Abstract
Blue shining fungus gnats (Diptera) had been long reported in the Waitomo caves of New Zealand (Arachnocampa luminosa Skuse), in stream banks of the American Appalachian Mountains (Orfelia fultoni Fisher) in 1939 and in true spore eating Eurasiatic Keroplatus Bosc species. This current report observes that similar blue light emitting gnat larvae also occur nearby the Betary river in the buffer zone of High Ribeira River State Park (PETAR) in the Atlantic Forest of Brazil, where the larvae were found when on fallen branches or trunks enveloped in their own secreted silk. The new species is named Neoceroplatus betaryiensis nov. sp. (Diptera: Keroplatidae: Keroplatinae: Keroplatini) based on a morphological analysis. Neoceroplatus betaryiensis nov. sp. larvae emit blue bioluminescence that can be seen from their last abdominal segment and from two photophores located laterally on the first thoracic segment. When touched, the larvae can actively stop its luminescence, which returns when it is no longer being agitated. The in vitro bioluminescence spectrum of N. betaryiensis nov. sp. peaks at 472 nm, and cross-reactivity of hot and cold extracts with the luciferin-luciferase from Orfelia fultoni indicate significant similarity in both enzyme and substrate of the two species, and that the bioluminescence system in the subfamily Keroplatinae is conserved.
Similar content being viewed by others
Introduction
The family Keroplatidae comprises of at least 92 genera and approximately 950 species1,2,3,4,5, distributed in all biogeographic regions. It is comprised of three subfamilies - Arachnocampinae, Macrocerinae, and Keroplatinae6, however Papp and Ševčik proposed a new subfamily7 named Sciarokeroplatinae based on a single species found the Oriental region. In the Neotropical region, there exist 32 genera and more than 200 species, of which 40 occur in Brazil5,8. Keroplatids inhabit mainly moist tropical forests usually associated with fungi. Adults can be collected in dark and humid places like stream banks and wet caves by sweeping in low vegetation, under hanging rocks or decaying logs. Usually, flies are caught by Malaise traps, UV lamp traps and occasionally in yellow pan traps4. Larvae are also present in moist and dark places, like caves, trunks, slits in stones, and have a variety of feeding habits. Predaceous larvae are seen in all subfamilies9, whereas mycophagy characterizes Keroplatinae4,10.
A few articles report on the function of bioluminescence of Diptera11,12,13. The larvae of luminous species such Archnocampa luminosa and Orfelia fultoni are carnivorous, however the Japanese Keroplatus nipponicus is fungivorous. Cannibalism is also common in the case of O. fultoni. Webs can be found in luminous and non-luminous mycetophilids. They are sticky and, in some cases, poisonous (i.e., contains oxalic acid as in the case of O. fultoni). Bigger arthropods such as cockroaches and ants were found caught in the webs of O. fultoni14. Sivinski performed experiments using transparent and blackened petri dishes covered with adhesive to verify whether light emission by O. fultoni can attract insects11. Most insects captured consisted of small Diptera (cecidomyids and phorids), which corroborated his hypothesis that preys were disoriented by the light emitted by the larva. In the case of a fungivorous species, like K. nipponicus, Sivinski hypothesized bioluminescence can perform other functions such as repelling negatively phototropic predators or as an aposematic signal12.
The subfamily Keroplatinae holds the highest number of genera (70) and species (677), in which it is possible to recognize two distinct tribes according to Matile2,15: Keroplatini and Orfeliini. The tribe Keroplatini contains 153 species in 21 genera16, which are characterized mainly by short, two-segmented palpi and laterally compressed or otherwise modified antennae. Of the few known immature specimens, the larva always has four anal lobes. Bioluminescence in Diptera is reported only in the Keroplatidae family in the genera Arachnocampa Edwards (Arachnocampinae), Keroplatus Bosc (Keroplatinae: Keroplatini), and Orfelia Costa (Keroplatinae: Orfeliini)4,16,17. Light emission by a species of Mallochinus Edwards of the Keroplatini group is uncertain18. Yet, according to Lloyd19 an unknown luminous species of Keroplatidae was found in New Guinea.
Notably, within the Keroplatidae there are at least two morphologically and biochemically distinct bioluminescent systems, namely those of Arachnocampa and O. fultoni Fisher20, whilst the bioluminescent system of Keroplatus species remains unknown. In Arachnocampa larvae, bioluminescence is produced by a lantern at the tip of the abdomen which is derived from Malpighian tubules20, and involves an ATP-dependent luciferase as in the case of beetle luciferases21. The chemical structure of the Arachnocampa luciferin was shown to derive from xanthurenic acid and tyrosine but has not yet been elucidated22. On the other hand, the bioluminescence system of O. fultoni, produced by translucent areas associated with rows of black bodies, involves an unknown heterodimeric 140 kDa luciferase, an unknown luciferin and a Substrate Binding Fraction (SBF), which apparently releases luciferin in the presence of reducing agents20. Notably, there is no cross-reaction between either the luciferin or the luciferase of Arachnocampa and Orfelia.
The genus Neoceroplatus Edwards is represented by twelve species worldwide, of which only N. samiri Khalaf is from the Neartic region. The other eleven species reportedly occur in the Neotropics4. Seven Neoceroplatus species are known: N. hodeberti Matile, N. lauroi Lane, N. punctipes Matile, N. minimax Edwards, N. dissimilis Matile, N. paicoenai Lane and N. spinosus Matile; the last four ones found in the State of São Paulo. Matile offers a taxonomic key for all these Neotropical species2. In this work, a new species of Keroplatini is described occurring in a conservation area of Atlantic Forest in São Paulo State named Betary Reserve, which is the first record of blue bioluminescent species of Diptera in the Neotropical region. Additionally, this work increases the number of known Neoceroplatus species to thirteen. Importantly, its luciferin and luciferase seem to be similar to the ones present in O. fultoni as attested by cross-reaction assays between both species.
Results and Discussion
Observation of larval behavior and bioluminescence
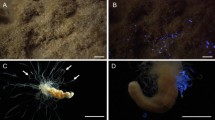
Larvae are usually found on fallen branches or tree trunks, where they are lodged between the wood and surrounded by their secreted mucus. They can also be found on tree trunks about a meter above the ground (Fig. 1). Typically, two or three larvae can share a single branch, although as many as 15 have once been collected from a single branch. Some larvae were also spotted in association with an unidentified species of brownish polypore mycelium. The larvae are very active, especially at night and can move constantly whilst completely covered by mucus. When disturbed, they quickly move under their mucus. Before pupation, they construct a cocoon on the surface of the log beneath either moss or fungi, where the pupae stay until the adult emerges (Fig. 2). Pupae are also bioluminescent when observed using a CCD camera (image not presented).
Different locations and habitats where larvae of Neoceroplatus betaryiensis nov. sp. were photographed. (A) Decaying log where larvae were collected. (B) Larvae on the surface of the log surrounded by a web-like mucus. (C) Association of a larva with a Favolus brasiliensis (Fr.) Fr. mushroom raised in a terrarium. (D) Photo of a typically translucid N. betaryiensis sp. nov. (E) Details of the larva head and (F) last abdominal segment.
Neoceroplatus betaryiensis nov. sp. larvae emit blue light from their last abdominal segment and from two photophores located laterally on the first thoracic segment (Fig. 3). Until now, it was not possible to ascertain whether the bioluminescence is associated to the black bodies as in the case Ofelia fultoni and possibly of Keroplatus spp., but with the presence of several dark granules spread along the body suggest this is a possibility. Light emission is turned off when the larva is touched and begins again a few minutes after this mechanical agitation ceases. We were not able to observe the eating habits of the larvae, although the absence of remnants of insects trapped in the web with the proximity of fungi suggest that the larvae could be fungivorous.
Unexpectedly, a different larva was once pulled out from the underside of a fallen leaf, showing diffuse blue bioluminescence apparently consisting of small brown photophores along the whole body (Fig. 4A,B). It exhibited bizarre behavior compared to other larvae observed, as it moved slower and exposed itself more than was previously observed. At first, it was thought to be another luminous dipteran species. However, when kept in a terrarium, the larva entered the pupal stage two weeks later and after eleven days, an ichneumonid parasitic wasp emerged from the pupa (Fig. 4C). Luminous mycetophilids are reportedly attacked by hymenopterous parasitoids12 and this diffuse bioluminescence could be the result of either a defensive reaction against the parasite or the consequence of internal organ damage spreading the photogenic material along the body of the larva. However, it may also belong to another new species that can emit light along the whole body as observed in Keroplatus nipponicus13.
Chemiluminescence and luciferin-luciferase cross-reaction
Whole Neoceroplatus betaryiensis nov. sp. larvae were homogenized in the extraction buffer. The resulting homogenate displayed considerable light emission. After centrifugation a weaker luminescence remained in the supernatant, indicating that the luciferin and luciferase are active and solubilized in these conditions. The supernatant or “cold extract” showed a gradual increase in light emission to a higher intensity upon the addition of DTT (dithiothreitol) as a reducing agent. This is similar to what occurs with crude extracts of Orfelia, consistent with the presence of the Substrate Binding Fraction (SBF) which retains luciferin. Addition of O. fultoni hot extract that contains the luciferin to Neoceroplatus cold extract (luciferase-rich fraction) also increased light emission. The spectrum obtained by cross-reacting the cold extract of N. betaryiensis nov. sp. and the hot extract of O. fultoni in the presence of DTT shows a maximum intensity of around 472 nm (Fig. 5), which matches the recently reported value for O. fultoni luciferin-luciferase system23. The addition of hot extract of N. betaryiensis nov. sp. to the purified luciferase of O. fultoni also resulted in blue light emission. These results indicate that N. betaryiensis nov. sp. and O. fultoni share either very similar or identical luciferin substrates and luciferase enzymes.
Molecular phylogenetic studies
A molecular phylogenetic analysis was conducted with N. betaryiensis, Orfelia fultoni and the non-luminescent Neoditomiya sp., using the mitochondrial gene of cytochrome oxidase I (COI). COI barcode-gene is useful to recognize closely related species, to investigate their relative phylogenetic position, and to analyze if there was an evolutionary relationship among the luciferin-luciferase systems of Keroplatinae. The molecular phylogenetic analysis showed that the fungus-gnat N. betaryiensis nov. sp. is positioned as a sister-group of the luminescent Keroplatus genus (Fig. 6), followed by the non-luminescent Neoditomyia (Lane & Sturm) and the luminescent O. fultoni, which is in agreement with the literature2,10. The placement of the non-bioluminescent Neoditomyia sp. as a sister-group of Keroplatinae subfamily was also observed. Interestingly, Orfelia-type luciferin and its Substrate Binding Protein (SBF) has been recently reported in Neoditomyia sp.23. Both the luciferin and SBF of Neoditomyia were able to cross-react with purified O. fultoni luciferase, resulting in an emission spectrum that overlaps with that of O. fultoni extracts. The cross-reactions between O. fultoni and N. betaryiensis nov. sp. strongly suggest that the same bioluminescent system is also shared by these genera. Altogether, the COI gene analysis and biochemical results indicate that Neoditomyia spp., Neoceroplatus betaryiensis and Orfelia fultoni, and by inference Keroplatus species, share the same bioluminescent system.
Phylogenetic tree of Keroplatidae bioluminescent and non-bioluminescent species using mitochondrial gene cytochrome oxidase I (COI). Species surrounded by a grey rectangle are bioluminescent. Species whose name is displayed in pale blue share the same specific luciferin and SBF and the species with name in dark blue, share the same specific luciferase and luciferin.
Taxonomical description
Neoceroplatus betaryiensis Falaschi, Johnson & Stevani nov. sp. (Figs 1B to 4B, S1A to S5D).
Material examined. Holotype: Male, BRAZIL, São Paulo, Iporanga, Reserva Betary, IPBio – Instituto de Pesquisas da Biodiversidade, 24°35′27″S 48°37′44″W, 120 m, manual collection on the underside of a leaf (on June 14th 2017 one larva was collected and on August 24th 2017 the adult male emerged), Domingos, A. H. R., Santos, I. & Johnson, G. A. cols. [MZUSP- MZ052800] (specimen pinned with terminalia on permanent slide). Paratypes: Two females, same data as holotype, except 01.v.2017 (larva collected) 24.v.2017 (adult female emerged) [MZUSP-MZ052801] (specimen pinned), [MZUSP-MZ052802 (specimen on permanent slide); one larva, same data as holotype, except 15.v.2017, [MZUSP-MZ052803] (in 80% ethanol); two larvae, same data as holotype, except 16.iv.2017, [MZUSP-MZ052804] (in 80% ethanol), [MZUSP-MZ052805] (in permanent slide); pupa exuvium, same data as holotype, except 24.viii.2017, [MZUSP-MZ052806] (in permanent slide); net remains, same data as holotype, except 24.viii.2017, [MZUSP-MZ052807] (in 80% ethanol).
Etymology: The specific epithet refers to the Betary brook, in whose banks the specimens were collected.
Diagnosis and comments
Neoceroplatus betaryiensis nov. sp. can be distinguished from the other Neotropical Neoceroplatus¸ especially from N. dissimilis, its closest species, by the shape of the genitalia, particularly the gonostylus (Figs S3E, S4B,C) and the absence of spines in the gonostylus as appears in N. paicoenai.
Male
Body length (without antennae): 5.2 mm (Fig. 2C). Wing length: 3.75 mm (Fig. S1F). Terminalia length: 0.28 mm (Figs S3A,C,E; S4A,B). Head: Brownish. Three ocelli in triangular position, each positioned on a callus. The lateral ocellus larger than middle one (Fig. S1D). The distance between the lateral ocelli two times its own diameter, separated from the edge of the eye at a distance slightly higher than its own diameter. Median ocellus approximately 3/4 smaller than lateral ocellus. Compound eyes are large, about 1.5 times higher than wide in lateral view, covered with microsetae. Antenna (Fig. S1A,B) three times higher than head, flattened laterally, yellowish-brown. Scape and pedicel cylindrical, both slightly wider than high, brownish. Flagellum expanded and flattened, with 14 flagellomeres, dorsal and ventral macrosetae covering flagellomeres. Terminal flagellomere, longer than wide, with a minute, thin yellow-whitish apical process at the apex, in a shallow notch (Fig. S1B). Labrum and labella weakly sclerotized; labellum exceeding the ventral edge of the eyes. Palpus bi-segmented. Second palpomere elongated, covered with setae laterally, pointed at the apex, as long as face and clypeus combined length; medially membranous, weakly sclerotized. Thorax: Mostly yellowish, with brownish spots laterally. Antepronotum brownish, covered with macrosetae; proepisternum brownish, bare; anterior spiracle surrounded by setae; anepisternum bare, apically yellowish and brownish at the base and internal edge; katepisternum and anepimerum bare, mostly yellowish, with a brownish spot proximally; laterotergite mostly brownish, covered with long and sparse macrosetae. Scutum brownish, densely covered with macrosetae. Scutellum brownish, covered with long macrosetae. Mediotergite bare, mostly brownish. Halter with brownish knob and stem mostly yellowish, with a row of setae. Wing: (Fig. S1F) Wing membrane infuscate brown to dark brown mainly on distal half, basal cells yellowish with small brown maculae; the minor hyaline area between R4 and R5, and C to M1. Microsetae irregularly scattered throughout wing. Veins brown to dark brown. Strong setation on C, R1, R4 and R5. Humeral lightly shorter than half of R1. Vein C exceeding apex of R5, at a distance beyond R5 that is roughly 1/4 the distance between R5 and M1 apex. R4 ending at R1 apex, bending posteriorly with almost right angle with R5. M1, M2, M4 and anal veins not reaching the wing margin. A1 as long as CuP vein. Legs: Mostly white to yellowish. Fore coxa white-yellowish with brownish spot and covered with macrosetae on its all anterolateral face. Mid coxa white-yellowish with two brownish spots covered with macrosetae anterolaterally; hind coxa white-yellowish with a large brownish spot covered with macrosetae only laterally. Femora white-yellowish, covered with setae, with an upper brownish spot antero-laterally. Tibiae and corresponding tarsi yellowish to pale brown, covered with regular rows of setae. Abdomen: Covered with macrosetae. Mostly yellowish ventrally and laterally, with large brownish areas dorsally. Sternites 3–6 with two brownish marks in each lateral margin (Fig. S2A–C). Terminalia: Mostly yellow (Fig. S3A,C,E) covered with long, dense macrosetae (Fig. S4A,B). Tergite 9 yellow-brownish, V-shaped, longer than wide (Figs S3C,G, S4A). Cerci yellow, poorly sclerotized, covered with dark setae (Figs S3C,G, S4A). Gonocoxites partially fused basally, yellowish, almost entirely covered with long and dark setae, and few short and dark setae (Fig. S3A,E). Gonostylus dark brown, prominent, shark fin-shaped, covered with long setae, and with dark, thicker bristles on inner side (Figs S3E, S4B,C), with an interiorly-directed, antero-dorsal lobe (Fig. S3E).
Female
Body length (without antennae): 5.9 mm (Fig. 2B). Wing length: 4 mm (Fig. S1G). Terminalia length: 0.39 mm (Figs S3B,D,F; S4D–E). As the male, except as follows. Head: Last flagellomere slightly wider than long, subquadrate (Fig. S1C). Wing: the minor hyaline area between R4 and R5, and C to M2 and the major hyaline area reaching the apex of CuA. Humeral roughly more than half of R1. Vein C at a distance beyond R5 that is more than one third the distance between R5 and M1. R4 divergent relative to R5 forming a 45°. Base of M4 weakly sclerotized. A1 longer than CuP vein (Fig. S1G). Abdomen: (Fig. S2D,E) Dorsally yellowish with two large brownish spots on tergites 2–7. Sternites 2–3 white-yellowish with one subtriangular brownish mark and rounded shaped on 4–5. Legs: Mid coxa whitish with two dark-brownish spots covered with macrosetae antero-laterally, and a row with four macrosetae at the lateral apex; hind coxa whitish with a large dark-brownish spot covered with macrosetae laterally (Fig. S1E). Femora whitish, the hind femora with two brownish spots antero-laterally. Terminalia: mostly brownish (Figs S3B,D,F; S4D,E) covered with long, dense macrosetae, without microsetae. Sternite 8 covered with dense setae, inner margin straight; tergite 8 covered with setae; tergite 10 well developed; cercus formed by one article, entirely covered with setae (Fig. S4D,E).
Immature
Body length: 20.1 mm (Figs 1D, 3A, 4A and S5C); Pupa exuvium: 6,95 mm (Fig. S5D). Overall morphology similar to other Keroplatini larvae. General color gray-whitish, head capsule brown, well sclerotized, subquadrate (Fig. S5A,B). No distinctive modification of the body cuticle, except anterior portion of body divided into four subquadrate areas, followed by narrow transverse lines towards posterior end (Figs 1D–F, 3A, 4A and S5C), giving a segmented texture to the integument. Cephalic capsule quadrangular, slightly longer than wide, very short in relation to body width (Figs 3C, S5A,B); a well-developed gena, ventral part of foramen magnum longer than wide. Antenna very reduced, flattened, ellipse-shaped, protruding above the antenna base, positioned more dorsally. Labrum in continuity with the clypeal area. Premandible with row of elongated, flexible teeth, supported by a pair of lateral chitinous arms. Mandible semicircular and bearing two rows of medially directed teeth; Mandible well developed. Maxilla elongated, rather parallel distally, with fifteen teeth on inner border. Cardo slender, transverse (Fig. S5A). Secondary annulation on abdominal segments. Posterior end with a pair of lobose triangular projections (Figs 1D,F and 3A).
Comments
The anterior photophores are located on the first thoracic segment of the larva and the posterior on the last abdominal segments, without visible external morphological structures.
In the holotype and paratypes of Chetoneura shennonggongensis (Amorim & Niu) from the Zoology Museum of University of São Paulo, Brazil24, there are no structures in the last segment similar to the “posterior papillae”, described by Matile from the genera Arachnocampa, Keroplatus, and Macrocera in either C. shennonggongensis or Neoceroplatus betaryiensis. These structures can be properly described and understood from deeper histological studies, which was not the aim of this study.
Conclusion
Here we report the discovery of the first bioluminescent species of fungus-gnats of the family Keroplatidae in the Neotropical region. Similar to the Palearctic and Oriental Keroplatus species, N. betaryiensis also lives under dead logs and is probably sporophagous. The bioluminescence is blue, and likely shares the same luciferin-luciferase system of the North-American Orfelia fultoni, and possibly of the Palearctic Keroplatus spp. These findings show how Neotropical biodiversity is still poorly known25, despite being recognized as the most diverse biogeographic region on the planet. Unfortunately, the anthropic pressure on natural areas has been increasing, causing disturbances in different habitats, with damage in megadiverse countries such as Brazil26. These threats affect especially small invertebrates, which have been extinguished at a much faster rate than their discovery and description27. This reinforces the need for conservation policies for areas such as the Betary Reserve, a place that provides new taxa for science, the Keroplatidae being one of them.
Methods
Diptera larva collection
The material presented here was collected by G.A.J., A.H.R.D. and I.S. on the property of the non-governmental organization Instituto de Pesquisas da Biodiversidade (IPBio), municipality of Iporanga, São Paulo State, Brazil. The Betary Reserve, the first branch of IPBio, is located between the geographical coordinates 24°35′16″S; 48°37′44″W, a preserved area of ca. 60 hectares of dense ombrophylous forest in an advanced regeneration status. The reserve is situated in the largest remaining continuous area of the Atlantic Forest, and within the protective zone of the Touristic State Park of High Ribeira River (PETAR). The fungus gnats were collected during a hot and rainy period, with relative humidity of 90%, and manually collected on fallen trees.
On May 1st 2017, four larvae were collected and kept in terraria, and on May 24th two female adults emerged. Another larva, with erratic behavior, collected on May 31, 2017 was parasitized by an ichneumonid wasp, which emerged on June 13th. In the laboratory the larvae were kept in large glass terraria with a thin fabric cover. The bottom of the terraria were covered with dried leaves, with branches leaning against the lateral glass walls, and with thicker branches that harbored mushrooms of the species Favolus brasiliensis (Fr.) Fr. It was observed that after a few days the larvae migrated to the bottom of the mushrooms, where they built their web. After a few days they went down to the leaves and about two weeks later they emerged as adults.
The female adults that emerged in the laboratory were primarily preserved in 70% ethanol. The larvae, which were preserved in KAAD solution (100 mL kerosene, 700 mL of absolute alcohol, 100 mL of acetic acid and 100 mL of colorless house detergent), were collected in the field. The larva remained in solution for 12 h and then 70% alcohol for 24 h. After that, the larva was removed and kept in 80% alcohol. Hot water prevented dryness and rapid “wrinkling” and KAAD preserved color.
Larvae and adult preservation and microscopic examination
The material examined in this study was deposited in the Diptera collection at the Museu de Zoologia da Universidade de São Paulo (MZUSP, São Paulo, Brazil). The collected material was initially preserved in 80% ethanol. The terminalia were detached from the abdomen, cleared in 10% KOH aqueous solution at 40 °C for 40–60 min and promptly washed first in glacial acetic acid and then in 80% ethanol for 15 min. Each specimen and wings were soaked for 15 min twice both in absolute ethanol and then in xylenes (mixture of o,m,p-xylene) Synth, 98.5%. Afterwards, each specimen was mounted in the permanent slide with Canadian balsam. Wing and terminalia were drawn after mounting on permanent slides. Photographs were taken with using a Leica DC camera either attached to a Leica MZ16 stereomicroscope or to a Leica DM2500 microscope. Stacking was performed with Helicon Focus 6 and edited with the Adobe Photoshop CC 2017. General illustrations of male and female terminalia were drawn with the help of a camera lucida attached to the microscope, vectorized using Adobe Illustrator CC 2017. The species was identified using the available identification key present in Matile2 and by comparison with the types housed at the MZUSP (with the closest species – Neoceroplatus dissimilis and the other with the type specimens available: N. dureti; N. hodeberti; N. lauroi; N. monostylus; N. paicoenai and N. spinosus). The morphological terminology used here follows the literature2,28.
Bioluminescence imaging
Imaging of bioluminescent larvae and pupae were done using a LightCapture II CCD camera (ATTO, Tokyo, Japan).
Chemiluminescence assay
Between 3 to 6 entire Neoceroplatus betaryiensis nov. sp. larvae were homogenized in 1 ml of Orfelia fultoni extraction buffer (0.10 M phosphate buffer, 1 mM EDTA, 1% Triton X-100, pH 7.0). Hot-cold extract assays were performed using a previously established method for O. fultoni20,23. The homogenate of the larvae was separated in two aliquots, one of which was centrifuged and the pellet discarded. The supernatant was left to react completely, with the remaining solution being termed the “cold extract”. The second aliquot was treated with 10 mM DTT and was heated at 98 °C for 5 minutes in an anoxic atmosphere. This solution was then centrifuged and the pellet discarded, leaving the “hot extract”. Purified O. fultoni luciferase and luciferin containing hot extracts were prepared using larvae collected in North Carolina (USA) by one of the authors (VRV). Light emission was measured in counts per second (cps) using an ATTO AB2200 luminometer (Tokyo, Japan). Chemiluminescence spectra was recorded using an Atto LumiSpectra spectroluminometer (Tokyo, Japan).
Molecular phylogenetic analysis
The phylogenetic analysis of Keroplatidae species was performed using the mitochondrial gene cytochrome oxidase I (COI). The nucleotide sequences were aligned using ClustalW algorithm29 on MEGA 6.0 software30. The jModelTest2 program31 was used to predict the best evolutionary model, which resulted in GTR + G + I substitution model. The phylogenetic analysis used by the software MrBayes 3.232, through two separately runs with 10,000,000 generations each. The first 25% of trees were discarded and concatenated to create a consensus tree.
References
Papavero, N. Family Keroplatidae (Ceroplatidae, incl. Macroceridae). In A Catalogue of the Diptera of the Americas South of the United States 19C, 1–22 (1978).
Matile, L. Recherches sur la systematique et l’evolution des Keroplatidae (Diptera, Mycetophilidae). Mem. Mus. Natn. Hist. Nat., ser A, Zool 148, 1–682 (1990).
Evenhuis, N. L. Catalogue of the fossil flies of the world (Insecta: Diptera). (Backhuys Press, Leiden, 1994).
Evenhuis, N. L. Catalog of the Keroplatidae of the World (Insecta: Diptera). Bishop Mus Bull Entomol 13, 1–178 (2006).
Oliveira, S. S., Falaschi, R. L., Urso-Guimarães, M. V. & Amorim, D. S. Lista das espécies de Bibionomorpha (Diptera) do Estado do Mato Grosso do Sul, Brasil. Iheringia Ser. Zool. 107, 1–8 (2017).
Matile, L. Description d’un Keroplatidae du crétacé moyen et données morphologiques et taxinomiques sur les Mycetophiloidea (Diptera). Ann. Soc. Entomol. Fr. 17, 99–123 (1981).
Papp, L. & Ševčik, J. Sciarokeroplatinae, a new subfamily of Keroplatidae (Diptera). Acta Zool. Hung. 51, 113–123 (2005).
Falaschi, R. L. Relações filogenéticas entre os Keroplatinae: posição de Orfeliini e relação entre seus gêneros (Diptera: Keroplatidae). (PhD Thesis, Ribeirão Preto, Universidade de São Paulo, 2012).
Mansbridge, G. H. On the biology of some Ceroplatinae and Macrocerinae (Diptera, Mycetophilidae). Trans. R. Entomol. Soc. Lond. 81, 75–92 (1933).
Matile, L. Phylogeny and evolution of the larval diet in the Sciaroidea (Diptera, Bibionomorpha) since the Mesozoic. In The Origin and Biodiversity in Insects: Phylogenetic Tests of Evolutionary Scenarios (ed. Grandcolas, P.). Mem. Mus. Nat. Hist. Nat. A 173, 273–303 (1997).
Sivinski, J. M. Prey attraction by luminous larvae of the fungus gnat Orfelia fultoni. Ecol. Entomol. 7, 443–446 (1982).
Sivinski, J. M. Phototropism, bioluminescence and the Diptera. Fla. Entomol. 81, 282–292 (1998).
Osawa, K., Sasaki, T. & Meyer-Rochow, V. B. New observations on the biology of Keroplatus nipponicus Okada 1938 (Diptera; Mycetophiloidea; Keroplatidae), a bioluminescent fungivorous insect. Entomol. Heute 26, 139–149 (2014).
Fulton, B. B. A luminous fly larva with spider traits. Ann. Entom. Soc. Am. 34, 289–302 (1941).
Matile, L. Note sur les Macrocerini (stat. nov.) et description d’un genre et de sept espèces de la région éthiopienne (Diptera, Mycetophilidae). Bull. Inst. Fond. Afr. Noire 34, 593–610 (1973).
Ševčík, J., Mantič, M. & Blagoderov, V. Two new genera of Keroplatidae (Diptera), with an updated key to the World genera of Keroplatini. Acta Entomol. Mus. Nat. Pragae 55, 387–399 (2015).
Baccetti, B., Crovetti, A. & Santini, L. Light-producing organs in Keroplatus tipuloides Bosc and K. reaumuri pentophthalmus Giglio-Tos (Diptera: Mycetophilidae). Int. J. Morphol. Embryol. 16, 169–176 (1987).
Oba, Y., Branham, M. A. & Fukatsu, T. The terrestrial bioluminescent animals of Japan. Zool. Sci. 28, 771–789 (2011).
Lloyd, J. E. Insect Bioluminescence. In Bioluminescence in Action (ed. Herring, P. J.). Academic Press, New York, 241–272 (1978).
Viviani, V. R., Hastings, J. W. & Wilson, T. Two bioluminescent diptera: the North American Orfelia fultoni and the Australian Arachnocampa flava. Similar niche, different bioluminescence systems. Photochem. Photobiol. 75, 22–27 (2002).
Trowell, S. C., Dacres, H., Dumancic, M. M., Leitch, V. & Rickards, R. W. Molecular basis for the blue bio- luminescence of the Australian glow-worm Arachnocampa richardsae, (Diptera: Keroplatidae). Biochem. Biophys. Res. Commun. 78, 533–539 (2016).
Watkins, O. C., Sharpe, M. L., Perry, N. B. & Krause, K. L. New Zealand glowworm (Arachnocampa luminosa) bioluminescence is produced by a firefly-like luciferase but an entirely new luciferin. Sci. Rep. 8, 3278 (2018).
Viviani, V. R., Amaral, D. T., Bevilaqua, V. R. & Falaschi, R. L. Orfelia-type luciferin and its associated storage protein in the non-luminescent cave worm Neoditomyia sp. (Diptera: Keroplatidae) from the Atlantic rainforest: biological and evolutionary implications. Photochem. Photobiol. 17, 1282 (2018).
Amorim, D. S., Niu, C. H., Li, X., Lei, C. H. & Clarke, A. K. Chetoneura shennonggongensis, a new species of cave-dwelling Keroplatini from China (Diptera: Keroplatidae), with a discussion of the position of Chetoneura. Zootaxa 1716, 59–68 (2008).
Lewinsohn, T. & Prado, P. I. Quantas espécies há no Brasil? Megadiversidade 1, 36–42 (2005).
Carvalho, C. J. B. Padrões de endemismo e a conservação da biodiversidade. Megadiversidade 5, 77–86 (2011).
Carvalho, M. R., Bockmann, F. A., Amorim, D. S. & Brandão, C. R. F. Systematics must embrace comparative biology and evolution, not speed and automation. J. Evol. Biol. 35, 97–104 (2008).
Cumming, J. M. & Wood, D. M. Adult morphology and terminology. In Manual of Central American Diptera (eds Brown, B. V., Borkent, A., Cumming, J. M., Wood, D. M., Woodley, N. E. & Zumbado, M. A.), NRC Research Press, Ottawa, 1, 9–50 (2009).
Higgins, D. et al. CLUSTAL W: improving the sensitivity of the progressive multiple sequence alignment through sequence weighting, position-specific gap penal- ties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680 (1994).
Tamura, K., Stecher, G., Peterson, D., Filipski, A. & Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 30, 2725–2729 (2013).
Darriba, D., Taboada, G. L., Doallo, R. & Posada, D. jModelTest 2: more models, new heuristics and parallel computing. Nat. Methods 9, 772 (2012).
Ronquist, F. et al. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Syst. Biol. 61, 539–542 (2010).
Acknowledgements
The authors are indebted to Dr. Carlos Lamas (MZUSP, Sao Paulo, Brazil), for access to the type material studied in this work and Dr. Diego Fachin (USP, Ribeirão Preto, Brazil) who very kindly revised the description text of the manuscript. We also thank Dr. Jay C. Dunlap (Dartmouth Geisel School of Medicine, USA) and Dr. Graham S. Timmins (University of New Mexico, USA) for careful reading of the manuscript. This work was funded by grants of Fundação de Amparo a Pesquisa do Estado de São Paulo (grants FAPESP 2010/05426-8 to VRV, 2013/16885-1 to CVS and 2017/22501-2 to AGO, EJHB and CVS), Brazilian National Research Council (CNPq/Universal Project 401867/2016-1 to VRV and 306460/2016-5 to EJHB) and by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES - Finance Code 001) to RLF. This work was also partially supported by funding from the Office of Naval Research Global through grant ONR N62909-17-1-2103 to CVS and AGO.
Author information
Authors and Affiliations
Contributions
I.S., A.H.R.D., G.A.J., A.G.S.M., I.B.V. and S.L.P. discovered, collected and conducted experiments in the field with N. betaryiensis nov. sp. A.H.R.D. took all macro photographs, excepting the ones depicted in Figures 1D–F and 4C, which were taken by G.A.J. R.L.F. was responsible for the identification, preparation, micrographies, description and designation of the type-species and morphological comparative analysis with the other described species of the studied genus. D.T.A. performed the molecular phylogenetic analysis. V.R.V. performed the cross-reaction experiments, recorded luminescence spectra purified Orfelia fultoni luciferase and supervised the biochemical and molecular aspects of bioluminescence. J.D.M., A.G.O., E.J.H.B. and C.V.S. planned, organized and evaluated critically the experiments, and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Falaschi, R.L., Amaral, D.T., Santos, I. et al. Neoceroplatus betaryiensis nov. sp. (Diptera: Keroplatidae) is the first record of a bioluminescent fungus-gnat in South America. Sci Rep 9, 11291 (2019). https://doi.org/10.1038/s41598-019-47753-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-47753-w
This article is cited by
-
Hidden diversity in the Brazilian Atlantic rainforest: the discovery of Jurasaidae, a new beetle family (Coleoptera, Elateroidea) with neotenic females
Scientific Reports (2020)
-
A new brilliantly blue-emitting luciferin-luciferase system from Orfelia fultoni and Keroplatinae (Diptera)
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.