Abstract
HilD is an AraC-like transcriptional regulator encoded in the Salmonella pathogenicity island 1 (SPI-1), which actives transcription of many genes within and outside SPI-1 that are mainly required for invasion of Salmonella into host cells. HilD controls expression of target genes directly or by acting through distinct regulators; three different regulatory cascades headed by HilD have been described to date. Here, by analyzing the effect of HilD on the yobH gene in Salmonella enterica serovar Typhimurium (S. Typhimurium), we further define an additional regulatory cascade mediated by HilD, which was revealed by previous genome-wide analyses. In this regulatory cascade, HilD acts through SprB, a LuxR-like regulator encoded in SPI-1, to induce expression of virulence genes. Our data show that HilD induces expression of sprB by directly counteracting H-NS-mediated repression on the promoter region upstream of this gene. Then, SprB directly activates expression of several genes including yobH, slrP and ugtL. Interestingly, we found that YobH, a protein of only 79 amino acids, is required for invasion of S. Typhimurium into HeLa cells and mouse macrophages. Thus, our results reveal a novel S. Typhimurium invasion factor and provide more evidence supporting the HilD-SprB regulatory cascade.
Similar content being viewed by others
Introduction
The genus Salmonella groups facultative anaerobic Gram-negative bacteria and is divided into two species, S. enterica and S. bongori. The former is responsible for diseases ranging from gastroenteritis to severe systemic infections in a wide range of hosts, and it comprises 6 subspecies that are further divided into serovars1,2. The broad-host-range S. enterica serovar Typhimurium (S. Typhimurium) is a common cause of gastroenteritis in humans and many animals worldwide; furthermore, it can also cause systemic infection in humans and some animals, including laboratory mice1,3,4. For this reason, S. Typhimurium is frequently used as a model for studying the host-pathogen interactions during infection with Salmonella.
During its evolution, Salmonella has acquired numerous DNA fragments through different horizontal transference events5,6; those encoding virulence factors are denominated Salmonella pathogenicity islands (SPIs)7,8,9. Up to 22 SPIs have been described among Salmonella serovars10, being SPI-1 and SPI-2 the most importantly involved in gastroenteritis and systemic disease, respectively2,8,9. SPI-1 is present in the two Salmonella species, in contrast, SPI-2 is present only in the S. enterica species, which supports that SPI-1 was acquired earlier than SPI-2 during the Salmonella pathogenicity evolution5,11.
SPI-1 is a ∼40 kb chromosomal region of Salmonella that contains 39 genes, which encode a type III secretion system (T3SS-1; a syringe-like molecular apparatus that extent from the membranes of bacteria), several effector proteins and their respective chaperones, as well as some transcriptional regulators8,12,13. Translocation of effector proteins into the cytoplasm of eukaryotic cells, through the T3SS-1, favors invasion of Salmonella into these cells by a trigger mechanism, which involves cytoskeletal rearrangements known as “membrane ruffles”8,12. Invasion of Salmonella into the intestinal epithelium induces strong intestinal inflammatory response leading to gastroenteritis; in turn, this generates different antimicrobial activities that displace most of the intestinal microbiota, which heighten the intestinal colonization by Salmonella2,9,14,15. Consistently with their role in intestinal disease, the SPI-1 genes are expressed in vivo when Salmonella resides in the intestinal lumen and in the cytosol of epithelial cells16,17. Moreover, expression of the SPI-1 genes is regulated by distinct molecules or conditions present in the intestine of humans and animals, such as short- and long-fatty acids, high osmolarity, bile, low level of aeration and neutral pH18,19,20,21,22,23. In laboratory conditions, the SPI-1 genes are expressed in nutrient-rich media like the lysogeny broth (LB), where they are expressed in the late exponential (early stationary) phase of growth, which somehow mimic the intestinal environment24,25,26.
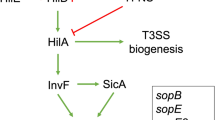
Expression of the SPI-1 genes is controlled by a myriad of global and Salmonella-specific regulators. HilD, an AraC-like transcription regulator encoded within SPI-1, is the apex of different regulatory cascades controlling expression of tens of Salmonella virulence genes2,27,28. HilD positively regulates expression of the SPI-1 genes and many other related genes encoded outside SPI-1, by inducing expression of HilA, an OmpR-ToxR-like transcriptional regulator, which in turn induces expression of InvF, also an AraC-like transcriptional regulator; HilA and InvF, both encoded in SPI-1, directly induce expression of the rest of SPI-1 genes2,29. HilD controls expression of hilA directly or through a feed-forward loop that it forms with HilC and RtsA, encoded within and outside SPI-1, respectively; although these three AraC-like regulators recognize the same DNA motif, HilD is dominant over HilC and RtsA30,31. On another hand, HilD positively controls expression of the FlhD4C2 complex, the master positive transcriptional regulator of the flagellar/chemotaxis genes, which are also required for the invasion phenotype of Salmonella32,33,34,35. Likewise, HilD induces expression of the SPI-2 genes, which are mainly required for replication of Salmonella within host cells, by acting on the ssrAB operon that codes for the SsrA/SsrB two-component system, the master regulator of SPI-225,36,37. Additionally, HilD directly controls expression of several other virulence genes28,38,39,40. For all cases characterized up to now, HilD induces expression of target genes by directly antagonizing repression mediated by the histone-like protein H-NS on the respective promoters36,37,41,42,43.
In addition to HilD, HilC, HilA and InvF, SPI-1 also codes for SprB, which belongs to the LuxR/UhaP family of transcriptional factors44. SprB is not required for expression of the SPI-1 genes, but it is expressed under the same conditions that favor expression of these genes28,44,45; furthermore, a previous study indicates that expression of SprB is positively regulated by HilA46. Firstly, SprB was shown to directly regulate expression of the siiABCDEF operon located in SPI-446; however, recent transcriptomic analyses support that SprB controls expression of several virulence and hypothetical genes, but not that of the siiABCDEF operon28.
Data from this study, together with previous results from genome-wide analyses28, define an additional regulatory cascade headed by HilD. In this regulatory cascade, HilD induces expression of SprB, which in turn activates expression of several target genes including yobH, slrP and ugtL; slrP and ugtL have been involved in Salmonella virulence. Our results show that HilD directly induces expression of sprB by antagonizing repression mediated by H-NS. Interestingly, we found that the yobH gene is required for invasion HeLa cells and mouse macrophages by S. Typhimurium, which reveals a novel invasion factor of Salmonella. Thus, the HilD-SprB regulatory cascade represents a novel pathway that controls expression of virulence genes in Salmonella.
Results
HilD positively controls the expression of yobH (SL1344_1770)
Previous RNA-sequencing (RNA-seq) analysis indicates that the HilD transcriptional regulator positively controls the expression of the S. Typhimurium SL1344_1770 gene28, which is located outside SPI-1 and codes for a hypothetical protein of 79 amino acids. Additional RNA-seq and co-expression analyses also support that HilD is involved in the expression of SL1344_177039,45. Orthologs of SL1344_1770, which show high sequence identity and a conserved genomic context, are denominated yobH in Escherichia coli and several other bacteria; thus, we kept the name of yobH for SL1344_1770.
To confirm the regulation of yobH by HilD, a transcriptional fusion of the intergenic region upstream of yobH to the cat (chloramphenicol acetyl transferase) reporter gene was constructed in the pKK232-8 plasmid. Specific activity from this fusion was quantified in the wild type (WT) S. Typhimurium strain SL1344 and its derivative ∆hilD mutant, grown in nutrient-rich lysogeny broth (LB) at 37 °C, conditions that induce the expression of genes regulated by HilD25,26,39. The activity of the yobH-cat fusion showed a 3-fold reduction in the ∆hilD mutant, compared with its expression in the WT strain; in addition, the expression of HilD from the pK6-HilD plasmid increased around 5-fold the activity of this fusion in the ∆hilD mutant (Fig. 1A). To investigate if yobH indeed codes for a protein and to further confirm the positive regulation of yobH by HilD, we tested the expression of the YobH-FLAG putative protein (YobH tagged with a 3XFLAG epitope) in the WT S. Typhimurium strain and its derivative ∆hilD mutant. A specific signal for YobH-FLAG was detected in the WT strain, with the expected size for this protein (Fig. 1B). The amount of YobH-FLAG was almost abolished in the ∆hilD mutant; as expected, it was restored at WT levels by the expression of HilD from the pK6-HilD plasmid (Fig. 1B). Taken together, these results show that HilD induces the expression of yobH.
HilD positively regulates the expression of yobH (SL1344_1770) in LB. (A) Activity of the yobH-cat transcriptional fusion from the pyobH-cat plasmid, was determined in the WT S. Typhimurium SL1344 strain and its isogenic ∆hilD mutant containing the pMPM-K6Ω vector or the pK6-HilD plasmid, with (+) and without (−) induction (0.001% L-arabinose). Means and standard deviations from three independent experiments performed in duplicate are shown. Statistically different values are indicated (***p < 0.001). (B) Expression of YobH-FLAG in WT S. Typhimurium SL1344 strain and its isogenic ∆hilD mutant containing the pMPM-K6Ω vector or the pK6-HilD plasmid, was analyzed by Western blotting by using an anti-FLAG monoclonal antibody. GroEL was detected as a loading control with an anti-GroEL polyclonal antibody. Blots were cropped from different parts of the same gel. CAT specific activity and YobH-FLAG expression were determined from samples of bacterial cultures grown for 9 h in LB at 37 °C.
YobH is involved in the S. Typhimurium invasion of host cells
HilD positively regulates expression of numerous genes mainly required for the invasion of Salmonella into host cells2,28,39,45. For instance, recently we found that HilD controls expression of the grhD1 invasion gene, which is located outside SPI-140. To determine whether YobH is required for this Salmonella virulence phenotype, we evaluated the invasion ability of the WT S. Typhimurium strain and its isogenic ∆yobH mutant in HeLa cells and RAW264.7 mouse macrophages. Additionally, we constructed a complemented ∆yobH mutant (∆yobH + yobH-FLAG-kan), by inserting yobH into the chromosome of the ∆yobH mutant as described in Fig. S1, which was also assessed in the invasion assays. Furthermore, the ∆hilD and ∆ssrB mutants were also tested in these assays as positive and negative controls, respectively; SsrB is a transcriptional regulator that is required for Salmonella intracellular replication but not for invasion of host cells2,40. The ∆yobH mutant showed a ∼3-fold decrease in the invasion of both HeLa cells and RAW264.7 macrophages with respect to the WT and the complemented ∆yobH + yobH-FLAG-kan strains (Fig. 2A,B). As expected, the ∆hilD mutant was unable to invade the HeLa cells and RAW264.7 macrophages, whereas the ∆ssrB mutant invaded these cells at similar levels to those showed by the WT and the complemented ∆yobH + yobH-FLAG-kan strains (Fig. 2A,B). Important to note, the number of bacteria present in the starting inoculums used in the invasion assays showed a variation of only 18% between the different strains tested (Fig. S2). Together, these results indicate that YobH is a novel invasion factor of S. Typhimurium.
YobH is involved in the S. Typhimurium invasion of HeLa cells and macrophages. Epithelial HeLa cells (A) and murine RAW 264.7 macrophages (B) were infected with the WT S. Typhimurium SL1344 strain and its isogenic ∆ssrB, ∆hilD, ∆yobH and ∆yobH + yobH-FLAG-kan (∆yobH complemented) mutants. Invasion was quantified by enumerating the intracellular CFUs at 1 h post-infection, using a gentamicin protection assay. Means and standard deviations from three independent experiments performed in duplicate are shown. Statistically different values are indicated (***p < 0.001; ****p < 0.0001).
HilD controls expression of yobH through SprB
We sought to determine if HilD regulates the yobH virulence gene directly or indirectly. For this, we performed electrophoretic mobility shift assays (EMSAs) by using affinity-purified maltose-binding protein (MBP)-HilD and a DNA fragment spanning the intergenic region upstream of yobH. DNA fragments carrying the regulatory region of hilA or sopB were also assessed in these assays as positive and negative controls, respectively; HilD binds to hilA but not to sopB25. As show in Fig. S3A, MBP-HilD did not shift the yobH fragment or that of sopB, even at the highest protein concentration tested (1 µM). In contrast, MBP-HilD shifted the positive control, hilA, at concentrations from 0.1 to 1 µM (Fig. S3B). These assays indicate that HilD does not interact with the regulatory region of yobH; alternatively, HilD could require an additional factor to bind to yobH.
To investigate whether HilD requires another S. Typhimurium regulator to induce the expression of yobH, we monitored the activity of the yobH-cat fusion in the WT E. coli MC4100 strain, which lacks HilD and the other Salmonella-specific regulators, in the presence of the pK6-HilD plasmid expressing HilD or in the presence of the pMPM-K6Ω vector. As a positive control, activity of the hilA-cat fusion was also tested; hilA is directly regulated by HilD47,48. As expected, the yobH-cat and hilA-cat fusions showed low or undetectable expression levels in E. coli (Fig. S3C,D). The activity of hilA-cat, but not that of yobH-cat, was induced by HilD in E. coli (Fig. S3C,D), indicating that an additional factor, present in S. Typhimurium SL1344 but not in E. coli MC4100, is required for the HilD-mediated expression of yobH.
Several studies have shown that HilD induces expression of a high number of virulence genes through distinct regulatory cascades involving the HilA, InvF, HilC, RtsA, SsrA/SsrB and FlhDC transcriptional regulators2,25,28,29,32,33. To determine if any of these regulators are required for the HilD-mediated expression of yobH, activity of the yobH-cat fusion was measured in the WT S. Typhimurium strain and its isogenic ∆hilA, ∆invF, ∆hilC, ∆rtsA, ∆ssrB and ∆flhDC mutants. As positive controls, the ∆SPI-1 and ∆hilD mutants were also tested; SPI-1 encodes HilD, HilA, InvF and HilC2. Activity of the yobH-cat fusion was affected in the ∆SPI-1 and ∆hilD mutants, but not in the ∆hilA, ∆invF, ∆hilC, ∆rtsA, ∆ssrB and ∆flhDC mutants, with respect to the WT strain (Fig. 3), suggesting that HilD induces expression of yobH through a regulatory cascade different to those well characterized before this study.
SprB is required for the expression of yobH in LB. Activity of the yobH-cat transcriptional fusion from the pyobH-cat plasmid, was determined in the WT S. Typhimurium SL1344 strain and its isogenic ∆SPI-1, ∆hilD, ∆sprB, ∆hilC, ∆hilA, ∆invF, ∆rtsA, ∆ssrB, ∆sinR and ∆flhDC mutants. CAT specific activity was quantified from samples of bacterial cultures grown for 9 h in LB at 37 °C. Means and standard deviations from three independent experiments performed in duplicate are shown. Statistically different values are indicated (***p < 0.001).
Previous RNA-seq analyses indicate that HilD induces expression of two additional Salmonella-specific transcriptional regulators, SprB and SinR28,45, encoded in SPI-1 and SPI-6, respectively. Moreover, we previously confirmed that HilD directly induces expression of sinR39. SprB has been involved in the expression of several S. Typhimurium virulence genes28,46, whereas SinR remains uncharacterized. To investigate whether SprB and/or SinR are involved in the HilD-mediated expression of yobH, we monitored the activity of the yobH-cat fusion in the ∆sprB and ∆sinR mutants. Surprisingly, the activity of this fusion was reduced in the ∆sprB mutant, as in the ∆SPI-1 and ∆hilD mutants, whereas it was not affected in the ∆sinR mutant (Fig. 3), suggesting that SprB is required for the expression of yobH. Expression of SprB from the pK6-SprB plasmid, under an arabinose inducible promoter, restored the activity of yobH-cat in both the ∆sprB and the ∆hilD mutants (Fig. 4A). In contrast, expression of HilD from the pK6-HilD plasmid induced the activity of yobH-cat in the ∆hilD mutant (Fig. 1A), but not in the ∆sprB mutant (Fig. 4A). Similarly, SprB restored the expression of YobH-FLAG in the ∆hilD mutant, whereas HilD was unable to induce the expression of YobH-FLAG in the ∆sprB mutant (Fig. 4B). Altogether, these results support that SprB acts downstream of HilD for the expression of yobH.
HilD induces the expression of yobH through SprB. (A) Activity of the yobH-cat transcriptional fusion from the pyobH-cat plasmid, was determined in the WT S. Typhimurium SL1344 strain and its isogenic ∆sprB mutant containing or not the pMPM-K6Ω vector, or the pK6-SprB or pK6-HilD plasmids, as well as in the ∆hilD mutant containing the pMPM-K6Ω vector or the pK6-SprB plasmid. Means and standard deviations from three independent experiments performed in duplicate are shown. Statistically different values are indicated (***p < 0.001). (B) Expression of YobH-FLAG in WT S. Typhimurium SL1344 strain and its isogenic ∆sprB mutant containing the pMPM-K6Ω vector or the pK6-HilD plasmid, as well as in the ∆hilD mutant containing the pMPM-K6Ω vector or the pK6-SprB plasmid, was analyzed by Western blotting by using an anti-FLAG monoclonal antibody. GroEL was detected as a loading control with an anti-GroEL polyclonal antibody. Blots were cropped from different parts of the same gel. CAT specific activity and YobH-FLAG expression were quantified from samples of bacterial cultures grown for 9 h in LB at 37 °C.
Results from a chromatin immunoprecipitation sequencing (ChIP-seq) analysis revealed that SprB binds to the regulatory region of yobH in vivo28. We sought to confirm the SprB binding on yobH by EMSAs; however, we were unable to purify the 6XHis-tagged SprB protein, probably due to its high insolubility. Alternatively, we investigated if SprB requires any other Salmonella-specific regulator to induce the expression of yobH. For this, the activity of the yobH-cat fusion was tested in the WT S. Typhimurium strain and in the WT E. coli MC4100 strain carrying the pMPM-K6Ω vector or the pK6-SprB plasmid. Activity of a cat transcriptional fusion of sirA, a gene expected to be not controlled by SprB, was also tested as negative control; an ortholog of sirA (uvrY) is present in E. coli K-1249,50. The presence of SprB induced the activity of yobH-cat in E. coli to levels similar to those reached by this fusion in the WT S. Typhimurium strain (Fig. 5A); in contrast, SprB did not affect the activity of the sirA-cat fusion (Fig. 5B). These results are in line with the notion that SprB directly activates expression of yobH.
SprB induces expression of yobH in the absence of Salmonella-specific regulators. Activity of the yobH-cat (A) and sirA-cat (B) transcriptional fusions from the pyobH-cat and psirA-cat plasmids, respectively, was determined in the WT S. Typhimurium SL1344 strain and in the WT E. coli MC4100 strain containing or not the pMPM-K6Ω vector or the pK6-SprB plasmid expressing SprB under an arabinose inducible promoter. CAT specific activity was quantified from samples of bacterial cultures grown for 9 h in LB containing 0.001% L-arabinose, at 37 °C. Means and standard deviations from three independent experiments performed in duplicate are shown. Statistically different values are indicated (***p < 0.001).
The results described above strongly suggest that HilD positively regulates the expression of sprB. To confirm this, we quantified the expression of sprB in the WT S. Typhimurium strain and its isogenic ∆hilD mutant. Expression of sprB seems to be controlled by both the regulatory region upstream of hilC, which generates a hilC-sprB transcript, and that located upstream of sprB28. HilD regulation on the promoter upstream of hilC has been extensively shown in previous studies28,30,38,48,51. Thus, we evaluated the effect of HilD on the regulatory region upstream of sprB by constructing and analyzing a sprB-cat transcriptional fusion carrying this region. As shown in Fig. 6A, activity of the sprB-cat fusion was 2-fold reduced in the ∆hilD mutant, compared with its activity in the WT strain; furthermore, expression of HilD from the pK6-HilD plasmid increased 3-fold the activity of this fusion in the ∆hilD mutant, indicating that HilD induces expression of sprB by also acting on the regulatory region upstream of this gene.
HilD positively controls expression of sprB. Activity of the sprB-cat transcriptional fusion from the psprB-cat plasmid, was determined in the WT S. Typhimurium SL1344 strain (A,B) and its isogenic ∆hilD mutant containing or not the pMPM-K6Ω vector or the pK6-HilD plasmid (A), as well as in the WT E. coli MC4100 strain containing or not the pMPM-K6Ω vector or the pK6-HilD plasmid (B). CAT specific activity was quantified from samples of bacterial cultures grown for 9 h in LB at 37 °C. Means and standard deviations from three independent experiments performed in duplicate are shown. Statistically different values are indicated (***p < 0.001). EMSAs were performed with purified MBP-HilD (0, 0.1, 0.5 and 1 µM) and a DNA fragment containing the regulatory region of sprB (C). A DNA fragment containing the regulatory region of ppK was used as a negative internal control. The DNA-protein complexes, indicated by an asterisk, were resolved in a nondenaturing 6% polyacrylamide gel and stained with ethidium bromide.
To investigate whether HilD regulates expression of sprB (by acting on the regulatory region upstream of this gene) directly or through an additional Salmonella-specific factor, we determined the activity of the sprB-cat fusion in the WT E. coli MC4100 strain carrying the pK6-HilD plasmid or the pMPM-K6Ω vector. As expected, activity of the sprB-cat fusion was 3-fold lower in the E. coli strain than in the WT S. Typhimurium strain (Fig. 6B). Expression of HilD from pK6-HilD induced 3-fold the activity of sprB-cat in the E. coli strain (Fig. 6B), supporting that HilD induces expression of sprB directly. In agreement to these results, EMSAs revealed that purified MBP-HilD binds to the regulatory region upstream of sprB, from a concentration of 0.5 µM, but it does not bind to the regulatory region upstream of ppK, used as a negative control in these assays (Fig. 6C). Furthermore, previous results from ChIP-seq analyses indicate that HilD binds to the intergenic region upstream of sprB in vivo28,38.
Collectively, these results indicate that HilD positively regulates the expression of yobH through SprB.
HilD counteracts H-NS-mediated repression on sprB
HilD induces expression of target genes mainly by counteracting H-NS-mediated repression on the respective promoters2,36. To know whether HilD induces expression of sprB by a similar way, we analyzed if inactivation of H-NS leads to HilD-independent expression of this gene. Since a Salmonella ∆hns mutant exhibits a severe growth defect52,53, we inactivated H-NS activity by the overexpression of the H-NSG113D dominant negative mutant, which is affected in DNA binding activity but still forms heterodimers with the WT H-NS monomers54. For this purpose, activity of the sprB-cat fusion was quantified in the WT S. Typhimurium strain and its isogenic ∆hilD mutant containing the pT6-HNS-G113D or pT6-HNS-WT plasmids, which express H-NSG113D and WT H-NS, respectively, or containing the empty vector pMPM-T6Ω. Expression of H-NSG113D, but not WT H-NS, increased the activity of sprB-cat in the ∆hilD mutant, at similar levels to those reached by this fusion in the WT strain (Fig. 7A). Consistently, activity of sprB-cat was also induced in an E. coli ∆hns mutant, compared with the WT E. coli strain (Fig. S4). Furthermore, EMSAs revealed that purified H-NS-FLAG-His (H-NS-FH) protein binds to the regulatory region upstream of sprB, from a concentration of 0.45 µM, but it does not bind to the regulatory region upstream of ppK, used as a negative control in these assays, even at the highest protein concentration tested (0.7 µM) (Fig. 7B). Previous genome-wide binding studies indicate that H-NS interacts with the region upstream of sprB in vivo52,53. In contrast to the observed for sprB, the activity of the yobH-cat fusion was not increased in the E. coli ∆hns mutant and H-NS-FH did not bind to the regulatory region upstream of yobH (Fig. S5). These results show that H-NS directly represses expression of sprB, but not of yobH, and that when the activity of H-NS is inactivated, or when H-NS is absent, expression of sprB becomes independent of HilD, which supports that HilD acts on this gene as an anti-H-NS factor.
HilD directly displaces H-NS-mediated repression on sprB. (A) Activity of the sprB-cat transcriptional fusion from the psprB-cat plasmid, was determined in the WT S. Typhimurium SL1344 strain and its isogenic ∆hilD mutant containing or not the pMPM-T6Ω vector, or the pT6-HNS-WT or pT6-HNS-G113D plasmids, with (+) and without (−) induction (0.1% L-arabinose). CAT specific activity was quantified from samples of bacterial cultures grown for 9 h in LB at 37 °C. Means and standard deviations from three independent experiments performed in duplicate are shown. Statistically different values are indicated (***p < 0.001). (B) EMSAs were performed with purified H-NS-FH (0, 0.2, 0.45 and 0.7 µM) and a DNA fragment containing the regulatory region of sprB. A DNA fragment containing the regulatory region of ppK was used as a negative internal control. The DNA-protein complexes, indicated by an asterisk, were resolved in a nondenaturing 6% polyacrylamide gel and stained with ethidium bromide. (C) Competitive nonradioactive EMSAs between H-NS and HilD on the regulatory region of sprB. Purified H-NS-FH protein was added at 0.6 µM (lanes 3 to 8) and purified MBP-HilD protein was added at 0.2, 0.4, 0.6, 0.8 and 1 µM (lanes 4 to 8, respectively). No proteins were added in lane 1 and MBP-HilD was added at 1 µM in lane 2. The DNA-protein complexes were resolved in a nondenaturing 6% polyacrylamide gel. The upper panel shows the protein-DNA complexes stained with ethidium bromide and the lower panel shows the immunoblot detection of H-NS-FH from the DNA-protein complexes. Blots for DNA or protein detection were cropped from different gels.
To determine whether HilD indeed displaces H-NS from sprB, we performed competitive EMSAs. A DNA fragment carrying the regulatory region of sprB was first incubated with a constant concentration of H-NS-FH (0.6 µM) and then increasing amounts of MBP-HilD (0.2, 0.4, 0.6, 0.8 and 1 µM) were added. Binding reactions containing only H-NS-FH or MBP-HilD were also tested. The DNA-protein complexes were detected by staining the DNA fragments with ethidium bromide; additionally, the presence of H-NS-FH on these complexes was detected by Western blot with anti-FLAG antibodies. As shown in Fig. 7C, the DNA-H-NS complex was shifted by the presence of MBP-HilD to a slower-migrating complex similar to that formed only by MBP-HilD (upper panel); furthermore, the immunoblots showed that the presence of MBP-HilD decreased the amount of H-NS-FH bound to the tested DNA fragment (lower panel), which indicates that HilD is able to remove H-NS from sprB.
Altogether, these results demonstrate that HilD induces expression of sprB by antagonizing H-NS-mediated repression on this gene.
The HilD-SprB regulatory cascade controls expression of the slrP and ugtL virulence genes
Previous RNA-seq analyses indicate that HilD and SprB positively controls expression of several other genes in common, in addition to yobH, including slrP and ugtL28, which have been involved in Salmonella virulence55,56,57,58,59. To further define if HilD and SprB also act in a cascade fashion on slrP and ugtL, we constructed and analyzed cat transcriptional fusions carrying the regulatory region of the slrP or ugtL genes. Activity of the slrP-cat and ugtL-cat fusions was quantified in the WT S. Typhimurium strain and its derivative ∆hilD mutant containing the pK6-SprB plasmid or the pMPM-K6Ω vector. As a negative control, an invF-cat transcriptional fusion was also assessed; HilD induces expression of invF through HilA27,60,61. The three fusions tested showed a decreased activity in the ∆hilD mutant, with respect to their activity in the WT strain (Fig. 8A–C). Expression of SprB from pK6-SprB induced activity of the slrP-cat and ugtL-cat fusions, but not that of the invF-cat fusion, in the ∆hilD mutant (Fig. 8A–C), supporting that HilD controls the expression of slrP and ugtL through SprB, which is in agreement with data from ChIP-seq analyses showing SprB binding, but not HilD binding, on the regulatory regions of slrP and ugtL28.
The HilD-SprB regulatory cascade induces expression of the slrP and ugtL genes. Activity of the slrP-cat, ugtL-cat and invF-cat transcriptional fusions from the pslrP-cat, pugtL-cat and pinvF-cat plasmids, was determined in the WT S. Typhimurium SL1344 strain and its isogenic ∆hilD mutant containing the pMPM-K6Ω vector or the pK6-SprB plasmid. CAT specific activity was quantified from samples of bacterial cultures grown for 9 h in LB at 37 °C. Means and standard deviations from three independent experiments performed in duplicate are shown. Statistically different values are indicated (***p < 0.001; ****p < 0.0001).
Thus, our results, together with previous studies, indicate that the regulatory cascade formed by HilD and SprB controls expression of a subset of Salmonella virulence genes, including yobH, slrP and ugtL.
Discussion
Acquisition of SPI-1 was a pivotal event for the evolution of Salmonella pathogenicity, not only by the virulence factors encoded in this island, which provide ability to invade host cells, but also by the additional factors for invasion encoded outside SPI-1 that have been recruited through the control of their expression by the SPI-1 regulator HilD2,28.
In this study, we identify a novel invasion factor, YobH, whose expression is controlled by HilD. Our results demonstrate that YobH is required for the S. Typhimurium invasion of HeLa cells and mouse macrophages. In agreement with these results, a previous analysis by transposon-directed insertion-site sequencing (TraDIS) supports that YobH plays a role in the intestinal colonization of S. Typhimurium in chicks and cows, but not in the systemic infection in the mouse model56. The yobH gene is located outside SPI-1, in a chromosomal region conserved in many bacteria, including S. bongori and E. coli K-12; YobH shares 79% sequence identity with its ortholog from E. coli K-12. A previous study indicates that HilD directly regulates expression of the flhDC operon, encoding the master regulator of the flagellar genes, which is also conserved in E. coli K-12 and many other bacteria32,33. YobH and its orthologs from different bacteria are on average 80 amino acids long and have no an assigned function; they are annotated as putative membrane, exported or uncharacterized proteins. Our preliminary results support that YobH is secreted in S. Typhimurium (data not shown). How is YobH secreted and what is the specific function of YobH for invasion, are topics of our current investigation.
HilD induces expression of a high number of target genes by acting directly or through distinct regulators, in growth conditions that somehow resemble the intestinal environment (SPI-1-inducing conditions), such as those that we assessed in this study2. At present, three different regulatory cascades headed by HilD have been well characterized: the HilD-HilA-InvF, HilD-SsrA/SsrB and HilD-FlhDC cascades2,25,27,32,33,36,37. Additionally, HilD forms a feed-forward positive loop with HilC and RtsA, which amplifies the activation of the HilD-HilA-InvF cascade30,31, and probably also the activation of the other regulatory cascades and genes controlled directly by HilD. Our data, together with previous results obtained from genome-wide expression and binding analyses28, define an additional cascade formed by HilD to induce expression of virulence genes. In this regulatory cascade, HilD induces expression of the yobH, slrP and ugtL virulence genes through SprB, a Salmonella-specific LuxR-like regulator encoded in SPI-1. Previous studies revealed that HilD and RtsA induce expression of slrP by an undefined way62,63. SlrP (Salmonella leucine-rich repeat protein) is an effector protein with ubiquitin ligase activity that is translocated into mammalian cells through both T3SS-1 and T3SS-263,64. Previous reports support that SlrP plays a role in the intestinal colonization of S. Typhimurium in chicks, pigs, cows and mice, but not in the systemic infection in the mouse model55,56. UgtL is an inner membrane protein that mediates resistance to antimicrobial peptides by modifying lipid A in the lipopolysaccharide57,59; furthermore, it is involved in the activation of the PhoP/PhoQ two-component regulatory system in response to mildly acidic pH58. UgtL is required by S. Typhimurium for killing58 and for the intestinal colonization of mice59; moreover, TraDIS analysis supports that UgtL is important for the intestinal colonization of S. Typhimurium in pigs56. Importantly to note, expression of both slrP and ugtL is also controlled directly by the PhoP/PhoQ two-component system, in growth conditions that somehow mimic the intracellular environment of host cells (SPI-2-inducing conditions)63,65, where the HilD-mediated regulation on target genes is not evident25,36,63; in contrast, expression of yobH seems to be not regulated by PhoP/PhoQ45. PhoP forms with the SlyA regulator a feed-forward loop that controls expression of ugtL in SPI-2-inducing conditions65,66. Thus, expression of both ugtL and slrP is controlled by at least two distinct regulatory mechanisms that act in response to different environmental conditions. HilD-SprB and PhoP-SlyA would induce expression of ugtL and slrP in different niches where the activity of these genes is required for the Salmonella infection of hosts. For instance, activity of UgtL is needed for the intestinal colonization and for the systemic infection of mice58,59. On another hand, it is tempting to speculate that HilD-SprB helps to reach the levels of UgtL required for the subsequent UgtL-mediated activation of the PhoP/PhoQ system in response to acidic pH58, a cue present in the intracellular environment. Following this idea, it has been shown that activated PhoP represses expression of hilD, hilA and rtsA, and thus the SP-1 invasion genes67; therefore, the HilD-SprB-UgtL-PhoP/PhoQ pathway could work as an additional negative feedback control in the complex and dynamic regulatory network governing expression of Salmonella invasion genes.
Global expression and binding analyses indicate that SprB positively controls expression of yobH, slrP, ugtL and 20 genes more28, all these genes located outside SPI-1, including the sifB, yhgE, yibP, SL1344_3112, SL1344_0336 and SL1344_0337 genes that have been associated to virulence56,68. Therefore, the HilD-SprB cascade represents an additional branch that further expands the HilD virulence regulon, connecting the activity of several genes located outside SPI-1 with the capability for invasion of host cells encoded within SPI-1.
Our results demonstrate that HilD positively controls expression of sprB by acting on the regulatory region upstream of this gene. Previous studies indicate that HilD can also control expression of sprB by acting on the hilC gene, located upstream of sprB; a hilC-sprB transcript was detected in a previous study28 and direct regulation of hilC by HilD is well documented28,30,38,48,51. We show that HilD induces expression of sprB by directly displacing the repressor H-NS from the regulatory region upstream of this gene; a mechanism that HilD follows to induce expression of other target genes36,37,41,42,43. H-NS represses expression of hilC43,53,69,70, which suggest that HilD induces expression of the hilC-sprB transcript also by antagonizing H-NS mediated repression. In contrast to HilD, which is required for the expression of target genes only in the presence of H-NS, we found that SprB is required for the expression of yobH even in the absence of H-NS, which supports that it does not act as an anti-H-NS factor. There is growing evidence to suggest that other LuxR-like regulators mainly act as classical activators, which induce expression of target genes by favoring binding of the RNA polymerase on promoters71,72,73,74. Whether SprB antagonizes a repressor different to H-NS or whether it acts as a classical activator remains to be elucidated.
Our data reveal a novel Salmonella invasion factor and further define an additional regulatory cascade mediated by HilD for the expression of Salmonella virulence genes. A model that summarizes the results from this study is depicted in Fig. 9.
Model for the expression of YobH, SlrP and UgtL mediated by the HilD-SprB regulatory cascade. H-NS represses expression of sprB by binding the two promoter regions transcribing this gene. HilD binds to and thus displaces the H-NS repressor complex from these promoter regions, which allows expression of SprB that finally activates transcription of the yobH, slrP and ugtL virulence genes. Transcription of sprB from the promoter upstream of hilC and the effect of HilD and H-NS on this promoter were reported previously28,30,43,48,69. The previously defined regulation of ugtL and slrP by SlyA and/or PhoP is not depicted in the model but it is described in text.
Methods
Bacterial strains and growth conditions
Bacterial strains used in this study are listed in Table S1. Bacterial cultures for the determination of chloramphenicol acetyl transferase (CAT) activity and for Western blot assays were grown in LB as described previously25,26,36. When necessary, the medium was supplemented with the following antibiotics: ampicillin (200 μg/ml), streptomycin (100 μg/ml), kanamycin (20 μg/ml) or tetracycline, (12 μg/ml).
Construction of plasmids
Plasmids and primers used in this study are listed in Tables S1 and S2, respectively. To generate the yobH-cat, sprB-cat, slrP-cat and ugtL-cat transcriptional fusions, the regulatory regions of yobH, sprB, slrP and ugtL were amplified by PCR using the primer pairs SL1770-FW22/SL1770-RV11, sprB-catF/sprB-catR, slrPB2-Fw22/slrPH3-Rv11 and ugtL-Fw/ugtL-Rv, respectively, and chromosomal DNA from the WT S. Typhimurium strain as template. The resulting PCR products were purified with the Zymoclean Gel DNA Recovery Kit (Zymo Research), digested with BamHI and HindIII enzymes and then cloned into the pKK232-8 vector75 digested with the same restriction enzymes. To construct the p2795-YobH-FLAG plasmid, the yobH::3XFLAG gene was amplified by PCR using the primer pair SL1770-FW22/1770-SalIRv and chromosomal DNA from the DTM128 strain as template. This PCR product was digested with SalI and BamHI enzymes and then cloned into the p2795 vector76 digested with the same restriction enzymes. To construct the pK6-SprB plasmid, the sprB structural gene was amplified by PCR using the primer pair sprB-K6NcoI/sprB-K6PstI and chromosomal DNA from the WT S. Typhimurium strain as template. This PCR product was digested with NcoI and PstI enzymes and then cloned into the pMPM-K6Ω vector77 digested with the same restriction enzymes. pK6-SprB expresses SprB from an arabinose-inducible promoter.
Construction of deletion and 3XFLAG-tagged S. Typhimurium mutant strains
Non-polar deletion of the sprB, sinR or yobH genes in the S. Typhimurium SL1344 strain was performed by the λRed recombinase system, as reported previously78, using the respective primers described in Table S2, thus generating the strains DTM121, DTM123 and DTM124, respectively. The chromosomal yobH gene was 3XFLAG-tagged in the S. Typhimurium SL1344 strain using a previously reported method based on the λRed recombinase system79, thus generating the DTM127 (yobH::3XFLAG-kan) strain. P22 transduction was used to transfer the yobH::3XFLAG-kan allele from the strain DTM127 into the strains JPTM25 and DTM122, generating the strains DTM129 and DTM131, respectively. The kanamycin resistance cassette was excised from the strains DTM121, DTM124, DTM127, DTM129 and DTM131, by using the pCP20 plasmid expressing the FLP recombinase, as described previously78, generating the strains DTM122, DTM125, DTM128, DTM130 and DTM132, respectively. The complemented DTM126 strain was generated by inserting the yobH::3XFLAG-kan into the chromosome of the DTM125 strain, using a previously reported method based on the λRed recombinase system76 and the p2795-YobH-FLAG plasmid. All modified strains were verified by PCR amplification and sequencing.
Chloramphenicol acetyltransferase (CAT) assays
The CAT activity and protein quantification to calculate CAT specific activities were determined as previously described80.
Statistical analysis
Data were analyzed with GraphPad Prism 5.0 software (GraphPad Inc., San Diego, CA) using One-Way analysis of variance (ANOVA) with the Tukey’s multiple comparison test. A P-value of <0.05 was considered significant.
Electrophoretic mobility shift assays (EMSAs)
Fragments spanning the regulatory regions of yobH, hilA, sopB, sprB and ppK were obtained by PCR amplification using the primer pairs SL1770-FW22/SL1770-RV11, hilA1FBamHI/hilA2RHindIII, SigDBH1F/SigDH3R, sprB-catF/sprB-catR and PPK-Fw1/PPK-Rv1, respectively, and chromosomal DNA from the WT S. Typhimurium strain as template. Binding reactions were performed as described previously25,40. For competitive EMSAs, the sprB fragment was first incubated with 0.6 µM H-NS–FH for 15 min and then incubated with increasing concentrations MBP-HilD for an additional 20 min. Binding mixtures were electrophoretically separated in 6% nondenaturing acrylamide in 0.5X Tris-borate-EDTA buffer, at room temperature. DNA bands were visualized by staining with ethidium bromide, in an Alpha-Imager UV transilluminator (Alpha Innotech Corp.).
Expression and purification of proteins
Expression and purification of MBP-HilD and H-NS-FH were performed as described previously25,40.
Western blotting
Western blot assays were performed as described previously26,36. Anti-FLAG M2 monoclonal antibodies (Sigma) were used at 1:2,000 or 1:3,000 dilutions, for detection of YobH-FLAG and H-NS-FH, respectively. Anti-GroEL polyclonal antibodies were used at a dilution of 1:100,000. Horseradish peroxidase-conjugated secondary antibodies (Pierce), anti-mouse or anti-rabbit, were used at a dilution of 1:10,000. Blots were developed by incubation with the Western Lightning Chemiluminescence Reagent Plus (Perkin-Elmer) and then exposition to KodaK X-Omat films.
Invasion assays
Invasion of HeLa cells or RAW264.7 macrophages was determined by gentamicin protection assays as described previously40,81. Briefly, HeLa cells or RAW264.7 macrophages were grown in high-glucose Dulbecco’s Modified Eagle Medium (GIBCO 12100-046) supplemented with 10 mM sodium pyruvate solution, 20 mM L-glutamine and 10% (v/v) heat-inactivated fetal bovine serum, at 37 °C and a 5% CO2 atmosphere, in 24-well tissue culture plates. Monolayers of HeLa cells or RAW264.7 macrophages, from each well, were infected during 10 min with the respective bacterial suspension obtained from LB cultures, using a multiplicity of infection (MOI) of 40:1 and 10:1 (bacterial to eukaryotic cells), respectively. After the time of infection, monolayers were washed and then incubated during 1 h in DMEM containing 50 μg/ml gentamicin to eliminate extracellular bacteria. DMEM was removed and the HeLa cells and RAW264.7 macrophages from each well were lysed in 1 ml and 200 μl of 0.2% (w/v) sodium deoxycholate in 1X PBS, respectively. To obtain the intracellular CFUs per well, serial dilutions of each cell lysate were plated onto LB agar supplemented with 100 μg/ml streptomycin. CFUs from the starting inoculums were also quantified.
Data Availability
All data generated or analyzed during the current study are available from the corresponding author on reasonable request.
References
Haraga, A., Ohlson, M. B. & Miller, S. I. Salmonellae interplay with host cells. Nat. Rev. Microbiol. 6, 53–66 (2008).
Fabrega, A. & Vila, J. Salmonella enterica Serovar Typhimurium Skills To Succeed in the Host: Virulence and Regulation. Clin. Microbiol. Rev. 26, 308–341 (2013).
Haselbeck, A. H. et al. Current perspectives on invasive nontyphoidal Salmonella disease. Curr. Opin. Infect. Dis. 30, 498–503, https://doi.org/10.1097/QCO.0000000000000398 (2017).
Eng, S.-K. et al. Salmonella: A review on pathogenesis, epidemiology and antibiotic resistance. Front. Life Sci. 8, 284–293 (2015).
Porwollik, S. & McClelland, M. Lateral gene transfer in. Salmonella. Microbes Infect. 5, 977–989 (2003).
Hensel, M. Evolution of pathogenicity islands of Salmonella enterica. Int. J. Med. Microbiol. 294, 95–102 (2004).
Marcus, S. L., Brumell, J. H., Pfeifer, C. G. & Finlay, B. B. Salmonella pathogenicity islands: big virulence in small packages. Microbes Infect. 2, 145–156 (2000).
dos Santos, A. M. P., Ferrari, R. G. & Conte-Junior, C. A. Virulence Factors in Salmonella Typhimurium: The Sagacity of a Bacterium. Curr. Microbiol. 76, 762–773, https://doi.org/10.1007/s00284-018-1510-4 (2019).
Ilyas, B., Tsai, C. N. & Coombes, B. K. Evolution of Salmonella-Host Cell Interactions through a Dynamic Bacterial Genome. Front. Cell. Infect. Microbiol. 7, 428 (2017).
Desai, P. T. et al. Evolutionary Genomics of Salmonella enterica Subspecies. MBio 4, e00579–12 (2013).
Groisman, E. A. & Ochman, H. How Salmonella became a pathogen. Trends Microbiol. 5, 343–9 (1997).
Moest, T. P. & Méresse, S. Salmonella T3SSs: successful mission of the secret(ion) agents. Curr. Opin. Microbiol. 16, 38–44 (2013).
Hansen-Wester, I. & Hensel, M. Salmonella pathogenicity islands encoding type III secretion systems. Microbes Infect. 3, 549–559 (2001).
Bäumler, A. J. & Sperandio, V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature 535, 85–93 (2016).
Gart, E. V. et al. Salmonella Typhimurium and Multidirectional Communication in the Gut. Front. Microbiol. 7, 1827 (2016).
Knodler, L. A. et al. Dissemination of invasive Salmonella via bacterial-induced extrusion of mucosal epithelia. Proc. Natl. Acad. Sci. USA 107, 17733–8 (2010).
Laughlin, R. C. et al. Spatial Segregation of Virulence Gene Expression during Acute Enteric Infection with Salmonella enterica serovar Typhimurium. MBio 5 (2014).
Altier, C. Genetic and Environmental Control of Salmonella Invasion. J. Microbiol. 43, 85–92 (2005).
Lawhon, S. D., Maurer, R., Suyemoto, M. & Altier, C. Intestinal short-chain fatty acids alter Salmonella typhimurium invasion gene expression and virulence through BarA/SirA. Mol. Microbiol. 46, 1451–1464 (2002).
Hung, C.-C. et al. The intestinal fatty acid propionate inhibits Salmonella invasion through the post-translational control of HilD. Mol. Microbiol. 87, 1045–1060 (2013).
Golubeva, Y. A., Ellermeier, J. R., Cott Chubiz, J. E. & Slauch, J. M. Intestinal Long-Chain Fatty Acids Act as a Direct Signal To Modulate Expression of the Salmonella Pathogenicity Island 1 Type III Secretion System. MBio 7, e02170–15 (2016).
Eade, C. R. et al. Bile Acids Function Synergistically To Repress Invasion Gene Expression in Salmonella by Destabilizing the Invasion Regulator HilD. Infect. Immun. 84, 2198–2208 (2016).
Kim, K., Golubeva, Y. A., Vanderpool, C. K. & Slauch, J. M. Oxygen‐dependent regulation of SPI1 type three secretion system by small RNAs in Salmonella enterica serovar Typhimurium. Mol. Microbiol. 111, 570–587 (2019).
Lundberg, U., Vinatzer, U., Berdnik, D., von Gabain, A. & Baccarini, M. Growth phase-regulated induction of Salmonella-induced macrophage apoptosis correlates with transient expression of SPI-1 genes. J. Bacteriol. 181, 3433–7 (1999).
Bustamante, V. H. et al. HilD-mediated transcriptional cross-talk between SPI-1 and SPI-2. Proc. Natl. Acad. Sci. USA 105, 14591–6 (2008).
Martínez, L. C. et al. Integration of a complex regulatory cascade involving the SirA/BarA and Csr global regulatory systems that controls expression of the Salmonella SPI-1 and SPI-2 virulence regulons through HilD. Mol. Microbiol. 80, 1637–1656 (2011).
Ellermeier, J. R. & Slauch, J. M. Adaptation to the host environment: regulation of the SPI1 type III secretion system in Salmonella enterica serovar Typhimurium. Curr. Opin. Microbiol. 10, 24–9 (2007).
Smith, C., Stringer, A. M., Mao, C., Palumbo, M. J. & Wade, J. T. Mapping the Regulatory Network for Salmonella enterica Serovar Typhimurium Invasion. MBio 7, e01024–16 (2016).
Golubeva, Y. A., Sadik, A. Y., Ellermeier, J. R. & Slauch, J. M. Integrating global regulatory input into the Salmonella pathogenicity island 1 type III secretion system. Genetics 190, 79–90 (2012).
Ellermeier, C. D., Ellermeier, J. R. & Slauch, J. M. HilD, HilC and RtsA constitute a feed forward loop that controls expression of the SPI1 type three secretion system regulator hilA in Salmonella enterica serovar Typhimurium. Mol. Microbiol. 57, 691–705 (2005).
Saini, S., Ellermeier, J. R., Slauch, J. M. & Rao, C. V. The Role of Coupled Positive Feedback in the Expression of the SPI1 Type Three Secretion System in. Salmonella. PLoS Pathog. 6, e1001025 (2010).
Singer, H. M., Kühne, C., Deditius, J. A., Hughes, K. T. & Erhardt, M. The Salmonella Spi1 virulence regulatory protein HilD directly activates transcription of the flagellar master operon flhDC. J. Bacteriol. 196, 1448–57 (2014).
Mouslim, C. & Hughes, K. T. The Effect of Cell Growth Phase on the Regulatory Cross-Talk between Flagellar and Spi1 Virulence Gene Expression. PLoS Pathog. 10, e1003987 (2014).
Eichelberg, K. & Galán, J. E. The flagellar sigma factor FliA (σ28) regulates the expression of Salmonella genes associated with the centisome 63 type III secretion system. Infect. Immun. 68, 2735–43 (2000).
Ibarra, J. A. et al. Induction of Salmonella pathogenicity island 1 under different growth conditions can affect Salmonella-host cell interactions in vitro. Microbiology 156, 1120–33 (2010).
Martínez, L. C., Banda, M. M., Fernández-Mora, M., Santana, F. J. & Bustamante, V. H. HilD induces expression of Salmonella pathogenicity island 2 genes by displacing the global negative regulator H-NS from. ssrAB. J. Bacteriol. 196, 3746–55 (2014).
Banda, M. M., Zavala-Alvarado, C., Pérez-Morales, D. & Bustamante, V. H. SlyA and HilD Counteract H-NS-Mediated Repression on the ssrAB Virulence Operon of Salmonella enterica Serovar Typhimurium and Thus Promote Its Activation by OmpR. J. Bacteriol. 201, e00530–18 (2019).
Petrone, B. L., Stringer, A. M. & Wade, J. T. Identification of HilD-regulated genes in Salmonella enterica serovar Typhimurium. J. Bacteriol. 196, 1094–101 (2014).
Martínez-Flores, I. et al. In silico clustering of Salmonella global gene expression data reveals novel genes co-regulated with the SPI-1 virulence genes through HilD. Sci. Rep. 6, 37858 (2016).
Banda, M. M. et al. HilD and PhoP independently regulate the expression of grhD1, a novel gene required for Salmonella Typhimurium invasion of host cells. Sci. Rep. 8, 4841 (2018).
Schechter, L. M., Jain, S., Akbar, S. & Lee, C. A. The small nucleoid-binding proteins H-NS, HU, and Fis affect hilA expression in Salmonella enterica serovar Typhimurium. Infect. Immun. 71, 5432–5 (2003).
Olekhnovich, I. N. & Kadner, R. J. Crucial roles of both flanking sequences in silencing of the hilA promoter in Salmonella enterica. J. Mol. Biol. 357, 373–86 (2006).
Olekhnovich, I. N. & Kadner, R. J. Role of nucleoid-associated proteins Hha and H-NS in expression of Salmonella enterica activators HilD, HilC, and RtsA required for cell invasion. J. Bacteriol. 189, 6882–90 (2007).
Eichelberg, K., Hardt, W.-D. & Galan, J. E. Characterization of SprA, an AraC-like transcriptional regulator encoded within the Salmonella typhimurium pathogenicity island 1. Mol. Microbiol. 33, 139–152 (1999).
Colgan, A. M. et al. The Impact of 18 Ancestral and Horizontally-Acquired Regulatory Proteins upon the Transcriptome and sRNA Landscape of Salmonella enterica serovar Typhimurium. PLoS Genet. 12, e1006258 (2016).
Saini, S. & Rao, C. V. SprB is the molecular link between Salmonella pathogenicity island 1 (SPI1) and SPI4. J. Bacteriol. 192, 2459–62 (2010).
Schechter, L. M. & Lee, C. A. AraC/XylS family members, HilC and HilD, directly bind and derepress the Salmonella typhimurium hilA promoter. Mol. Microbiol. 40, 1289–99 (2001).
Olekhnovich, I. N. & Kadner, R. J. DNA-Binding Activities of the HilC and HilD Virulence Regulatory Proteins of Salmonella enterica Serovar Typhimurium. J. Bacteriol. 184, 4148–4160 (2002).
Pernestig, A. K., Melefors, O. & Georgellis, D. Identification of UvrY as the cognate response regulator for the BarA sensor kinase in Escherichia coli. J. Biol. Chem. 276, 225–31 (2001).
Goodier, R. I. & Ahmer, B. M. SirA orthologs affect both motility and virulence. J. Bacteriol. 183, 2249–58 (2001).
Lucas, R. L. & Lee, C. A. Roles of hilC and hilD in regulation of hilA expression in Salmonella enterica serovar Typhimurium. J. Bacteriol. 183, 2733–45 (2001).
Navarre, W. W. Selective Silencing of Foreign DNA with Low GC Content by the H-NS Protein in Salmonella. Science. 313, 236–238 (2006).
Lucchini, S. et al. H-NS Mediates the Silencing of Laterally Acquired Genes in Bacteria. PLoS Pathog. 2, e81 (2006).
Ueguchi, C., Suzuki, T., Yoshida, T., Tanaka, K. & Mizuno, T. Systematic Mutational Analysis Revealing the Functional Domain Organization of Escherichia coli Nucleoid Protein H-NS. J. Mol. Biol. 263, 149–162 (1996).
Tsolis, R. M. et al. Identification of a Putative Salmonella enterica Serotype Typhimurium Host Range Factor with Homology to IpaH and YopM by Signature-Tagged Mutagenesis. Infect. Immun. 67, 6385–93 (1999).
Chaudhuri, R. R. et al. Comprehensive Assignment of Roles for Salmonella Typhimurium Genes in Intestinal Colonization of Food-Producing Animals. PLoS Genet. 9, e1003456 (2013).
Shi, Y., Cromie, M. J., Hsu, F.-F., Turk, J. & Groisman, E. A. PhoP-regulated Salmonella resistance to the antimicrobial peptides magainin 2 and polymyxin B. Mol. Microbiol. 53, 229–241 (2004).
Choi, J. & Groisman, E. A. Activation of master virulence regulator PhoP in acidic pH requires the Salmonella-specific protein UgtL. Sci. Signal. 10, eaan6284 (2017).
Goto, R., Miki, T., Nakamura, N., Fujimoto, M. & Okada, N. Salmonella Typhimurium PagP- and UgtL-dependent resistance to antimicrobial peptides contributes to the gut colonization. PLoS One 12, e0190095 (2017).
Bajaj, V., Hwang, C. & Lee, C. A. hilA is a novel ompR/toxR family member that activates the expression of Salmonella typhimurium invasion genes. Mol. Microbiol. 18, 715–727 (1995).
Bajaj, V., Lucas, R. L., Hwang, C. & Lee, C. A. Co-ordinate regulation of Salmonella typhimurium invasion genes by environmental and regulatory factors is mediated by control of hilA expression. Mol. Microbiol. 22, 703–714 (1996).
Ellermeier, C. D. & Slauch, J. M. RtsA and RtsB coordinately regulate expression of the invasion and flagellar genes in Salmonella enterica serovar Typhimurium. J. Bacteriol. 185, 5096–108 (2003).
Cordero-Alba, M. & Ramos-Morales, F. Patterns of expression and translocation of the ubiquitin ligase SlrP in Salmonella enterica serovar Typhimurium. J. Bacteriol. 196, 3912–22 (2014).
Miao, E. A. et al. A conserved amino acid sequence directing intracellular type III secretion by Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 97, 7539–44 (2000).
Shi, Y., Latifi, T., Cromie, M. J. & Groisman, E. A. Transcriptional control of the antimicrobial peptide resistance ugtL gene by the Salmonella PhoP and SlyA regulatory proteins. J. Biol. Chem. 279, 38618–25 (2004).
Perez, J. C., Latifi, T. & Groisman, E. A. Overcoming H-NS-mediated transcriptional silencing of horizontally acquired genes by the PhoP and SlyA proteins in Salmonella enterica. J. Biol. Chem. 283, 10773–83 (2008).
Palmer, A. D., Kim, K. & Slauch, J. M. PhoP-mediated repression of the SPI1 T3SS in Salmonella enterica serovar Typhimurium. J. Bacteriol. JB.00264–19, https://doi.org/10.1128/JB.00264-19 (2019).
Freeman, J. A., Ohl, M. E. & Miller, S. I. The Salmonella enterica Serovar Typhimurium Translocated Effectors SseJ and SifB Are Targeted to the Salmonella-Containing Vacuole. Infect. Immun. 71, 418–27 (2003).
Ono, S. et al. H-NS is a part of a thermally controlled mechanism for bacterial gene regulation. Biochem. J. 391, 203–13 (2005).
Baños, R. C. et al. Differential Regulation of Horizontally Acquired and Core Genome Genes by the Bacterial Modulator H-NS. PLoS Genet. 5, e1000513 (2009).
Ball, A. S. & Kessel, J. C. The master quorum‐sensing regulators LuxR/HapR directly interact with the alpha subunit of RNA polymerase to drive transcription activation in Vibrio harveyi and Vibrio cholerae. Mol. Microbiol. 111, 1317–1334, https://doi.org/10.1111/mmi.14223 (2019).
White, C. E. & Winans, S. C. Identification of amino acid residues of the Agrobacterium tumefaciens quorum-sensing regulator TraR that are critical for positive control of transcription. Mol. Microbiol. 55, 1473–86 (2005).
Finney, A. H., Blick, R. J., Murakami, K., Ishihama, A. & Stevens, A. M. Role of the C-Terminal Domain of the Alpha Subunit of RNA Polymerase in LuxR-Dependent Transcriptional Activation of the lux Operon during Quorum Sensing. J. Bacteriol. 184, 4520–8 (2002).
Stevens, A. M., Fujita, N., Ishihama, A. & Greenberg, E. P. Involvement of the RNA Polymerase α-Subunit C-Terminal Domain in LuxR-Dependent Activation of the Vibrio fischeri Luminescence Genes. J. Bacteriol. 181, 4704–7 (1999).
Brosius, J. Plasmid vectors for the selection of promoters. Gene 27, 151–160 (1984).
Husseiny, M. I. & Hensel, M. Rapid method for the construction of Salmonella enterica Serovar Typhimurium vaccine carrier strains. Infect. Immun. 73, 1598–605 (2005).
Mayer, M. P. A new set of useful cloning and expression vectors derived from pBlueScript. Gene 163, 41–46 (1995).
Datsenko, K. A. & Wanner, B. L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97, 6640–5 (2000).
Uzzau, S., Figueroa-Bossi, N., Rubino, S. & Bossi, L. Epitope tagging of chromosomal genes in. Salmonella. Proc. Natl. Acad. Sci. USA 98, 15264–9 (2001).
Puente, J. L., Bieber, D., Ramer, S. W., Murray, W. & Schoolnik, G. K. The bundle-forming pili of enteropathogenic Escherichia coli: transcriptional regulation by environmental signals. Mol. Microbiol. 20, 87–100 (1996).
Pérez-Morales, D. et al. The transcriptional regulator SsrB is involved in a molecular switch controlling virulence lifestyles of Salmonella. PLOS Pathog. 13, e1006497 (2017).
Acknowledgements
This work was supported by grants from the Dirección General de Asuntos del Personal Académico de la UNAM/México (IN202418) and from the Consejo Nacional de Ciencia y Tecnología (CONACYT)/México (254531) to V.H.B. M.M.B. was supported by a pre-doctoral fellowship from CONACYT (403748). R.M. was supported by a master fellowship from CONACYT (367095). We thank D. Pérez-Morales for constructing the DTM123 strain and the slrP-cat and ugtL-cat transcriptional fusions; F.J. Santana and M. Fernández-Mora for technical assistance; I. Martínez-Flores for critical reading of the manuscript; and J.C.D. Hinton and J.L. Puente for sharing results from transcriptomics before publication.
Author information
Authors and Affiliations
Contributions
V.H.B. designed and supervised the research; M.M.B. and R.M. performed experimental research; M.M.B., R.M. and V.H.B. analyzed data; and M.M.B. and V.H.B. wrote the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Banda, M.M., Manzo, R. & Bustamante, V.H. HilD induces expression of a novel Salmonella Typhimurium invasion factor, YobH, through a regulatory cascade involving SprB. Sci Rep 9, 12725 (2019). https://doi.org/10.1038/s41598-019-49192-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-49192-z
This article is cited by
-
Regulation of gene expression by protein lysine acetylation in Salmonella
Journal of Microbiology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.