Abstract
Small numbers of hematopoietic stem cells (HSCs) balance self-renewal and differentiation to produce the diversity and abundance of cell types that make up the blood system. How nutrients are recruited to support this massive differentiation and proliferation process remains largely unknown. The unique metabolism of adult HSCs, which rely on glycolysis and glutaminolysis, suggests a potential role for the post-translational modification O-GlcNAc as a critical nutrient signal in these cells. Glutamine, glucose, and other metabolites drive the hexosamine biosynthetic pathway (HBP) ultimately leading to the O-GlcNAc modification of critical intracellular targets. Here, we used a conditional targeted genetic deletion of the enzyme that removes O-GlcNAc, O-GlcNAcase (OGA), to determine the consequences of blocked O-GlcNAc cycling on HSCs. Oga deletion in mouse HSCs resulted in greatly diminished progenitor pools, impaired stem cell self-renewal and nearly complete loss of competitive repopulation capacity. Further, early T cell specification was particularly sensitive to Oga deletion. Loss of Oga resulted in a doubling of apoptotic cells within the bone marrow and transcriptional deregulation of key genes involved in adult stem cell maintenance and lineage specification. These findings suggest that O-GlcNAc cycling plays a critical role in supporting HSC homeostasis and early thymocyte development.
Similar content being viewed by others
Introduction
The mammalian hematopoietic system is comprised of diverse cell types that each have unique functions. There are erythrocytes that carry oxygen, lymphocytes that are the cornerstones of adaptive immunity and myeloid cells, that have unique roles ranging from innate immunity to blood clotting. As abundant and diverse as these cells are, they all originate from a limited pool of hematopoietic stem cells (HSCs) residing in the bone marrow. HSCs are required to maintain homeostasis despite constant insults, like bleeding or infection. To do this, HSCs must strike a balance between quiescence to maintain HSC pools and proliferation and differentiation. One way that HSCs regulate this balance is through metabolic reprogramming, in fact, HSCs rely heavily on glycolysis and glutaminolysis to meet their unique energy needs1,2. However, what regulates how nutrients are recruited and support HSC homeostasis remain largely unknown. Here, we provide evidence that cycling of the nutrient-sensitive post-translational modification O-GlcNAc plays a key role in this process.
Glucose, glutamine, and other nutrient-derived metabolites help fuel the hexosamine biosynthetic pathway (HBP), ultimately contributing to multiple glycan classes including the post-translational modification O-GlcNAc. O-GlcNAc is a dynamic modification attached to serines and threonines of thousands of intracellular proteins3. Added to proteins by O-GlcNAc transferase (OGT) and removed by O-GlcNAcase (OGA), O-GlcNAc cycling integrates nutrient availability with molecular processes like transcription, translation, proteostasis and signaling4. Because it plays diverse roles in cell physiology, O-GlcNAcylation is poised to influence cells reliant on glycolysis and glutaminolysis like HSCs and T cells. In fact, Ogt is essential for embryonic stem cell (ESC) maintenance, differentiation and lineage specification5,6,7. O-GlcNAc cycling has been linked to polycomb repression8,9,10,11 and trithorax activation8,12,13 which act to define and shape early cell fate decisions. Many of the core components of the pluripotency network, including the Yamanaka factors OCT4, SOX2 and cMYC6,14 have been identified as O-GlcNAc modified. Indeed, mouse deletion models have shown that the enzymes of O-GlcNAc cycling are vital for early development, with Ogt deletion being embryonic lethal5,15, and Oga deletion perinatal lethal16. Further, a recent report suggested O-GlcNAc cycling influences in vitro erythrocyte differentiation17.
Downstream of HSCs, the T cell lineage also utilizes glucose and glutamine throughout development in the thymus. In fact, dynamic increases in protein O-GlcNAc levels has been defined at key stages of T cell development18. Dynamic O-GlcNAcylation has been shown to be essential for generation of mature CD4+ and CD8+ T cells15,18. Although O-GlcNAc addition is required for thymic development15,18, it remains unclear how blocked O-GlcNAc turnover by selective loss of Oga might impact early thymocytes. Moreover, the role of dynamic O-GlcNAcylation had not been investigated in earlier hematopoietic cell populations and hematopoiesis. Because O-GlcNAc cycling is a crucial feature in cell populations reliant on glycolysis and glutaminolysis, we anticipated that stable O-GlcNAcylation would disrupt HSC homeostasis. In fact, O-GlcNAc has been shown to regulate key factors in HSC maintenance like HIF1α19 and cMYC14,18. Here, we investigated the consequences of blocking O-GlcNAc turnover on HSC homeostasis and T cell development. We found that mice in which Oga had been selectively deleted in HSCs had depleted HSC and progenitor populations with diminished self-renewal and competitive repopulation capacity. Loss of Oga resulted in an increase in apoptosis and transcriptional analysis revealed that nutrient transport and FGF signaling likely contributed to HSC dysfunction in mutant HSCs. Our data suggested that the processes of O-GlcNAc addition and removal each play a key part in regulating critical steps of hematopoiesis. These findings have important implications for the role of hexosamine signaling in the maintenance of stem cell populations giving rise to immune homeostasis.
Results
Tissue specific Oga deletion resulted in diminished HSC and progenitor pools
Previous inquiries into the role of O-GlcNAc cycling in stem cells have largely focused on altering Oga or Ogt in cultured embryonic stem cells. However, little is known about the function of O-GlcNAc cycling in an in vivo adult stem cell population. To test our hypothesis that O-GlcNAc cycling supports HSC function and immune homeostasis, we decided to specifically delete Oga in the hematopoietic lineage and assess the consequences on HSC maintenance and differentiation. Our laboratory previously generated a floxed allele of the Oga locus in mouse (Ogafl/fl)16. To make a hematopoietic lineage specific Oga mutant mouse, we bred the Ogafl/fl with the Vav-iCre transgenic mouse20 from Jax (stock number 008610). The resulting mouse (OgaVav-Cre) deleted Oga in HSCs. To ensure Oga deletion, we performed a fluorescence based OGA activity assay21 and found diminished OGA activity in bone marrow from OgaVav-Cre mice compared to their wildtype littermates (Fig. 1a). To test whether there was an increase in O-GlcNAc resulting from the Oga deletion, we assessed O-GlcNAc levels in bone marrow and liver by western blot. Here, we found elevated O-GlcNAc levels specifically in bone marrow from the OgaVav-Cre mice (Fig. 1b,c). Importantly, we were unable to detect Oga transcripts in Lin−Sca+Kit+ bone marrow cells from these mice (Supplementary Fig. S5), nor was there a change in endogenous Vav expression in these mutant cells (Supplementary Table S1). These experiments confirmed tissue-specific Oga deletion in HSCs from the OgaVav-Cre mice, resulting in increased O-GlcNAcylation.
Deletion of Oga in hematopoietic stem cells. (a) OGA activity was quantified with an established in vitro fluorescence assay21 using lysates from wildtype (WT) or OgaVav-Cre bone marrow, N = 3, error bars represent standard deviation. (b) O-GlcNAc was assessed by western blot using lysates from liver and bone marrow of WT and OgaVav-Cre (Vav-Cre) mice using RL2 for O-GlcNAc and β-Actin as loading control. Lysates from liver and bone marrow were run out on separate gels and transferred to nitrocellulose membranes (full length blots can be found in Supplemental Fig. S7) that were cut at 55 kDa to allow for simultaneous quantitation of O-GlcNAc and β-Actin. (c) Quantitation of western blot from panel b with O-GlcNAc intensity normalized to β-Actin intensity, with error bars representing range. Black bars represent wildtype and blue bars represent OgaVav-Cre.
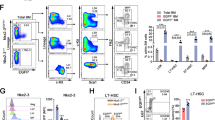
The hematopoietic system is comprised of increasingly specified progenitor cells, ultimately terminating in a fully differentiated lineage. Each of these distinct cell populations display unique cell surface markers that allow analysis by flow cytometry. In order to assess the consequences of Oga deletion on HSC maintenance and differentiation we analyzed HSC and progenitor cell populations in bone marrow from OgaVav-Cre mice in comparison to their wildtype littermates. The OgaVav-Cre mice had a significant decrease in total bone marrow cells as compared to wildtype (Fig. 2a). Using flow cytometry analysis, we detected significantly decreased progenitor and HSC populations, including the LK (Lin−Kit+) and the LSK (Lin−Sca-1+Kit+) progenitor populations (Fig. 2b,c). When gated on the LSK, further analysis indicated that the majority of the deficiency of the LSK population resulted from ~50% decrease in the long-term HSC (LT-HSC, CD150+Flt3−) and lymphoid-primed multipotent progenitor (LMPP, CD150+Flt3+) populations (Fig. 2b,c). Reduced LT-HSC pools suggested that deletion of Oga impaired maintenance of these stem cells. Investigation of further specified progenitor populations also uncovered significant reductions in common lymphoid progenitors (CLP, KitintFlt3+CD127+) (Fig. 2b,c) and granulocyte-macrophage progenitors (GMP, Lin−Kit+CD16/32+CD34+) (Fig. 2b,c). Further analysis of the CLP population using Ly6D, a marker of B cell progenitors (BLP)22, indicated significant decreases in this population as compared to wildtype (Fig. 2b,c). Although total cell numbers were dramatically decreased in the mutant mice, the only population that was significantly decreased in relative frequency was CLP, indicating that the lymphoid lineage was particularly sensitive to Oga deletion (Supplementary Fig. S1a). Together, these data indicated that OGA was required for normal HSC maintenance and that without OGA there were substantial decreases in the HSC pools as well as further differentiated progenitor cell populations.
Reduced bone marrow progenitor populations in OgaVav-Cre mice. (a) Quantitation of total number of bone marrow cells from the indicated genotype. (b) Representative flow cytometric analysis, with boxes depicting gating, of long-term hematopoietic stem cells (LT-HSC, Lin−Sca1+Kit+ CD150+Flt3−), short-term hematopoietic stem cells (ST-HSC, Lin−Sca1+Kit+ CD150−Flt3−), multipotent progenitors (MPP, Lin−Sca1+Kit+ CD150−Flt3+) and lymphoid primed MPP (LMPP, Lin−Sca1+Kit+ CD150−Flt3+), common lymphoid progenitors (CLP, Lin−KitintFlt3+CD127+), B cell-biased lymphoid progenitors (BLP, Lin−KitintFlt3+CD127+Ly6d+) granulocyte/macrophage progenitors (GMP, Lin−Kit+Sca−CD16/32+CD34+) and common myeloid progenitors (CMP, Lin−Kit+Sca−CD16/32−CD34+). (c) Graphs depicting the absolute numbers of the indicated cell populations for the indicated genotype. Black bars represent wildtype and blue bars represent OgaVav-Cre. N = 4–6, error bars represent standard deviation, *p < 0.05, **p < 0.01 as determined by t-test.
Oga deficiency resulted in impaired thymocyte development
O-GlcNAc addition by the activity of OGT has an established role in T cell development and activation15,18,23,24,25,26,27. Early thymic progenitor cells are CD4 and CD8 double negative (DN), and T cell receptor (TCR) negative. DN thymocytes are further subdivided based on the expression of surface markers. DN1 cells are heterogeneous and can give rise to T cells, NK cells, B cells and macrophages. TCR rearrangement begins in DN2 cells. Commitment to the T cell lineage occurs in the transition from the DN2 to DN3 stage. DN3 cells that have successfully rearranged their TCRβ-chain, form a pre-TCR complex with a pre-TCRα chain28. This pre-TCR enforces β-selection and supports proliferation and differentiation, transitioning cells from DN3 to DN4. This process increases glucose and glutamine uptake with a concomitant increase in O-GlcNAc levels18. In fact, mice that conditionally deleted Ogt had a phenotype consistent with a failure of β-selection and had decreased numbers of thymocytes as well as decreased mature CD4+ and CD8+ T cells15,18.
While OGT had been previously shown to be important for T cell development, it has remained unknown how blocked O-GlcNAc cycling might impact this process. To answer this question, we systematically investigated thymocyte development in the OgaVav-Cre mice as compared to wildtype littermates. OgaVav-Cre mice had significantly decreased numbers of thymocytes (Fig. 3a). Consistent with the decrease in lymphoid progenitor populations, analysis of thymocyte populations by flow cytometry revealed a significant decrease in the cell number of all populations (Fig. 3b,c), including all DN populations, as well as the CD4/CD8 double positive (DP) and single positive populations. Most strikingly, there was also a significant decrease in frequency in the earliest detectable thymic progenitors (early ETP, Lin−Kit+CD25−Flt3+) as well as the DN2a and DN2b (Lin−Kit+CD25+CD44+) pools (Fig. 3b, Supplementary Fig. S1a). Further analysis of the spleen to assess peripheral populations indicated decreased splenocytes as well as decreased populations of CD8, CD4 T cells and B cells (Fig. 4a–c). These data suggest that the early HSC loss resulted in decreased cell populations that were maintained throughout development and in movement to the periphery.
Decreased thymocytes in OgaVav-Cre mice. (a) Quantitation of total number of thymocytes from the indicated genotype. (b) Representative flow cytometric analysis, with boxes depicting gating of thymus cells including early thymic progenitors (ETP, Kit+CD25−) DN2/3 (CD25+), Early ETPs (Kit+CD25−Flt3+), DN2a (CD25+Kit+CD44+), DN2b (CD25+KitintCD44+), DN3 (CD25+Kit−CD44−) and analysis of double positive (DP) CD8+ and CD4+ T cells. (c) Graphs depicting the absolute numbers of the indicated cell populations for the indicated genotype. Black bars represent wildtype and blue bars represent OgaVav-Cre. N = 4–6, error bars represent standard deviation, *p < 0.05, **p < 0.01, ***P < 0.001, ****P < 0.0001 as determined by t-test.
Reduced peripheral lymphoid populations in OgaVav-Cre mice. (a) Quantitation of total number of splenocytes from the indicated genotype. (b) Representative flow cytometric analysis of CD8+, CD4+ T cells, and B cell (CD4−CD8−B220+) populations from mouse spleen from the indicated genotype. (c) Graph depicting the absolute numbers of the indicated cell populations for the indicated genotype. Black bars represent wildtype and blue bars represent OgaVav-Cre. N = 6, error bars represent standard deviation, *p < 0.05, **p < 0.01, ***P < 0.001, as determined by t-test.
Impaired self-renewal and competitive repopulation capacity of OgaVav-Cre HSCs
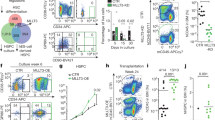
Two key characteristics of HSCs is the ability to self-renew and differentiate throughout the lifetime of an organism. Despite substantially reduced numbers of bone marrow HSCs, the surviving HSCs were able to differentiate (Figs 2–4). Accordingly, we observed normal in-vitro T cell differentiation from LSKs isolated from the OgaVav-Cre mice (Supplemental Fig. S2). Thus, we next wanted to directly test the impacts of OGA deletion on self-renewal and repopulation capacity. In order to examine the ability of Oga deficient HSCs to self-renew, we turned to an in-vitro colony forming unit (CFU) assay. For this experiment we plated whole bone marrow cells as well as sorted LT-HSCs (CD150+ LSK cells) isolated from OgaVav-Cre and wildtype mice. After 10–14 days of growth in methylcellulose, we found ~20% decrease in the number of colonies from the OgaVav-Cre derived cells versus the wildtype for both the whole bone marrow and LT-HSCs (Fig. 5a,b). To assess self-renewal, these cells were re-plated in methylcellulose and incubated again to form colonies. After re-plating, there was a greatly diminished ability of the OgaVav-Cre cells in the colony forming assay as compared to wildtype. Cells derived from whole bone marrow had ~80% decrease in number of colonies formed compared to wildtype and the LT-HSCs had ~50% decrease (Fig. 5a,b). After plating for a third time, the LT-HSCs had ~80% decrease in number of colonies formed as compared to wildtype (Fig. 5b). There were not enough OgaVav-Cre whole bone marrow cells to allow for a third plating. The decreases in colony numbers were not enriched for a specific cell-type (Supplementary Fig. S3). Therefore, these findings support the conclusion that blocked O-GlcNAc cycling impaired self-renewal ability of HSCs.
Impaired self-renewal and diminished competitive repopulation capacity of OgaVav-Cre HSCs. (a) 3 × 104 bone marrow cells were plated in methylcellulose from the indicated genotype and colonies were counted 10–14 days after plating. For 2nd plating, 1 × 104 cells were plated with colonies counted at 10–14 days after re-plating. Number of wildtype colonies were set to 100% and number of mutant colonies were graphed accordingly. (b) Bone marrow cells from the indicated genotype were sorted to obtain LT-HSCs (Lin−Sca1+Kit+ CD150+Flt3−). 500 LT-HSCs were plated in methylcellulose and number of colonies were counted at 10–14 in culture. For re-plating, 1 × 104 cells were re-plated and counted 10–14 days in culture. Number of wildtype colonies were set to 100% and number of mutant colonies were graphed accordingly. (a,b) Black bars represent wildtype and blue bars represent OgaVav-Cre. N = 3, error bars represent standard deviation, *p < 0.05, **p < 0.01 as determined by student’s t-test. (c,d) CD45.1+ mice were irradiated and reconstituted with wildtype (WT) or OgaVav-cre bone marrow progenitors (CD45.2+) mixed with equal numbers of WT CD45.1+ bone marrow progenitors. CD45.1 and CD45.2 were used to distinguish between donor and WT competitor-derived cells from bone marrow after 10 weeks of reconstitution by flow cytometry. (c) Representative plot showing donor chimerism in the LSK compartment at 10 weeks of reconstitution. (d) Donor chimerism in HSC, LSK and one downstream cell lineage (granulocytes). (c,d) Black bars represent wildtype and blue bars represent OgaVav-Cre. N = 3, error bars represent standard deviation, P = 0.001 as determined by 2-way ANOVA.
In order to directly assess competitive fitness of the OgaVav-Cre HSCs, we set up competitive bone marrow chimera mice. Here, we assessed the ability of OgaVav-Cre cells to engraft and reconstitute the hematopoietic system while in competition with wildtype cells. CD45.1+ mice were irradiated and reconstituted with wildtype or OgaVav-Cre bone marrow progenitor cells (CD45.2+) mixed with equal numbers of wildtype CD45.1+ bone marrow progenitor cells. CD45.1 and CD45.2 were used to distinguish between donor and wildtype competitor-derived cells by flow cytometry. We first analyzed blood granulocytes 5 weeks after reconstitution. We found that both wildtype as well as heterozygote donor cells were able to effectively compete with wildtype recipient cells, whereas the OgaVav-Cre did not contribute (Supplementary Fig. S4). Next, we did a more thorough analysis at 10 weeks after reconstitution and found that wildtype donor cells competed effectively with the wildtype recipient cells to reconstitute ~45%, 25% and 33% of the HSC, LSK, and granulocytes (Fig. 5c,d) of the chimeric mice, respectively. However, OGA depleted cells only reconstituted ~1% of the HSC, LSK, and granulocytes in the chimeric mice (Fig. 5c,d). These data suggested severe impairment of repopulation ability resulting from a decline in HSC maintenance in the mutants at both 5 and 10 weeks after the competitive bone marrow chimeras were initiated.
Increased apoptosis in OgaVav-Cre bone marrow populations
To better define the underlying causes of the HSC deficiency in the OgaVav-Cre mice, we assessed proliferation and apoptosis of the mutant LSKs versus wildtype. Both a decrease in proliferation and an increase in proliferation can lead to a loss of HSC populations and diminished competitive repopulation capacity. Whereas a decrease in proliferation gives the wildtype competitors a distinct advantage, a highly proliferative HSC pool causes HSC exhaustion because these cells should be largely quiescent29. Alternatively, an increase in apoptosis would also deplete cell populations, impair self-renewal and give a competitive advantage to wildtype cells. To assess these possibilities, we performed cell cycle analysis by DAPI staining on LSK cells isolated from wildtype or OgaVav-Cre mice and both had similar cell cycle profiles (Fig. 6a, Supplemental Fig. S5a).
Normal proliferation but increased apoptosis of OgaVav-Cre bone marrow progenitors. (a) Percentage of LSK cells in the indicated cell cycle stage from each genotype was measured by DAPI staining and quantified by flow cytometry. Representative flow cytometric analysis can be found in Supplemental Fig. S3a. (b) Apoptosis was determined for the indicated genotypes and cell populations, by Annexin V positive and PI negative staining quantified by flow cytometry analysis. Representative flow cytometric analysis can be found in Supplemental Fig. S3b. Black bars represent wildtype and blue bars represent OgaVav-Cre. N = 3, error bars represent standard deviation, *p < 0.05, **p < 0.01 as determined by t-test.
Next, we analyzed apoptosis in the LSK populations by Annexin V and PI staining. The OgaVav-Cre cells showed approximately double the number of apoptotic LSK cells as their wildtype counterparts (Fig. 6b, Supplemental Fig. S5b), with an increase in apoptosis from about 2.5% to 5%. To assess if this effect on apoptosis was specific to the stem cell population we also analyzed Annexin V and PI staining in all lineage negative populations as well as the lineage positive population, and found a similar doubling of apoptotic cells, from about 2% to 4%. This increase in apoptosis without any change in proliferation rate likely contributed to the competitive disadvantage of the mutant cells as well as the decrease in cell populations.
Transcriptional deregulation of genes in OgaVav-Cre progenitor cells
Our data indicated that hyper-O-GlcNAcylation resulted in increased apoptosis, HSC dysfunction and a decline in stem cell maintenance. Both stem cell maintenance and differentiation require tight transcriptional regulation. In fact, O-GlcNAc is essential for proper transcriptional control by directly modifying RNA POLII30,31,32, modifying histones33,34,35,36 and through interactions with epigenetic regulators like PcG8,9,10,11, TRX8,12,13, and TET37,38,39,40 proteins. Interestingly, microarray analysis of Oga−/− mouse embryonic fibroblasts (MEFs) indicated transcriptional deregulation of genes necessary for HSC maintenance including Hif1α, Tal1, Lyl1 and Cdkn1c16. Therefore, we hypothesized that in addition to the increased apoptosis, transcriptional deregulation also contributed to HSC dysfunction of Oga deficient HSCs. To assess whether there were transcriptional changes in the Oga mutants, we performed RNA-Seq on sorted LSK cells derived from the bone marrow of OgaVav-Cre mice in comparison to wildtype. We identified 41 significantly downregulated and 12 upregulated genes (Fig. 7a,b, Supplemental Table S1). Bioinformatic analysis using Genomatix indicated enrichment of pathways involved in HSC function within the deregulated genes. Interestingly, 3 out of the 10 most significantly deregulated gene ontology (GO) terms (Fig. 7c) had to do with nutrient uptake and signaling (glucose transport, glycoprotein transport and glutamine transport). HSCs rely significantly on glycolysis and glutaminolysis for their energy needs and changes in glucose and glutamine uptake could have consequences on metabolic regulation. Further, the most significantly upregulated (Slc1a5) and downregulated (Fgf3) genes (Fig. 7b,d, Supplemental Fig. S6) have defined roles in self-renewal and lineage specification of adult stem cells41,42. Thus, these data suggested that O-GlcNAc cycling contributes to transcriptional networks regulating signaling and nutrient uptake in hematopoietic stem cells.
Transcriptional deregulation of genes involved in stem cell function. RNA-Seq was performed on sorted LSK cells from 3 OgaVav-Cre and 3 wildtype mice. Genomatix analysis called 41 significantly downregulated and 12 significantly upregulated genes represented as a heatmap (using RPKMs) (a) and volcano plot (b). (b) Oga was excluded from volcano plot for scale, genes with a log2(foldchange) ± >1.25 and p < 0.005 are labelled. (c) Top 10 enriched gene ontology terms (GO) among the deregulated genes as determined by Genomatix. (d) Representative IGV view of the most significantly down regulated (Fgf3) and upregulated (Slc1a5) genes.
Discussion
Stem cells are characterized by their unique metabolic profiles and transcriptional networks. Their reliance on glycolysis allows for the production of metabolic intermediates that provide the building blocks for growth, and contributes to survival in a hypoxic environment. Tight transcriptional regulation enforces the pluripotency state of stem cells and permits controlled fate specification. At the nexus of these processes is O-GlcNAcylation, which integrates metabolism with cell physiology. Physiologically, the regulation of O-GlcNAc cycling is quite complex. Levels of O-GlcNAc are regulated in a number of ways including; (1) feedback inhibition of UDP-GlcNAc levels43, (2) microRNAs44,45,46, (3) a unique intron mechanism which limits the levels of OGT produced47 and (4) varying levels and activities of OGA. This last mechanism involving the regulated expression of O-GlcNAcase is certain to play a pivotal role in regulating the physiological levels of O-GlcNAc. Here, we focused on deleting Oga to assess the impact of O-GlcNAc cycling on HSC homeostasis and early T cell development.
Our lab, and others, have shown that OGA is essential for normal differentiation of mouse embryonic stem cells6,7. The Oga gene is located in the highly evolutionarily conserved Lbx1/Ladybird NK cluster. The Lbx genes have essential roles in development, including key functions in neural and mesodermal specification48. Despite having a clear role in embryonic stem cell biology, it’s in vivo role in adult stem cells had not previously been investigated. Because knocking out Ogt or Oga in the mouse is lethal5,15,16, we analyzed a tissue-specific deletion of Oga. This was done by crossing the Ogafl/fl mice with Vav-iCre mice to conditionally delete Oga in HSCs. Thus, while OGT could properly modify proteins, this modification could not be removed in the hematopoietic lineage of these mutant mice. HSCs differentiate towards increasingly specified progenitors, with distinct cell surface markers that allowed for quantitation by flow cytometry. We found that OgaVav-Cre mice had a substantially decreased HSC population as well as diminished numbers of intermediate progenitors. In-vitro CFU assays indicated that the Oga deficient HSCs had impaired self-renewal compared to wildtype. Further, when put in competition with wildtype HSCs, the mutant HSCs were unable to contribute to repopulation of an irradiated mouse. Together these data suggested that OGA was required for normal HSC function and that elevated O-GlcNAc had consequences on HSC maintenance, fitness and competitive repopulation capacity.
O-GlcNAc is required in stem cells to maintain the pluripotency transcriptional network, and has been proposed to be an essential component regulating stem cell self-renewal by modifying transcription factors like OCT4, cMYC and SOX26. Previous transcriptional analysis of Oga−/− MEFs found significant enrichment of deregulated genes associated with immunity and metabolism16 suggesting a possible regulatory role for O-GlcNAc cycling in the hematopoietic lineage. In fact, a recent report suggested a link between transcriptional regulation via O-GlcNAc cycling with in vitro erythropoiesis17. To further define a possible mechanism for HSC dysfunction, we performed RNA-Seq and qRT-PCR confirmation to uncover deregulated gene expression in LSK cells. Similar to the MEF transcriptional data, we also found a significant enrichment of genes involved in development and nutrient signaling as deregulated. Importantly, the most significantly down regulated and up regulated genes in the OGA depleted LSK cells have defined roles in adult stem cell maintenance and lineage specification. One such gene was Fgf3, a canonic member of the fibroblast growth factor family. FGF signaling plays critical roles in adult stem cell self-renewal41 and specifying primitive hematopoietic stem cells in the embryo49,50. While most FGFs are secreted, FGF3 is both secreted and transported to the nucleus where it plays a role in controlling cell growth51,52,53.
The metabolic state, including glucose and glutamine metabolism, of HSCs is important for both self-renewal and lineage specification and commitment. Our RNA-Seq analysis found deregulation of factors involved in both glucose and glutamine transport. The most highly upregulated gene, Slc1a5, is a glutamine transporter required in HSCs for erythyroid specification42. Further, two genes involved in glucose consumption, Clip354 and Slc5a955 were significantly downregulated in the Oga mutant LSK cells. HSCs require a distinct metabolic profile for self-renewal and differentiation. Our study suggests that interference with O-GlcNAc cycling disturbed the transcriptional network regulating nutrient uptake in the LSK cells.
Interestingly, glutamine enters the HBP and increased glutamine uptake could increase O-GlcNAcylation. Thus, upregulation of Slc1a5 expression in the LSK cells suggests a positive feedback loop whereby increased O-GlcNAcylation results in upregulation of glutamine transporters and possibly increasing flux through the HBP. Other studies have also described an O-GlcNAc dependent positive feedback loop for metabolites entering the HBP. For example, O-GlcNAcylation of cMYC in T cells increased Glut1 expression in mouse T cells. With increased Glut1 there was increased glucose uptake, ultimately contributing towards O-GlcNAcylation of cMYC18.
Aside from transcription factors and epigenetic regulators known to be O-GlcNAc modified that are involved in HSC regulation, analysis of about 150 mouse mutants with HSC phenotypes has highlighted the role of specific pathways involved in HSC homeostasis29. Interestingly, O-GlcNAc cycling is intimately involved in these pathways which include cell cycle56, TGF-β57,58, PTEN/AKT59, and WNT60,61 signaling pathways. Thus it was likely that blocked O-GlcNAc cycling influenced these pathways contributing to increased apoptosis and disruption of HSC homeostasis. Unfortunately, due to limited material of these very early cell populations, further biochemical characterization was difficult.
Analysis of the thymus revealed that early thymocytes were particularly sensitive to OGA deletion. The ETP and DN2 populations were the only populations analyzed that also had significant decreases in frequency in the mutant mice. This suggested that although the primary defect in these mice was in HSC maintenance, there was a secondary defect in the earliest stages of thymocyte development. Dynamic changes in O-GlcNAc had been demonstrated in early thymocyte development, with OGT being identified as a critical factor in these cells15,18. Here, we have identified removal of O-GlcNAc as an important component of early thymic development. Thus, we conclude that both the addition and the cycling of O-GlcNAc support normal T cell development.
Our data highlights the role dynamic O-GlcNAc cycling plays in HSC maintenance. We note that both the inability to compete in a bone marrow chimera assay and the greatly diminished numbers of early ETPs might suggest impaired homing could have contributed to the mutant phenotypes. Proper homing requires an exquisite interplay between chemokines, chemokine receptors, intracellular signaling, adhesion molecules and proteases. In fact, key cell adhesion molecules involved in homing, vascular cell adhesion molecule 1 (VCAM-1) and intracellular adhesion molecule (ICAM-1), have been previously reported to be indirectly regulated by O-GlcNAc through modification of transcription factors like NFκB62, SP163 and TNFα64. In-depth analysis of our RNA-seq data from mutant LSKs revealed a small but significant increase in basal cell adhesion molecule (BCAM) (Supplemental Table 1). However, BCAM does not have a known role in homing, and bioinformatic analysis did not reveal cell adhesion as an enriched pathway. Further, as described above, altering O-GlcNAc cycling and transcription of nutrient transporters could alter flux through the HBP which has the potential to impact extracellular N-linked glycosylation. In fact, glycosylation of cell surface molecules is required for normal homing65,66. Thus, the interplay between intracellular and extracellular glycosylation, while quite interesting, will require further research.
Together, these experiments suggested that the processes of O-GlcNAc addition and removal each play a key role in regulating hematopoiesis. Loss of Oga in mouse HSCs resulted in an abnormal stem cell population with numbers of hematopoietic progenitors and further differentiated T and B cells remaining low. Furthermore, Oga deficient HSCs had impaired self-renewal, decreased repopulation capacity and an altered transcriptional profile. This is the first report to uncover O-GlcNAc cycling as a critical component of adult stem cell maintenance in vivo and has important implications for the role of hexosamine signaling in the maintenance of adult stem cell populations giving rise to immune homeostasis.
Methods
Mice
The Vav-iCre transgenic mouse20 was purchased from Jax (stock number 008610). The hematopoietic specific Oga deletion was obtained by breeding the Ogafl/fl described previously16 with the Vav-iCre. Genotyping for the floxed allele was confirmed using primer Oga 7F and Oga7R. Presence of Cre was confirmed using primers oIMR9266 and oIMR9267 (see Supplemental Table 2 for primer sequences). All mice were analyzed between 4–12 weeks of age. Both males and females were used in analysis. The animals were maintained according to the animal protocol # K023-LCBB-16 approved by the NIDDK Animal Care and Use Committee, National Institutes of Health.
Oga assay
OGA assay was performed as described previously21 using 30ug of lysates. To control for any background fluorescence of the FDGlcNAc substrate itself, a no lysate control was subtracted from all samples. Fluorescence was measured in 1-s intervals at the excitation wavelength of 485 nm and emission wavelength of 520 nm on a Polarstar Omega (BMG Labtech). All assays were performed in triplicate.
Western blot
Protein was extracted with T-PER tissue protein extraction reagent (Thermo-Fisher). Lysates were run on a 4–12% Bis-Tris gel and transferred to a nitrocellulose membrane. Membranes were blocked with Odyssey blocking buffer (Odyssey, number 927–40000) for 1 h, incubated with primary antibodies rabbit anti-actin (Abcam) and mouse anti-O-GlcNAc (RL2) (Thermo Fisher MA1-072) overnight at 4 °C. Secondary antibodies were Odyssey IR dye-conjugated. Membranes were imaged and band intensities were analyzed with Odyssey imaging equipment.
Antibodies and flow cytometry
Cell suspensions were counted after ACK lysis using a Nexcelom Cellometer and incubated with a mix of purified rat, mouse and hamster IgG before addition of specific antibodies. Antibodies specific for B220 (RA3-6B3), CD19 (1D3), Mac-1 (M1/70), Gr-1 (8C5), CD11c (N418), Ter119 (TER119), NK1.1 (PK136), CD3ε (2C11), CD4 (GK1.5), CD8α (53-6.72), CD8β (H35-17.2), TCRβ (H57), TCRγδ (GL-3), Kit (2B8), Sca-1 (D7), CD150 (mShad150), CD34 (RAM34), CD16/32 (93), IL-7Rα (A7R34), CD25 (PC61.5), CD44 (IM7), Thy-1.2 (53-2.1), Ly6d were from Thermo Fisher, and anti-Flt3 (A2F10) was from BD. The lineage ‘cocktail’ (Lin) is a mix of the following antibodies: anti- B220, CD19, Mac-1, Gr-1, CD11c, Ter119, NK1.1, CD3ε, CD8α, CD8β, TCRβ and TCRγδ. Live/dead discrimination was performed by staining with DAPI. Annexin V and PI were from Thermo Fisher. Samples were acquired using an LSRFortessa flow cytometer (BD) and analyzed using FlowJo software (Tree Star). All analyses are presented on singlet live cells. Total cell numbers were determined by multiplying total cell count by the frequency of the specific population.
In-vitro colony forming assays
Bone marrow was isolated from OgaVav-Cre and wildtype littermates and after ACK lysis were seeded at a density of 3 × 104 cells/replicate or sorted to obtain CD150+LSK cells and seeded at a density of 500 cells/replicate in methocult M3434 (Stemcell Technologies). Colonies propogated in culture were scored at 10–14 days. Cells were resuspended and 1 × 104/replicate were replated.
Competitive chimera
Bone marrow cells were isolated from OGA deficient mice and littermate controls, lineage-depleted, and injected into lethally irradiated (850 rads) CD45.1 recipient mice with competitor CD45.1 bone marrow cells.
RNA-Seq
Bone marrow cells collected from 3 OgaVav-Cre and 3 wildtype female mice were sorted for Lin−Sca+Kit+ cells using a BD FACSAria Fusion Flow cytometer and the following antibodies: Lineage Cocktail FITC (R&D Biosystems), Sca-1(D7)APC (ebiosciences), c-Kit (2B8)PE (ebiosciences), FVD (ebiosciences). At least 2 × 104 cells were collected for each sample. RNA was extracted using RNeasy plus micro kit (Qiagen) following manufacturers protocol. RNA quality was confirmed using Bioanalyzer (Agilent technologies) all RIN scores were >7. RNA libraries were prepped using NEBNext Ultra RNA Library Prep Kit for Illumina and the NEBNext Poly(A) mRNA Magnetic Isolation Module (New England Biolabs). Single-end 50 bp reads were generated by a HiSeq2500 sequencer. Raw reads were aligned to the mouse genome (mm9) and bioinformatic analysis was done using Genomatix. Significance was determined by DESeq2 parametric Wald test67. Significant transcripts had an adjusted p-value < 0.05 and log2(fold-change) > 1 or <−1. Visualization was done using Partek Flow (Partek), R (R Core Team) and IGV68. RNA-Seq data are available at GEO under accession number GSE116620.
qRT-PCR
cDNA libraries prepared for RNA-Seq were diluted 1:5. Fast SYBR Green Master Mix (Applied Biosystems) was used for amplification following manufacturers protocol using 2 ul of cDNA and the appropriate primer (Supplemental Table 2) on an 7900HT fast real-time PCR system (Applied Biosystems). Each reaction was performed in technical quadruplicate. Relative gene expression was normalized to the geometric mean of Rplp0, Eef2, Rpl38 (Supplemental Table 2). Significance was determined using an unpaired t test. P < 0.05.
In vitro T cell differentiation
OP9, OP9Dl1 and LSKs were cultured in α-MEM media supplemented with 20% FBS, glutamine, penicillin and streptomycin. For T cell–differentiation assays, LSK were cultured on irradiated OP9-Dl1 stromal layers in the presence of IL-7 (1 ng/ml) and Flt3L (5 ng/ml). All cytokines were purchased from PeproTech. Cultures were analyzed 15 days later for the presence of CD45.2+ hematopoietic progeny. Thy1-2+ CD25+ cells were considered as T lineage cells.
Statistical analysis
Graphpad prism was used for all statistics. A two-tailed paired t test or a two way anova was used to determine significance as indicated in figure legends. P values less than .05 was considered statistically significant.
Data Availability
RNA-Seq data are available at GEO under accession number GSE116620.
References
Simsek, T. et al. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell stem cell 7, 380–390, https://doi.org/10.1016/j.stem.2010.07.011 (2010).
Takubo, K. et al. Regulation of glycolysis by Pdk functions as a metabolic checkpoint for cell cycle quiescence in hematopoietic stem cells. Cell stem cell 12, 49–61, https://doi.org/10.1016/j.stem.2012.10.011 (2013).
Ma, J. & Hart, G. W. O-GlcNAc profiling: from proteins to proteomes. Clin Proteomics 11, 8, https://doi.org/10.1186/1559-0275-11-8 (2014).
Hanover, J. A., Chen, W. & Bond, M. R. O-GlcNAc in cancer: An Oncometabolism-fueled vicious cycle. Journal of bioenergetics and biomembranes, https://doi.org/10.1007/s10863-018-9751-2 (2018).
Shafi, R. et al. The O-GlcNAc transferase gene resides on the X chromosome and is essential for embryonic stem cell viability and mouse ontogeny. Proceedings of the National Academy of Sciences of the United States of America 97, 5735–5739, https://doi.org/10.1073/pnas.100471497 (2000).
Jang, H. et al. O-GlcNAc regulates pluripotency and reprogramming by directly acting on core components of the pluripotency network. Cell stem cell 11, 62–74, https://doi.org/10.1016/j.stem.2012.03.001 (2012).
Olivier-Van Stichelen, S., Wang, P., Comly, M., Love, D. C. & Hanover, J. A. Nutrient-driven O-linked N-acetylglucosamine (O-GlcNAc) cycling impacts neurodevelopmental timing and metabolism. The Journal of biological chemistry 292, 6076–6085, https://doi.org/10.1074/jbc.M116.774042 (2017).
Chu, C. S. et al. O-GlcNAcylation regulates EZH2 protein stability and function. Proceedings of the National Academy of Sciences of the United States of America 111, 1355–1360, https://doi.org/10.1073/pnas.1323226111 (2014).
Love, D. C., Krause, M. W. & Hanover, J. A. O-GlcNAc cycling: emerging roles in development and epigenetics. Seminars in cell & developmental biology 21, 646–654, https://doi.org/10.1016/j.semcdb.2010.05.001 (2010).
Sinclair, D. A. et al. Drosophila O-GlcNAc transferase (OGT) is encoded by the Polycomb group (PcG) gene, super sex combs (sxc). Proceedings of the National Academy of Sciences of the United States of America 106, 13427–13432, https://doi.org/10.1073/pnas.0904638106 (2009).
Gambetta, M. C., Oktaba, K. & Muller, J. Essential role of the glycosyltransferase sxc/Ogt in polycomb repression. Science (New York, N.Y.) 325, 93–96, https://doi.org/10.1126/science.1169727 (2009).
Akan, I., Love, D. C., Harwood, K. R., Bond, M. R. & Hanover, J. A. Drosophila O-GlcNAcase Deletion Globally Perturbs Chromatin O-GlcNAcylation. The Journal of biological chemistry 291, 9906–9919, https://doi.org/10.1074/jbc.M115.704783 (2016).
Ding, X. et al. Mixed Lineage Leukemia 5 (MLL5) Protein Stability Is Cooperatively Regulated by O-GlcNac Transferase (OGT) and Ubiquitin Specific Protease 7 (USP7). PloS one 10, e0145023, https://doi.org/10.1371/journal.pone.0145023 (2015).
Chou, T. Y., Dang, C. V. & Hart, G. W. Glycosylation of the c-Myc transactivation domain. Proceedings of the National Academy of Sciences of the United States of America 92, 4417–4421 (1995).
O’Donnell, N., Zachara, N. E., Hart, G. W. & Marth, J. D. Ogt-dependent X-chromosome-linked protein glycosylation is a requisite modification in somatic cell function and embryo viability. Molecular and cellular biology 24, 1680–1690 (2004).
Keembiyehetty, C. et al. Conditional knock-out reveals a requirement for O-linked N-Acetylglucosaminase (O-GlcNAcase) in metabolic homeostasis. The Journal of biological chemistry 290, 7097–7113, https://doi.org/10.1074/jbc.M114.617779 (2015).
Zhang, Z. et al. O-GlcNAc homeostasis contributes to cell fate decisions during hematopoiesis. The Journal of biological chemistry 294, 1363–1379, https://doi.org/10.1074/jbc.RA118.005993 (2019).
Swamy, M. et al. Glucose and glutamine fuel protein O-GlcNAcylation to control T cell self-renewal and malignancy. Nature immunology 17, 712–720, https://doi.org/10.1038/ni.3439 (2016).
Ferrer, C. M. et al. O-GlcNAcylation Regulates Cancer Metabolism and Survival Stress Signaling via Regulation of the HIF-1 Pathway. Molecular Cell 54, 820–831, https://doi.org/10.1016/j.molcel.2014.04.026 (2014).
de Boer, J. et al. Transgenic mice with hematopoietic and lymphoid specific expression of Cre. European journal of immunology 33, 314–325, https://doi.org/10.1002/immu.200310005 (2003).
Kim, E. J., Kang, D. O., Love, D. C. & Hanover, J. A. Enzymatic characterization of O-GlcNAcase isoforms using a fluorogenic GlcNAc substrate. Carbohydrate research 341, 971–982, https://doi.org/10.1016/j.carres.2006.03.004 (2006).
Inlay, M. A. et al. Ly6d marks the earliest stage of B-cell specification and identifies the branchpoint between B-cell and T-cell development. Genes Dev 23, 2376–2381, https://doi.org/10.1101/gad.1836009 (2009).
Kearse, K. P. & Hart, G. W. Lymphocyte activation induces rapid changes in nuclear and cytoplasmic glycoproteins. Proceedings of the National Academy of Sciences of the United States of America 88, 1701–1705 (1991).
Golks, A., Tran, T. T., Goetschy, J. F. & Guerini, D. Requirement for O-linked N-acetylglucosaminyltransferase in lymphocytes activation. The EMBO journal 26, 4368–4379, https://doi.org/10.1038/sj.emboj.7601845 (2007).
Ramakrishnan, P. et al. Activation of the transcriptional function of the NF-kappaB protein c-Rel by O-GlcNAc glycosylation. Sci Signal 6, ra75, https://doi.org/10.1126/scisignal.2004097 (2013).
Lund, P. J., Elias, J. E. & Davis, M. M. Global Analysis of O-GlcNAc Glycoproteins in Activated Human T Cells. Journal of immunology (Baltimore, Md.: 1950) 197, 3086–3098, https://doi.org/10.4049/jimmunol.1502031 (2016).
Woo, C. M. et al. Mapping and Quantification of Over 2000 O-linked Glycopeptides in Activated Human T Cells with Isotope-Targeted Glycoproteomics (Isotag). Molecular & cellular proteomics: MCP 17, 764–775, https://doi.org/10.1074/mcp.RA117.000261 (2018).
Shah, D. K. & Zuniga-Pflucker, J. C. An overview of the intrathymic intricacies of T cell development. Journal of immunology (Baltimore, Md.: 1950) 192, 4017–4023, https://doi.org/10.4049/jimmunol.1302259 (2014).
Rossi, L. et al. Less is more: unveiling the functional core of hematopoietic stem cells through knockout mice. Cell stem cell 11, 302–317, https://doi.org/10.1016/j.stem.2012.08.006 (2012).
Comer, F. I. & Hart, G. W. Reciprocity between O-GlcNAc and O-phosphate on the carboxyl terminal domain of RNA polymerase II. Biochemistry 40, 7845–7852 (2001).
Kelly, W. G., Dahmus, M. E. & Hart, G. W. RNA polymerase II is a glycoprotein. Modification of the COOH-terminal domain by O-GlcNAc. The Journal of biological chemistry 268, 10416–10424 (1993).
Ranuncolo, S. M., Ghosh, S., Hanover, J. A., Hart, G. W. & Lewis, B. A. Evidence of the involvement of O-GlcNAc-modified human RNA polymerase II CTD in transcription in vitro and in vivo. The Journal of biological chemistry 287, 23549–23561, https://doi.org/10.1074/jbc.M111.330910 (2012).
Sakabe, K., Wang, Z. & Hart, G. W. Beta-N-acetylglucosamine (O-GlcNAc) is part of the histone code. Proceedings of the National Academy of Sciences of the United States of America 107, 19915–19920, https://doi.org/10.1073/pnas.1009023107 (2010).
Fujiki, R. et al. GlcNAcylation of histone H2B facilitates its monoubiquitination. Nature 480, 557–560, https://doi.org/10.1038/nature10656 (2011).
Zhang, S., Roche, K., Nasheuer, H. P. & Lowndes, N. F. Modification of histones by sugar beta-N-acetylglucosamine (GlcNAc) occurs on multiple residues, including histone H3 serine 10, and is cell cycle-regulated. The Journal of biological chemistry 286, 37483–37495, https://doi.org/10.1074/jbc.M111.284885 (2011).
Fong, J. J. et al. beta-N-Acetylglucosamine (O-GlcNAc) is a novel regulator of mitosis-specific phosphorylations on histone H3. The Journal of biological chemistry 287, 12195–12203, https://doi.org/10.1074/jbc.M111.315804 (2012).
Chen, Q., Chen, Y., Bian, C., Fujiki, R. & Yu, X. TET2 promotes histone O-GlcNAcylation during gene transcription. Nature 493, 561–564, https://doi.org/10.1038/nature11742 (2013).
Zhang, Q. et al. Differential regulation of the ten-eleven translocation (TET) family of dioxygenases by O-linked beta-N-acetylglucosamine transferase (OGT). The Journal of biological chemistry 289, 5986–5996, https://doi.org/10.1074/jbc.M113.524140 (2014).
Vella, P. et al. Tet proteins connect the O-linked N-acetylglucosamine transferase Ogt to chromatin in embryonic stem cells. Mol Cell 49, 645–656, https://doi.org/10.1016/j.molcel.2012.12.019 (2013).
Shi, F. T. et al. Ten-eleven translocation 1 (Tet1) is regulated by O-linked N-acetylglucosamine transferase (Ogt) for target gene repression in mouse embryonic stem cells. The Journal of biological chemistry 288, 20776–20784, https://doi.org/10.1074/jbc.M113.460386 (2013).
Coutu, D. L. & Galipeau, J. Roles of FGF signaling in stem cell self-renewal, senescence and aging. Aging 3, 920–933, https://doi.org/10.18632/aging.100369 (2011).
Oburoglu, L. et al. Glucose and glutamine metabolism regulate human hematopoietic stem cell lineage specification. Cell stem cell 15, 169–184, https://doi.org/10.1016/j.stem.2014.06.002 (2014).
Milewski, S. Glucosamine-6-phosphate synthase–the multi-facets enzyme. Biochimica et biophysica acta 1597, 173–192 (2002).
Luo, P., He, T., Jiang, R. & Li, G. MicroRNA-423-5p targets O-GlcNAc transferase to induce apoptosis in cardiomyocytes. Molecular medicine reports 12, 1163–1168, https://doi.org/10.3892/mmr.2015.3491 (2015).
Vaiana, C. A., Kurcon, T. & Mahal, L. K. MicroRNA-424 Predicts a Role for beta-1,4 Branched Glycosylation in Cell Cycle Progression. The Journal of biological chemistry 291, 1529–1537, https://doi.org/10.1074/jbc.M115.672220 (2016).
Liu, Y. et al. MicroRNA-24-1 suppresses mouse hepatoma cell invasion and metastasis via directly targeting O-GlcNAc transferase. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 91, 731–738, https://doi.org/10.1016/j.biopha.2017.05.007 (2017).
Park, S. K. et al. A Conserved Splicing Silencer Dynamically Regulates O-GlcNAc Transferase Intron Retention and O-GlcNAc Homeostasis. Cell reports 20, 1088–1099, https://doi.org/10.1016/j.celrep.2017.07.017 (2017).
Wotton, K. R., Weierud, F. K., Dietrich, S. & Lewis, K. E. Comparative genomics of Lbx loci reveals conservation of identical Lbx ohnologs in bony vertebrates. BMC evolutionary biology 8, 171, https://doi.org/10.1186/1471-2148-8-171 (2008).
Lee, Y. et al. FGF signalling specifies haematopoietic stem cells through its regulation of somitic Notch signalling. Nature communications 5, 5583, https://doi.org/10.1038/ncomms6583 (2014).
Pouget, C. et al. FGF signalling restricts haematopoietic stem cell specification via modulation of the BMP pathway. Nature communications 5, 5588, https://doi.org/10.1038/ncomms6588 (2014).
Antoine, M., Reimers, K., Dickson, C. & Kiefer, P. Fibroblast growth factor 3, a protein with dual subcellular localization, is targeted to the nucleus and nucleolus by the concerted action of two nuclear localization signals and a nucleolar retention signal. The Journal of biological chemistry 272, 29475–29481 (1997).
Kiefer, P. & Dickson, C. Nucleolar association of fibroblast growth factor 3 via specific sequence motifs has inhibitory effects on cell growth. Molecular and cellular biology 15, 4364–4374 (1995).
Reimers, K. et al. NoBP, a nuclear fibroblast growth factor 3 binding protein, is cell cycle regulated and promotes cell growth. Molecular and cellular biology 21, 4996–5007, https://doi.org/10.1128/mcb.21.15.4996-5007.2001 (2001).
Yang, X. et al. MicroRNA-593-3p regulates insulin-promoted glucose consumption by targeting Slc38a1 and CLIP3. Journal of molecular endocrinology 57, 211–222, https://doi.org/10.1530/jme-16-0090 (2016).
Tazawa, S. et al. SLC5A9/SGLT4, a new Na+-dependent glucose transporter, is an essential transporter for mannose, 1,5-anhydro-D-glucitol, and fructose. Life sciences 76, 1039–1050, https://doi.org/10.1016/j.lfs.2004.10.016 (2005).
Liu, C. & Li, J. O-GlcNAc: A Sweetheart of the Cell Cycle and DNA Damage Response. Frontiers in endocrinology 9, 415, https://doi.org/10.3389/fendo.2018.00415 (2018).
Pathak, S. et al. O-GlcNAcylation of TAB1 modulates TAK1-mediated cytokine release. The EMBO journal 31, 1394–1404, https://doi.org/10.1038/emboj.2012.8 (2012).
Mondoux, M. A. et al. O-linked-N-acetylglucosamine cycling and insulin signaling are required for the glucose stress response in Caenorhabditis elegans. Genetics 188, 369–382, https://doi.org/10.1534/genetics.111.126490 (2011).
Hanover, J. A., Krause, M. W. & Love, D. C. The hexosamine signaling pathway: O-GlcNAc cycling in feast or famine. Biochimica et biophysica acta 1800, 80–95, https://doi.org/10.1016/j.bbagen.2009.07.017 (2010).
Olivier-Van Stichelen, S. et al. O-GlcNAcylation stabilizes beta-catenin through direct competition with phosphorylation at threonine 41. FASEB journal: official publication of the Federation of American Societies for Experimental Biology 28, 3325–3338, https://doi.org/10.1096/fj.13-243535 (2014).
Wu, J. et al. O-GlcNAc transferase is critical for transducin-like enhancer of split (TLE)-mediated repression of canonical Wnt signaling. The Journal of biological chemistry 289, 12168–12176, https://doi.org/10.1074/jbc.M114.553859 (2014).
Yang, W. H. et al. NFkappaB activation is associated with its O-GlcNAcylation state under hyperglycemic conditions. Proc Natl Acad Sci USA 105, 17345–17350, https://doi.org/10.1073/pnas.0806198105 (2008).
Zhang, Y., Qu, Y., Niu, T., Wang, H. & Liu, K. O-GlcNAc modification of Sp1 mediates hyperglycaemia-induced ICAM-1 up-regulation in endothelial cells. Biochem Biophys Res Commun 484, 79–84, https://doi.org/10.1016/j.bbrc.2017.01.068 (2017).
Rajapakse, A. G., Ming, X. F., Carvas, J. M. & Yang, Z. O-linked beta-N-acetylglucosamine during hyperglycemia exerts both anti-inflammatory and pro-oxidative properties in the endothelial system. Oxid Med Cell Longev 2, 172–175 (2009).
Sackstein, R. et al. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat Med 14, 181–187, https://doi.org/10.1038/nm1703 (2008).
Takagaki, S. et al. Galactosyl carbohydrate residues on hematopoietic stem/progenitor cells are essential for homing and engraftment to the bone marrow. Sci Rep 9, 7133, https://doi.org/10.1038/s41598-019-43551-6 (2019).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome biology 15, 550, https://doi.org/10.1186/s13059-014-0550-8 (2014).
Robinson, J. T. et al. Integrative genomics viewer. Nature Biotechnology 29, 24, https://doi.org/10.1038/nbt.1754, https://www.nature.com/articles/nbt.1754#supplementary-information (2011).
Acknowledgements
We would like to acknowledge Drs Dona Love, Ludmila Jirmanov and Jonathan Ashwell for their initial analysis of the Oga−/− mice finding decreases in thymocyte and splenocyte numbers. We also acknowledge Drs Michelle R. Bond and Stephanie Olivier-Van Stichelen for their help with visualization of RNA-Seq data. We additionally thank the NHLBI Flow Cytometry Core Facility, in particular Drs Pradeep Dugar and Ankit Saxena, the CCR Flow Cytometry Core Facility, the NIDDK Genomics Core Facility for technical support. Funding was provided by NIDDK Intramural funds and the Intramural Research Program of the Center for Cancer Research at the National Cancer Institute.
Author information
Authors and Affiliations
Contributions
All authors helped design research and analyze data; L.K.A., C.H. and A.D. performed experiments; L.K.A. wrote the manuscript; J.A.H. edited the manuscript; all authors read and commented on the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abramowitz, L.K., Harly, C., Das, A. et al. Blocked O-GlcNAc cycling disrupts mouse hematopoeitic stem cell maintenance and early T cell development. Sci Rep 9, 12569 (2019). https://doi.org/10.1038/s41598-019-48991-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-48991-8
This article is cited by
-
Tools, tactics and objectives to interrogate cellular roles of O-GlcNAc in disease
Nature Chemical Biology (2022)
-
Dietary glucosamine overcomes the defects in αβ-T cell ontogeny caused by the loss of de novo hexosamine biosynthesis
Nature Communications (2022)
-
O-GlcNAcylation and its role in the immune system
Journal of Biomedical Science (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.