.1030 (1993).
Koren, S. et al. Canu: scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome research 27, 722–736, https://doi.org/10.1101/gr.215087.116 (2017).
Lin, H. C. et al. AGORA: Assembly Guided by Optical Restriction Alignment. BMC bioinformatics 13, 189, https://doi.org/10.1186/1471-2105-13-189 (2012).
Acknowledgements
The authors thank Christophe Boury and Charlotte Mouden for whole genome sequencing and assembling, performed at the Genome Transcriptome Facility of Bordeaux. We thank Brigitte Tauzin, Marie Gardette and Nadège Hénin for technical assistance. We finally thank Sébastien Rodrigue and Florence Tardy for reading the manuscript.
Author information
Authors and Affiliations
Contributions
C.B., C.L., F.R., C.C., E.D.F., B.H. and S.P. designed the research. F.R., C.L.R., H.R. and E.S. performed the experiments. F.R., C.L. and C.B. contributed to writing of the paper.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rideau, F., Le Roy, C., Sagné, E. et al. Random transposon insertion in the Mycoplasma hominis minimal genome. Sci Rep 9, 13554 (2019). https://doi.org/10.1038/s41598-019-49919-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-49919-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.