Abstract
We evaluated the survival effects and biochemical profiles of levosimendan in septic rats after partial hepatectomy and investigated its effects in cultured hepatocytes. Thirty-two rats underwent 70% hepatectomy and were randomised equally into four groups, followed by lipopolysaccharide (LPS) injection (250 µg/kg, i.v.) after 48 h. Levosimendan was given (i.p.) 1 h before LPS injection [group (A) levosimendan 2 mg/kg; (B) 1; (C) 0.5; (D) vehicle]. Survival at 7 days was increased significantly in group A compared with that in group D [A: 63%; B: 38%; C: 13%; D: 0%]. In serum, levosimendan decreased the level of tumour necrosis factor-α, interleukin (IL)-1β, IL-6 and nitric oxide (NO). In remnant livers, levosimendan inhibited inducible nitric oxide synthase (iNOS) gene expression. In primary cultured rat hepatocytes stimulated by IL-1β, levosimendan suppressed NO production by inhibiting iNOS promoter activity and stability of its mRNA.
Similar content being viewed by others
Introduction
Levosimendan is a calcium sensitiser licensed in numerous countries to treat decompensated heart failure1. It acts by: (i) increasing the sensitivity of troponin C to calcium in myocardial cells, leading to inotropy; (ii) opening mitochondrial adenosine triphosphate (ATP)-sensitive potassium channels in smooth muscle cells, resulting in vasodilation2. Moreover, it is known that levosimendan treatment leads to reduction in proinflammatory cytokines and apoptosis signaling pathways in patients with heart failure3. Furthermore, in experimental sepsis model induced by cecal ligation and puncture (CLP), levosimendan showed cardioprotective effects through preventing cardiac inflammation4. The composite actions of levosimendan as an inotrope and anti-inflammatory support raise the theoretical possibility that levosimendan may have a value as a treatment of sepsis5. In animal studies, accumulating evidences suggest that levosimendan may mitigate multiple organ injuries besides the heart in conditions of septic shock or ischemia–reperfusion2,5,6, including lung injury in CLP model7,8,9, renal failure in LPS-induced endotoxemia10 and liver injury in hepatic ischemia–reperfusion11. In terms of underlying mechanisms, levosimendan exerted anti-inflammatory effects through probably decreasing nitric oxide (NO) release in sepsis2,12. However, the inhibitory effect of levosimendan on proinflammatory cytokine production cannot be solely attributed to alterations in the NF-κB pathway12. In a few limited case series and trials have shown a beneficial potential of levosimendan on cardiac13, renal14, pulmonary15 and hepatic16 function in patients with sepsis. However, the survival and organ protective benefits of levosimendan in patients with septic shock were not demonstrated in one randomised controlled clinical trial6. The optimal indications and protocols of levosimendan for sepsis treatment have not been established.
Sepsis after major hepatectomy is a major issue. There is general agreement that 70% hepatectomy alone is not fatal in rodents17, but intravenous injection of a sub-lethal dose of lipopolysaccharide (LPS) 48 h after partial hepatectomy (‘PH/LPS’-model) is associated with high mortality18,19,20,21. Reduced phagocytic function of the reticuloendothelial system after hepatectomy is considered to enhance endotoxin sensitivity22. LPS has a direct effect on macrophages (or Kupffer cells) to activate nuclear factor (NF)-κB, which induces expression of proinflammatory cytokines and inducible nitric oxide synthase (iNOS). The latter produces an excess of NO, which has been implicated in tissue injury and assumed to be one of the triggers leading to septic shock and multiple-organ failure23.
Previously, we reported that fibronectin18, pirfenidone19, edaravone20 and sivelestat21 improved survival and prevented liver injury in PH/LPS-model rats. These agents commonly exerted survival benefits if they were administered before LPS injection and had inhibitory effects on iNOS induction in hepatocytes24,25,26. In primary cultured rat hepatocytes, interleukin (IL)-1β stimulates production of iNOS and NO markedly in the absence of other cytokines27, and prevention of expression of those proinflammatory mediators is a reliable indicator of liver protection28.
We hypothesised that levosimendan pretreatment improves the survival of PH/LPS-model rats by preventing the endotoxin-induced systemic inflammatory response and liver injury. In addition to experiments using the PH/LPS-model, we conducted analyses of IL-1β-stimulated primary cultured rat hepatocytes, as a simple in vitro model of liver injury, in the presence or absence of levosimendan for better understanding of the intracellular mechanisms involved.
Results
Effect of levosimendan pretreatment on survival of PH/LPS-model rats
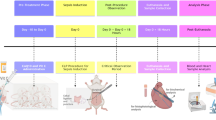
A scheme of the experimental protocol of PH/LPS is shown in Fig. 1. Although we have less than 10% failure rate of PH/LPS model during the induction of anaesthesia and laparotomy, there was no rat to be lost during the interval between randomisation after laparotomy and LPS injection. Thirty-two operated rats were randomised equally into four groups and evaluated the survival during 7 days after LPS injection (Fig. 2). Rats administered vehicle (group D) began to die at 6 h and all rats died within 1 day after LPS injection. Survival of groups A, B and C at 7 days was 63%, 38% and 13%, respectively. The significant difference among four groups was confirmed (P < 0.01). According to post hoc analysis, survival of group A was significantly improved compared with group D (P < 0.01). A dose of 2 mg/kg was used in subsequent in vivo experiments.
Experimental protocol of PH/LPS. Rats were treated with lipopolysaccharide (LPS, 250 µg/kg, i.v.) 48 h after 70% hepatectomy (PH/LPS). Levosimendan (Levo) or vehicle [saline containing 2% dimethyl sulfoxide (DMSO)] was administered (i.p.) 1 h before LPS injection. Survival of 32 rats was evaluated during 7 days. Samples from 20 rats were obtained at 0 h, 1 h or 4 h after LPS administration and analysed for an exploratory experiment.
Effect of levosimendan on expression of cytokines, NO and transaminase in serum
The levels of tumour necrosis factor (TNF)-α, IL-1β, IL-6, NO, aspartate transferase (AST) and alanine transaminase (ALT) in serum were inhibited significantly (P < 0.01, 0.01, 0.02, 0.02, 0.04 and 0.02, respectively) by levosimendan at 4 h (Fig. 3a–f).
Effects of levosimendan on expression of cytokines, NO and transaminases in serum. Biochemical analyses of serum samples for (a) TNF-α, (b) IL-1β, (c) IL-6, (d) NO, (e) ALT and (f) AST are shown. Each graph consists of 5 bars representing 0 h (48 h after 70% hepatectomy without LPS or levosimendan treatment) as well as 1 h and 4 h after LPS treatment with levosimendan or vehicle. * and n.s. stand for P < 0.05 and not significant, respectively, between the shown pair.
Effect of levosimendan on expression of NF-κB, iNOS and cytokines in remnant livers
The level of NF-κB in remnant liver was activated by LPS injection at 1 h, and then attenuated at 4 h, after LPS injection was examined by electrophoretic mobility shift assays (EMSAs). Activation of NF-κB tended to be decreased by levosimendan at 4 h after LPS injection, without significant differences (P = 0.056) (Fig. 4a). iNOS expression in remnant livers was inhibited significantly (P = 0.01) by levosimendan at 4 h (Fig. 4b). Levosimendan inhibited expression of the mRNA of TNF-α, IL-1β and IL-6 significantly (P = 0.03, 0.03 and 0.03, respectively) at 1 h, but insignificantly (P = 0.8, 0.09 and 0.15, respectively) at 4 h (Fig. 4c–f). iNOS mRNA was inhibited significantly (P = 0.03) at 4 h, but insignificantly (P = 0.1) at 1 h. Expression of cytokine-induced neutrophil chemoattractant (CINC)-1 mRNA tended to be inhibited at both 1 h and 4 h, without significant differences (P = 0.1 and 0.2) (Fig. 4g). Expression of IL-10 mRNA tended to be increased at both 1 h and 4 h, without significant differences (P = 0.6 and 0.4) (Fig. 4h).
Effects of levosimendan on expression of NF-κB, iNOS and cytokines in livers. (a) The result of EMSA for remnant-liver samples is shown (upper), which consists of representatives of PH/LPS with vehicle at 0 h (3 rats), PH/LPS with vehicle at 1 h (3 rats), PH/LPS with levosimendan (Levo) at 1 h (4 rats), PH/LPS with vehicle at 4 h (4 rats) and PH/LPS with Levo at 4 h (5 rats). Full-length gels and full sample data are shown in a Supplementary information file. The density of blots in each group was quantified by densitometry (lower). (b) Results of western blotting for remnant-liver samples using primary antibodies against iNOS (upper) and β-tubulin (lower) at 1 h (representative 2 samples) and 4 h (5 samples in each group) after LPS treatment with levosimendan or vehicle are shown (Full-length gels are shown in a Supplementary Information file). (c-h) RT-PCR results for (c) TNF-α, (d) IL-1β, (e) IL-6, (f) iNOS, (g) CINC-1 and (h) IL-10. Each graph consists of five bars representing 0 h (48 h after 70% hepatectomy without LPS or levosimendan treatment), 1 h and 4 h after LPS treatment with levosimendan or vehicle. * and n.s. stand for P < 0.05 and not significant, respectively, between the shown pair.
Effect of levosimendan on histopathological changes
Histopathology revealed the change in regeneration of rat livers 48 h after 70% hepatectomy: ballooning hepatocytes and spreading of lipid droplets (Fig. 5a). After 4 h of LPS injection, focal necrotic hepatocytes were prominent at the centrilobular zone and midzone in both groups of PH/LPS with vehicle and levosimendan (Fig. 5b,c). Few myeloperoxidase (MPO)-positive cells were infiltrated in livers 48 h after 70% hepatectomy: (0.3 cells/mm2, Fig. 5d). Severe infiltration of MPO-positive cells was recognized in specimens of rat livers after 4 h of LPS injection with vehicle (Fig. 5e). Levosimendan pretreatment did not inhibit the infiltration of MPO-positive cells significantly in remnant livers (P = 0.7) (Fig. 5f,g). Apoptotic bodies were evaluated by terminal deoxynucleotidyl transferase-mediated dUTP-digoxigenin nick-end labelling (TUNEL) staining, and few positive nuclei were detected in rats 48 h after 70% hepatectomy (4 per 1,000 nuclei, Fig. 5h). The difference in the percentage of TUNEL-positive cells in all nuclei was not significant in the absence (Fig. 5i) or presence of levosimendan pretreatment (P = 0.9) (Fig. 5j,k).
Effects of levosimendan on liver histopathology. Histopathology of remnant liver specimens of H&E staining (a–c), MPO staining (d–f) and TUNEL staining (h–j) are shown. Figure (a,d,h) are specimens of rats after 48 h of PH; figure (b,e,i) are rats after 4 h of LPS injection with vehicle; figure (c,f,j) are rats after 4 h of LPS injection with levosimendan. Graph (g) shows the result of MPO-positive cell counts (per mm2) in each group. Graph (h) shows the result of TUNEL-positive cell counts (per mm2) in each group. Each figure is a representative of each condition, and data of each graph represent the mean ± SE (n = 3–5 specimens/group). N.s. stands for not significant. Bar = 100 microns.
Effect of levosimendan on induction of expression of NO, iNOS protein and iNOS mRNA in IL-1β-stimulated cultured hepatocytes
In the culture medium, simultaneous administration of levosimendan (20 µM) with IL-1β (1 nM) reduced the level of nitrite (NO metabolite) time-dependently, which was increased by single administration of IL-1β (Fig. 6a). Levosimendan reduced the production of NO and iNOS protein dose-dependently, and decreased production to a near-basal level at a concentration of 20 µM (Fig. 6b, upper and middle). The level of lactate dehydrogenase (LDH) in the culture medium was not increased by ≤20 µM of levosimendan (Fig. 6b, lower). A dose of 20 µM was used in subsequent in vitro experiments. Reverse transcription-polymerase chain reaction (RT-PCR) revealed that levosimendan reduced expression of iNOS mRNA in each hour (Fig. 6c).
Effects of levosimendan on NO and iNOS induction in IL-1β-stimulated primary cultured hepatocytes. (a) Effects of levosimendan (Levo, 20 µM) on NO production for the indicated times (IL-1β only, open circles ○; IL-1β and Levo, closed circles ●; Levo only, closed triangles ▲; control, open triangles Δ). (b) Effects of Levo (1–20 µM) for 8 h on NO production (upper), iNOS and β-tubulin levels (middle, full-length gels are shown in a Supplementary Information file), and LDH activity (lower). (c) Effects of Levo (20 µM) on expression of iNOS mRNA for the indicated times. *P < 0.05 vs. IL-1β alone. n = 3 dishes/point or indication.
Effect of levosimendan on the activity of iNOS promoters, iNOS antisense transcription and intranuclear level of NF-κB in IL-1β-stimulated hepatocytes
The scheme of the constructs containing firefly luciferase controlled by the iNOS promoter (pRiNOS-Luc-SVpA and pRiNOS-Luc-3′UTR) is shown in Fig. 7a. Levosimendan inhibited relative luciferase activities on both constructs, which were increased by IL-1β single administration (Fig. 7b). RT-PCR revealed that levosimendan inhibited expression of the iNOS antisense transcript at 3 h and 6 h (Fig. 7c). EMSAs with nuclear extracts did not show an inhibitory effect of levosimendan on NF-κB activation (Fig. 7d). Further, we could not detect significant influences of levosimendan on NF-κB nuclear translocation, IκB degradation and phosphorylation of NF-κB p65 (Ser536) (Supplementary information file; M, K, and L).
Effects of levosimendan on transactivation of iNOS promoters, iNOS AST expression, and binding of nuclear extracts to NF-κB consensus oligonucleotide. (a) Promoter region of iNOS (schematic). Two reporter constructs consisting of the rat iNOS promoter (1.0 kb), a luciferase gene, and the SV40 poly(A) region (pRiNOS-Luc-SVpA) or iNOS 3′-UTR (pRiNOS-Luc-3′UTR). “An” indicates the presence of a poly(A) tail. The iNOS 3′-UTR contains AREs (AUUU(U)A × 6), which contribute to mRNA stabilisation. (b) Relative luciferase activity of pRiNOS-Luc-SVpA and pRiNOS-Luc-3′UTR. *P < 0.05 vs. IL-1β alone (n = 6 dishes/indication). (c) Expression of iNOS AST for the indicated times. *P < 0.05 vs. IL-1β alone (n = 3 dishes/indication). (d) Nuclear extracts were analysed by EMSA (full-length gel is shown in a Supplementary Information file).
Effect of levosimendan on the mRNA expression of pro-inflammatory cytokines in IL-1β-stimulated hepatocytes
RT-PCR revealed that levosimendan suppressed expression of the mRNA of TNF-α, CINC-1 and the type-I IL-1 receptor (IL-1RI) at certain times (Fig. 8).
Discussion
Two experimental models of sepsis with acute liver injury can be employed: (i) simultaneous administration of D-galactosamine/LPS29,30,31; (ii) PH/LPS. The lethal activity of endotoxins is enhanced considerably under both models, but the PH/LPS-model exhibits more severe and refractory symptoms32, and is closer to a specific clinical situation.
A pilot study revealed that a 50% lethal dose of LPS for this model was ≈100 µg/kg, and that >90% of rats died at a LPS dose of 250 µg/kg (i.v.) (T. O., unpublished observation). We chose the doses of levosimendan by reference to a similar study of ischemia–reperfusion injury in rat mesenteries33. In our preliminary study, administration of levosimendan (2 mg/kg) 1 h after LPS injection showed no effect on survival (data not shown). In contrast, pretreatment of levosimendan increased the survival of PH/LPS-model rats in a dose-dependent fashion, though a significant difference was only found between group A (doses of 2 mg/kg) and group D (vehicle) by post hoc analysis. Levosimendan pretreatment prevented an increase in expression of proinflammatory cytokines in serum and their mRNAs in remnant livers. Expression of iNOS in remnant livers and NO in serum (which are proinflammatory mediators) was also inhibited by levosimendan pretreatment. Those effects would probably involve inhibition of NF-κB activation, because NF-κB has an important role as a transcriptional factor of iNOS gene28. However, levosimendan did not inhibit NF-κB activation significantly shown in EMSA experiments in remnant livers. We should mention of the limited number of experimental animals we used and there probably existed the influence of other transcriptional factors such as hypoxia-inducible factor-1α34 or nuclear respiratory factor 235. According to a reported study of septic mice, Wang et al. concluded that levosimendan did not inhibit the LPS-induced activation of NF-κB significantly, which is a similar result to our study8. Levosimendan demonstrated a hepatoprotective effect in that levels of transaminases in serum decreased significantly in the levosimendan group 4 h after LPS injection. However, histopathology revealed that levosimendan did not inhibit both the infiltration of MPO-positive cells (i.e., necrotic change) and TUNEL-positive cells (i.e., apoptotic change). The results of histopathology will cause controversy whether a hepatoprotective effect of levosimendan determined the survival benefit in our study. We assume that D-galactosamine/LPS-model would be essential for examining a heaptoprotective effect of levosimendan against LPS-induced acute liver injury36, but this model would not surely represent for septic shock37. As a limitation, we could not adopt a blinded maneuver of each group for a practical reason when we injected LPS and/or levosimendan. However, two researchers (T. S. and T. O.) assured the quality of experiments of PH/LPS.
In IL-1β-stimulated primary cultured hepatocytes, levosimendan suppressed NO production in a time- and dose-dependent fashion through inhibition of iNOS gene expression. We set the concentration of levosimendan at 20 μM in the experiments, because the levels of LDH in culture medium were slightly elevated at the concentration of 100 μM of levosimendan (data not shown), which implied cytotoxicity caused by the overdose of levosimendan, but levosimendan had no such effects at 1–20 μM. The experiments with iNOS promoter constructs demonstrated that levosimendan inhibited iNOS expression during the synthesis and stabilisation of mRNA. iNOS promoter activity measured with the constructs represented the intensity of NF-κB-dependent transcription because both constructs have two NF-κB binding sites (κB) in each promoter area. However, EMSAs revealed that the binding activity of nuclear extracts to the NF-κB consensus oligonucleotide was not inhibited by levosimendan. We conducted the additional experiments to investigate the NF-κB nuclear translocation, IκB degradation and phosphorylation of NF-κB p65 (Ser536), which are the important signalling steps to stimulate NF-κB activation. However, we could not detect significant influences of levosimendan on these steps (Supplementary file). From the results above, we concluded that levosimendan did not inhibit the activating steps of NF-κB in cultured hepatocytes. This result suggests that levosimendan might affect the synthesis of iNOS mRNA through signalling pathways and transcription factors other than NF-κB. We found that the iNOS antisense-transcript had a key role in stabilising iNOS mRNA by interacting with the 3′-ultratranslated region (UTR) and adenylate-uridylate-rich sequence elements-binding proteins37. Levosimendan demonstrated an inhibitory effect on expression of iNOS antisense transcripts. An anti-inflammatory profile of levosimendan was also shown in hepatocytes because of inhibition of the mRNA expression of TNF-α, CINC-1 and IL-1RI. Note that our in vitro study was not a complete reproduction of PH/LPS-model in two points that we did not use the direct cultured hepatocytes from all groups in PH/LPS-model, and we used a single cytokine (IL-1β) to stimulate the hepatocytes. The results from our in vitro study should be considered as reference to understand the anti-inflammatory mechanism of levosimendan.
Some in vitro studies have shown that levosimendan can down-regulate iNOS induction and NO production in response to inflammatory stimuli in macrophages12, cardiac fibroblasts38 and hepatocytes39. Differences in the signalling events leading to activation of iNOS transcription between cell types might exist. Sareila et al. reported that levosimendan did not affect the activation, nuclear translocation or DNA binding of NF-κB in J774 macrophages, but inhibited NF-κB-dependent transcription in L929 fibroblasts12. Okada et al. reported that levosimendan inhibited IL-1β-induced apoptosis via activation of the phosphatidylinositol-4, 5-bisphosphate 3-kinase/Akt pathway in the cardiac fibroblasts of adult rats38. The data from those studies are similar to our results.
As an in vivo model of sepsis, CLP-model7,8 has previously been used to show a survival benefit of levosimendan. Authors selected continuous infusion via a catheter in the jugular vein7 or an intraperitoneal osmotic pump8 of levosimendan, whereas we used intraperitoneal bolus administration. One may argue that intraperitoneal bolus administration of levosimendan does not represent the clinical situation accurately. However, the sepsis model caused by LPS injection does not fully represent human sepsis because LPS causes a much earlier peak of expression of pro-inflammatory cytokines compared with that seen in human sepsis. A survival curve of PH/LPS model is more precipitous that the majority of positive control rats died at 6 h after LPS injection compared with CLP-model that two-thirds of controlled rats survived at 9 h after operation7. Levosimendan has a half-life of ≈1 h but its active metabolite, OR-1896, has a half-life of 80 h40, which could cover the duration of effect of a LPS bolus administration. Continuous infusion of levosimendan in the PH/LPS-model may merit further study.
As in vivo model of liver injury, Grossini et al. reported that levosimendan protected against ischemia–reperfusion injury through mechanisms related to NO production and mitochondrial ATP-dependent potassium-channel function11. Taken together, levosimendan would have a beneficial effect in liver surgery/transplantation. The results of our study lead us to recommend levosimendan pretreatment for sepsis management after acute liver injury.
Methods
Animals
All animal experiments were undertaken in accordance with the Guidelines for the care and use of laboratory animals (National Institutes of Health, Bethesda, MD, USA). The study protocol was approved by the Animal Care Committee of Kansai Medical University (Permission numbers: 17-023(01) and 18-027(01)).
Rats (specific pathogen-free) were purchased from Charles River Laboratories Japan (Yokohama, Japan) and maintained in a room at 22 °C under a 12-h light–dark cycle with a diet of γ-irradiated CRF-1 (Oriental Bioservice, Kyoto, Japan) and water ad libitum.
Drugs
Levosimendan was purchased from Wako Pure Chemical Industries (Osaka, Japan). Levosimendan was resolved in dimethyl sulfoxide (DMSO) and stored at −80 °C. For PH/LPS experiments, resolved levosimendan was diluted by 1 ml of normal saline for each rat so that the DMSO concentration was 2%. Isoflurane, pentobarbital sodium, collagenase, Transaminase CII-test kit, LDH-Cytotoxicity Assay kit and PicaGene Luminescence kit were from Wako Pure Chemical Industries. LPS (Escherichia coli O111:B4) and mouse anti-β-tubulin were from Sigma–Aldrich Japan (Tokyo, Japan).
Recombinant human IL-1β (2 × 107 U/mg protein) was purchased from MyBioSource (San Diego, CA, USA). Enzyme-linked immunosorbent assay (ELISA) kits for TNF-α, IL-1β and IL-6 were from Life Technologies Japan (Tokyo, Japan). Rabbit anti-iNOS, TRIzol™ Reagent, and UltraPure™ DNase/RNase-Free Distilled Water were from Thermo Scientific (Waltham, MA, USA). ECL western blotting detection reagents were from GE Healthcare Japan (Tokyo, Japan). Luminate Forte Western Horseradish Peroxidase (HRP) was from Merck Japan (Tokyo, Japan). Oligo (dT) Primer (25 ng), 5 × RT Buffer, dNTPs Mixture, RNase Inhibitor and Rever Tra Ace® were from Toyobo (Osaka, Japan). Magnet-assisted transfection (MATra) Reagent was from IBA (Gottingen, Germany). Beta-Glo kits, mouse immunoglobulin κ light chain was from Promega (Fitchburg, WI, USA). An In situ Apoptosis Detection kit (MK500) was from Takara Bio (Shiga, Japan). An Anti-myeloperoxidase rabbit polyclonal antibody (A0398) was from DAKO (Glostrup, Denmark).
Creation of the PH/LPS model
The procedure for 70% partial hepatectomy is based on experiments described elsewhere41. Briefly, male Sprague–Dawley rats (8 weeks; 310 ± 10 g) were anaesthetised using pentobarbital sodium (40 mg/kg, i.p.) and isoflurane (0–2%). A laparotomy was done with a midline incision (≈3 cm). The left lateral and left median lobe of the liver were removed after ligation, followed by wound closure. Operated rats were randomised immediately and equally into four groups: (A) levosimendan 2 mg/kg; (B) levosimendan 1 mg/kg; (C) levosimendan 0.5 mg/kg; (D) vehicle (normal saline). Forty-eight hours after surgery, 250 µg/kg body weight of LPS in saline was injected into the penile vein. Levosimendan (i.p.) was given 1 h before LPS injection. Survival was evaluated during 7 days after LPS injection, and then rats were killed by isoflurane. As an exploratory experiment, samples of blood and remnant liver were taken from Sprague–Dawley rats 0 h, 1 h and 4 h after LPS injection with or without levosimendan pretreatment (n = 3–5 in each group). A scheme of the experimental protocol is shown in Fig. 1.
Isolation and culture of primary hepatocytes
The isolation and culture of rat hepatocytes is based on experiments described elsewhere42,43. Hepatocytes were isolated from livers of Wister rats (200–220 g) by collagenase perfusion via the portal vein, followed by centrifugation (50 × g, 70 sec, 4 °C; four times). Isolated hepatocytes were suspended at 6 × 105 cells/mL in Williams’ E (WE) culture medium, supplemented with 10% newborn calf serum, Hepes (5 mM), penicillin (100 U/mL), streptomycin (100 μg/mL), fungisone (0.25 μg/mL), aprotinin (0.1 μg/mL), dexamethasone (10 nM) and insulin (10 nM). The cells were seeded into 35- or 100-mm plastic dishes (2 or 10 mL/dish; Falcon Plastic, Oxnard, CA, USA) and cultured at 37°C in a CO2 incubator under a humidified atmosphere of 5% CO2 in air for 2 h. The medium (1.5 mL/35-mm dish) was replaced with fresh serum-free and hormone-containing WE medium (first medium change), then with fresh serum- and hormone-free WE medium at 5 h (second medium change), and the cells were cultured overnight. As cells were cultured two days or more before use in experiments, fresh serum-free and hormone-containing WE medium was used in the second medium change, with this medium subsequently changed every day. Then, cells were treated with recombinant human IL-1β (1 nM) in the presence or absence of levosimendan.
Biochemical analyses
Serum levels of TNF-α, IL-1β and IL-6 were measured using commercial ELISA kits. The sum of nitrite and nitrate (stable metabolites of NO) in the serum, or nitrite in the culture medium, was measured using the Griess reagent method44. Serum levels of AST and ALT were determined using commercial kits. LDH activity in the culture medium was measured using a commercial kit according to manufacturer instructions.
Western blotting
Protein extracts of liver sections and hepatocytes were prepared for western blotting, as described previously29. They were subjected to a 7.5% gel, and electroblotted. Immunostaining was done using primary antibodies against iNOS and β-tubulin (internal control), followed by visualisation with ECL Western Blotting Detection Reagents for iNOS and Luminate Forte Western HRP for β-tubulin. The bands corresponding to each protein were quantified by densitometry using ImageJ (San Diego, CA, USA)45.
RT-PCR
Total RNAs of liver sections and hepatocytes were extracted in TRIzol Reagent using the guanidinium–phenol–chloroform method46. cDNA was synthesised from 1 µg of total RNA from each sample with Oligo(dT)20 Primer (25 ng), 5 × RT Buffer (5 µl), 10 mM of dNTPs Mixture (2.5 µl), RNase Inhibitor (0.5 µl), Rever Tra Ace (100 U) and UltraPure™ DNase/RNase-Free Distilled Water. The conditions of thermal cycling using iCycler (Bio-Rad Laboratories, Hercules, CA, USA) were 42 °C for 60 min and 95 °C for 5 min.
Real-time PCR was done using SYBR Green and primers for each gene. Primer sequences were synthesised by Eurofins Genomics (Tokyo, Japan) (Table 1). The conditions of thermal cycling using Rotor-Gene Q (Qiagen, Stanford, VA, USA) were 95 °C for 5 min followed by 40 cycles of 95 °C for 5 s and 60 °C for 10 s. Collection and analyses of data were done using the software included with the system. mRNA levels of each gene were measured as CT threshold levels and normalised to those of eukaryotic elongation factor-1α.
Transfection and luciferase assay
Transfection of cultured hepatocytes was undertaken as described previously47. Briefly, on day-0, hepatocytes were cultured for 7 h before being subjected to MATra. Reporter plasmid pRiNOS-Luc-SVpA or pRiNOS-Luc-3′UTR (1 µg) and the cytomegalovirus promoter-driven β-galactosidase plasmid pCMV-LacZ (1 ng) as an internal control were mixed with 1.5 µg of MATra-A reagent in 200 µL of Williams’ E medium. After incubation for 15 min on a magnetic plate, the medium was replaced and cultured overnight, and then treated with IL-1β in the presence or absence of levosimendan. Activities of luciferase and β-galactosidase in cell extracts were measured using PicaGene and Beta-Glo kits, respectively. Luciferase activity was normalised by β-galactosidase activity. Fold activation was calculated by dividing luciferase activity by control activity (without IL-1β and levosimendan).
EMSA
EMSA was carried out as described previously48,49 with a minor modification, as described elsewhere20,50. Nuclear extracts were prepared from frozen liver at −80 °C or cultured hepatocytes. Binding reactions were undertaken by incubating the nuclear extracts in reaction buffer (20 mM of HEPES-KOH, pH 7.9; containing 1 mM of EDTA, 60 mM of KCl, 10% glycerol, and 1 µg of poly[dI-dC]) with a probe (40,000 dpm) for 20 min at room temperature. Products were electrophoresed on a 4.8% polyacrylamide gel in high-ionic-strength buffer, and dried gels were analysed by autoradiography. An NF-κB consensus oligonucleotide (5′-AGTTGAG GGGA-CTTTCCCAGGC) from the mouse immunoglobulin κ light chain was purchased and labelled with [γ-32P]-ATP and T4 polynucleotide kinase. Protein was measured using the Bradford method51. Bands corresponding to NF-κB were quantified by densitometry using ImageJ45.
Histopathology
Specimens of remnant liver were fixed in 10% formalin solution and embedded in paraffin. Sections (3–5 µm) were cut and stained with haematoxylin and eosin. Neutrophil infiltration was evaluated by the counts of MPO-positive cells using the Anti-myeloperoxidase rabbit polyclonal antibody (A0398) per 20 HPFs under light microscopy. Apoptotic bodies were evaluated by TUNEL staining using the In Situ Apoptosis Detection kit (MK500) per 20 HPFs under light microscopy. The Labeling Index of TUNEL-positive cells per 1,000 hepatocyte nuclei was counted in duplicate.
Statistical analyses
Comparison of rats’ survival among four groups was analysed statistically by one-way ANOVA, followed by Tukey-Kramer method (JMP® 14, SAS Institute Inc., Cary, NC, USA). The results of in vitro studies in the figures are representative of at least three independent experiments that yielded similar findings. Data are the mean ± standard error (SE). Differences were analysed using the Student’s t-test and P < 0.05 was considered significant.
References
Nieminen, M. S. et al. Levosimendan: current data, clinical use and future development. Heart Lung Vessel 5, 227–245 (2013).
Farmakis, D. et al. Levosimendan beyond inotropy and acute heart failure: Evidence of pleiotropic effects on the heart and other organs: An expert panel position paper. Int. J. Cardiol. 222, 303–312, https://doi.org/10.1016/j.ijcard.2016.07.202 (2016).
Parissis, J. T. et al. Effects of levosimendan on circulating pro-inflammatory cytokines and soluble apoptosis mediators in patients with decompensated advanced heart failure. Am. J. Cardiol. 95, 923–924, https://doi.org/10.1016/j.amjcard.2004.01.073 (2005).
Yamashita, S. et al. Cardioprotective and functional effects of levosimendan and milrinone in mice with cecal ligation and puncture-induced sepsis. Naunyn-Schiedeberg’s Arch. Pharmacol. 391, 1021–1032, https://doi.org/10.1007/s00210-018-1527-z (2018).
Hattori, Y., Hattori, K., Suzuki, T. & Matsuda, N. Recent advances in the pathophysiology and molecular basis of sepsis-associated organ dysfunction: Novel therapeutic implications and challenges. Pharmacol. Ther. 177, 56–66, https://doi.org/10.1016/j.pharmthera.2017.02.040 (2017).
Gordon, A. C. et al. Levosimendan for the prevention of acute organ dysfunction in sepsis. N. Engl. J. Med. 375, 1638–1648, https://doi.org/10.1056/NEJMoa1609409 (2016).
Tsao, C. M. et al. Levosimendan attenuates multiple organ injury and improves survival in peritonitis-induced septic shock: studies in a rat model. Crit. Care Med. 35, 1376–1382, https://doi.org/10.1186/s13054-014-0652-4 (2007).
Wang, Q. et al. Anti-inflammatory profile of levosimendan in cecal ligation-induced septic mice and in lipopolysaccharide-stimulated macrophages. Crit. Care Med. 43, e508–520, https://doi.org/10.1097/CCM.0000000000001269 (2015).
Ji, M. et al. Effects of combined levosimendan and vasopressin on pulmonary function in porcine septic shock. Inflammation 35, 871–880, https://doi.org/10.1007/s10753-011-9388-3 (2012).
Zager, R. A., Johnson, A. C., Lund, S., Hanson, S. Y. & Abrass, C. K. Levosimendan protects against experimental endotoxemic acute renal failure. American Journal of Physiology. Renal Physiology 290, F1453–F1462, https://www.physiology.org/doi/full/10.1152/ajprenal.00485.2005 (2006).
Grossini, E. et al. Protective effects elicited by levosimendan against liver ischemia/reperfusion injury in anesthetized rats. Liver Transpl. 20, 361–375, https://doi.org/10.1002/lt.23799 (2014).
Sareila, O. et al. Effect of levo- and dextrosimendan on NF-kappaB-mediated transcription, iNOS expression and NO production in response to inflammatory stimuli. Br. J. Pharmacol. 155, 884–895, https://doi.org/10.1038/bjp.2008.328 (2008).
Matejovic, M., Krouzecky, A., Radej, J. & Novak, I. Successful reversal of resistant hypodynamic septic shock with levosimendan. Acta Anaesthesiologica Scandinavica 49, 127–128, https://doi.org/10.1111/j.1399-6576.2005.00541.x (2005).
Morelli, A. et al. Effects of levosimendan on systemic and regional hemodynamics in septic myocardial depression. Intensive Care Med. 31, 638–644, https://doi.org/10.1007/s00134-005-2619-z (2005).
Morelli, A. et al. Effects of levosimendan on right ven- tricular afterload in patients with acute respiratory distress syndrome: a pilot study. Crit. Care Med. 34, 2287–2293, https://doi.org/10.1097/01.CCM.0000230244.17174.4F (2006).
Memiş, D., Inal, M. T. & Sut, N. The effects of levosimendan vs dobutamine added to dopamine on liver functions assessed with noninvasive liver function monitoring in patients with septic shock. J. Crit. Care 27, 318.e1–6, https://doi.org/10.1016/j.jcrc.2011.06.008 (2012).
Martins, P. N., Theruvath, T. P. & Neuhaus, P. Rodent models of partial heaptectomies. Liver international 28, 3–11, https://doi.org/10.1111/j.1478-3231.2007.01628.x (2008).
Saito, T. et al. Protective effect of fibronectin for endotoxin-induced liver injury after partial hepatectomy in rats. J. Surg. Res. 124, 79–84, https://doi.org/10.1016/j.jss.2004.10.018 (2005).
Tsuchiya, H. et al. Pirfenidone prevents endotoxin-induced liver injury after partial hepatectomy in rats. J. Hepatol. 40, 94–101, https://doi.org/10.1016/j.jhep.2003.09.023 (2004).
Tsuji, K. et al. Free radical scavenger (edaravone) prevents endotoxin-induced liver injury after partial hepatectomy in rats. J. Hepatol. 42, 94–101, https://doi.org/10.1016/j.jhep.2004.09.018 (2005).
Kwon, A. H. & Qiu, Z. Neutrophil elastase inhibitor prevents endotoxin-induced liver injury following experimental partial hepatectomy. Br. J. Surg. 94, 609–619, https://doi.org/10.1002/bjs.5625 (2007).
Arii, S. et al. Changes in the reticuloendothelial phagocytic function after partial hepatectomy. J. Lab. Clin. Med. 105, 668–672 (1985).
Szabó, C. & Módis, K. Pathophysiological roles of peroxynitrite in circulatory shock. Shock 34, 4–14, https://doi.org/10.1097/SHK.0b013e3181e7e9ba (2010).
Nakanishi, H. et al. J Hepatol. Pirfenidone inhibits the induction of iNOS stimulated by interleukin-1beta at a step of NF-kappaB DNA binding in hepatocytes. J. Hepatol. 41, 730–736, https://doi.org/10.1016/j.jhep.2004.07.007 (2004).
Araki, Y. et al. Sivelestat suppresses iNOS gene expression in proinflammatory cytokine-stimulated hepatocytes. Dig. Dis. Sci. 56, 1672–1681, https://doi.org/10.1007/s10620-010-1520-y (2011).
Yoshida, H. et al. Edaravone prevents iNOS expression by inhibiting its promoter transactivation and mRNA stability in cytokine-stimulated hepatocytes. Nitric Oxide 18, 105–112, https://doi.org/10.1016/j.niox.2007.11.003 (2008).
Kitade, H. et al. Interleukin-1β markedly stimulates nitric oxide formation in the absence of other cytokines or lipopolysaccharide, in primary cultured rat hepatocytes, but not in Kupffer cells. Hepatology 23, 797–802, https://doi.org/10.1053/jhep.1996.v23.pm0008666334 (1996).
Kaibori, M., Okumura, T., Sato, K., Nishizawa, M. & Kon, M. Inducible nitric oxide synthase expression in liver injury: Liver-protective effects on primary rat hepatocytes. Inflammation & Allergy-Drug Targets 14, 77–83, https://doi.org/10.2174/1871528114666160330113227 (2015).
Tanaka, H. et al. Na+/H+ exchanger inhibitor, FR183998, has protective effect in lethal acute liver failure and prevents iNOS induction in rats. J. Hepatol. 48, 289–299, https://doi.org/10.1016/j.jhep.2007.09.017 (2008).
Hijikawa, T. et al. Insulin-like growth factor 1 prevents liver injury through the inhibition of TNF-alfa and iNOS induction in D-galactosamine and LPS-treated rats. Shock 29, 740–747, https://doi.org/10.1097/shk.0b013e31815d0780 (2008).
Tanaka, Y. et al. Alpha-lipoic acid exerts a liver protective effect in acute liver injury rats. J. Surg. Res. 193, 675–683, https://doi.org/10.1016/j.jss.2014.08.057 (2015).
Okuyama, T. et al. A sense oligonucleotide to inducible nitric oxide synthase mRNA increases the survival rate of rats in septic shock. Nitric Oxide 72, 32–40, https://doi.org/10.1016/j.niox.2017.11.003 (2018).
Polat, B. et al. The effect of levosimendan in rat mesenteric ischemia/reperfusion injury. J. Invest. Surg. 26, 325–333, https://doi.org/10.3109/08941939.2013.806615 (2013).
Kietzmann, T. & Gorlach, A. Reactive oxygen species in the control of hypoxia-inducible factor-mediated gene expression. Semin. Cell Dev. Biol. 16, 474–486, https://doi.org/10.1016/j.semcdb.2005.03.010 (2005).
Itoh, K., Tong, K. I. & Yamamoto, M. Molecular mechanism activating Nrf2-Keap1 pathway in regulation of adaptive response to electrophiles. Free Radic. Biol. Med. 36, 1208–1213, https://doi.org/10.1016/j.freeradbiomed.2004.02.075 (2004).
Galanos, C., Freudenberg, M. A. & Reutter, W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc. Natl. Acad. Sci. USA 76, 5939–5943 (1979).
Mignon, A. et al. LPS challenge in D-galactosamine-sensitized mice accounts for caspase-dependent fulminant hepatitis, not for septic shock. Am. J. Respir. Crit. Care Med. 159, 1308–1315, https://doi.org/10.1164/ajrccm.159.4.9712012 (1999).
Okada, M. & Yamawaki, H. Levosimendan inhibits interleukin-1β- induced apoptosis through activation of Akt and inhibition of inducible nitric oxide synthase in rat cardiac fibroblasts. Eur. J. Pharmacol. 769, 86–92, https://doi.org/10.1016/j.ejphar.2015.10.056 (2015).
Grossini, E. et al. Levosimendan inhibits peroxidation in hepatocytes by modulating apoptosis/autophagy interplay. PLoS One 10, e0124742, https://doi.org/10.1371/journal.pone.0124742 (2015).
Kivikko, M. et al. Pharmacokinetics of levosimendan and its metabolites during and after a 24-hour continuous infusion in patients with severe heart failure. Int. J. Clin. Pharmacol. Ther. 40, 465–471, https://doi.org/10.5414/CPP40465 (2002).
Higgins, G. M. & Anderson, R. M. Experimental pathology of the liver. Arch. Pathol. 12, 186–202 (1931).
Seglen, P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 13, 29–83, https://doi.org/10.1016/S0091-679X(08)61797-5 (1976).
Kanemaki, T., Kitade, H., Hiramatsu, Y., Kamiyama, Y. & Okumura, T. Stimulation of glycogendegradation by prostaglandin E2 in primary cultured rat hepatocytes. Prostaglandins 45, 459–474, https://doi.org/10.1016/0090-6980(93)90122-N (1993).
Green, L. C. et al. Analysis of nitrate, nitrite and (15N) nitrate in biological fluids. Anal. Biochem. 126, 131–138, https://doi.org/10.1016/0003-2697(82)90118-X (1982).
Rasband, W. S. ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA, http://imagej.nih.gov/ij/, 1997–2012.
Chomczynski, P. & Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162, 156–159, https://doi.org/10.1016/0003-2697(87)90021-2 (1987).
Matsui, K. et al. Natural antisense transcript stabilizes inducible nitric oxide synthase messenger RNA in rat hepatocytes. Hepatology 47, 686–697, https://doi.org/10.1002/hep.22036 (2008).
Essani, N. A., McGuire, G. M. Manning, A. M. & Jaeschke, H. Endotoxin-induced activation of the nuclear transcription factor B and expression of E-selectin messenger RNA in hepatocytes, Kupffer cells, and endothelial cells in vivo. J. Immunol. 156, 2956–2963, http://www.jimmunol.org/content/156/8/2956 (1996).
Schreiber, E., Matthias, P., Müller, M. M. & Schaffner, W. Rapid detection of octamer binding proteins with mini-extracts, prepared from a small number of cells. Nucleic Acids Res. 17, 6419 (1989).
Oda M, et al. Vicinal dithiol-binding agent, phenylarsine oxide, inhibits inducible nitric oxide synthase gene expression at a step of nuclear factor-κB DNA binding in hepatocytes. J. Biol. Chem. 275, 4369–4373, http://www.jbc.org/content/275/6/4369.long (2000).
Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254, https://doi.org/10.1016/0003-2697(76)90527-3 (1976).
Acknowledgements
Authors thank Dr. Mitsuaki Ishida (Department of Pathology and Laboratory Medicine, Kansai Medical University) for analysing liver specimens by histopathology; Kyodo Byori. (Kobe, Japan) for staining the liver specimens; and Arshad Makhdum, PhD, from Edanz Group (www.edanzediting.com/ac), for editing a draft of this manuscript. Prof. A-Hon Kwon and his colleagues had established the experimental model of PH/LPS. This study was supported by the research fund of the Department of Surgery of Kansai Medical University.
Author information
Authors and Affiliations
Contributions
T.S. carried out the experiments, acquired and analysed the data, and wrote the major part of the manuscript. Y.H. helped create the PH/LPS-model under the supervision of M.K. H.M. and H.H. assisted in the isolation and culture of primary hepatocytes. M.N. helped in the experiments on antisense transcripts and provided reporter plasmids. T.O. was a mentor of this study and attended all experiments. All authors approved the final version of the manuscript for submission.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sakaguchi, T., Hashimoto, Y., Matsushima, H. et al. Levosimendan pretreatment improves survival of septic rats after partial hepatectomy and suppresses iNOS induction in cytokine-stimulated hepatocytes. Sci Rep 9, 13398 (2019). https://doi.org/10.1038/s41598-019-48792-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-48792-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.