Abstract
Stress tolerance and adaptation to stress are known to facilitate species invasions. Many invasive species are also pests and insecticides are used to control them, which could shape their overall tolerance to stress. It is well-known that heavy insecticide usage leads to selection of resistant genotypes but less is known about potential effects of mild sublethal insecticide usage. We studied whether stressful, sublethal pyrethroid insecticide exposure has within-generational and/or maternal transgenerational effects on fitness-related traits in the Colorado potato beetle (Leptinotarsa decemlineata) and whether maternal insecticide exposure affects insecticide tolerance of offspring. Sublethal insecticide stress exposure had positive within-and transgenerational effects. Insecticide-stressed larvae had higher adult survival and higher adult body mass than those not exposed to stress. Furthermore, offspring whose mothers were exposed to insecticide stress had higher larval and pupal survival and were heavier as adults (only females) than those descending from control mothers. Maternal insecticide stress did not explain differences in lipid content of the offspring. To conclude, stressful insecticide exposure has positive transgenerational fitness effects in the offspring. Therefore, unsuccessful insecticide control of invasive pest species may lead to undesired side effects since survival and higher body mass are known to facilitate population growth and invasion success.
Similar content being viewed by others
Introduction
Invasive species pose a serious threat not only to agriculture, and the health of humans and animals, but also to biodiversity and ecosystem functioning1,2. Thus, it is important to understand the ecological and evolutionary factors that influence invasion success. Stress tolerance and adaptation to stressful environments are among the most important factors contributing to invasion success3,4. Stress can be defined as changes in the external or internal environment that threaten the maintenance of homoeostasis5,6. High stress-tolerance or organismal flexibility (e.g. behavioural or physiological) may contribute to invasion success by enabling invasive species to persist under unfavourable environmental conditions and allow time for adaptation to occur3,4. Stress may also be adaptive (via the process of genetic assimilation) by releasing phenotypic variation that contributes to fitness, or by facilitating developmental expression of beneficial traits that are phenotypically neutral under normal conditions7,8. Thus, in order to prevent invasions, it is important to understand how invasive species respond to stress and what the evolutionary consequences of stress are.
Many invasive species are insect pests and insecticides are commonly used to control them. However, insecticides do not only form a strong selection pressure but can also be a major stress factor when exposure is sublethal. Insect pests can be exposed to sublethal levels of insecticide in several ways; for example, as a result of an improper application or due to the degradation of the insecticide by abiotic factors, such as sunlight, rainfall or temperature9. Studies exploring sublethal insecticide exposures in insecticide resistance are rare but relevant due to their potential consequences also at the community level (i.e. community stress due to the changes in the species interactions)10. While insecticide stress generally has negative fitness effects11, it can at times be advantageous and increase fitness12,13,14. This phenomenon, where exposure to low levels of stress can induce stimulatory effects but is lethal at higher exposure levels, is known as hormesis14. This phenomenon has been demonstrated on maize weevil (Sitophilus zeamais) where exposure to sublethal doses of pyrethroid insecticide lead to a peak in the net reproductive rate15. Nevertheless, both positive and negative insecticide stress-induced modifications can have adaptive importance as these may be carried over to the next generation and persist across multiple generations7,16,17 through transgenerational effects.
Transgenerational effects occur when the phenotype of the offspring is influenced by the phenotype or environment of its parents18,19,20. Mousseau and Fox18 suggest that these transgenerational effects are most often seen between mother and offspring. This is because mothers can contribute to offspring development through a range of inputs via nutrition of the egg, transfer of immune factors or epigenetic mechanisms18,21,22. Transgenerational stress effects have received attention because of their significance from an evolutionary point of view23. However, to date, only a few studies have examined transgenerational stress in the context of insect pest invasions13,16. Considering the harm that invasive pest species pose to the environment and agriculture, it is important to study how insecticide stress affects performance and population dynamics within a generation, and whether sublethal doses lead to transgenerational cost or benefits.
The Colorado potato beetle (Leptinotarsa decemlineata Say.) is a notorious pest of potato (Solanum tuberosum). The beetle is native to Mexico and the south-western parts of the United States but can nowadays be found from the sub-tropical to temperate northern hemisphere24,25, and it is predicted to continue to expand its range rapidly26,27. The control of the beetle is heavily based on insecticides28,29. It is an excellent species to study insecticide stress within- and across generations in the context of invasive species. Due to its complex life-history combined with high selection pressure and rapid adaptation, the beetle has developed resistance to most classes of insecticides28,30. This means, that for already resistant populations, an insecticide application is likely to cause stress instead of lethal effects.
In the present study we investigated within- and transgenerational (maternal) effects of sublethal insecticide stress on several fitness-associated traits; survival, development time, body mass and lipid content. By rearing beetles for two generations we could investigate within-generational responses to insecticide stress in the first and second generation while transgenerational effects of maternal insecticide stress were evaluated in the second-generation beetles. Furthermore, we investigated whether transgenerational insecticide stress exposure influenced the offspring´s tolerance to the same stressor. The investigation of transgenerational effects is relevant for invasive pest insects, including the Colorado potato beetle, because they can have multiple generations per year that are exposed to the same insecticide. Based on hormesis (insecticides are known to have hormetic effects) and our previous findings16, exposure to insecticide stress could induce positive transgenerational effects. Offspring whose mothers were exposed to insecticide stress should be heavier and accumulate more lipids than offspring descending from mothers not exposed to the stress. Possible effects on body mass are important because higher body mass is associated with higher reproductive performance, survival and overall increased fitness (including overwintering survival)31. All these traits are relevant as they can facilitate the invasion of the beetle towards northern latitudes as well as generally increase its severity as a pest.
Results
Within-generational insecticide effects on survival in the first generation
In the first generation, after being exposed to insecticide stress for 24 hours, larval survival was 95% and 97% in the insecticide and control groups, respectively (β21; Fig. 1; Table 1). This high survival confirms that the insecticide exposure was sublethal. Total larval survival (i.e. survival after being exposed to insecticide for 24 h until pupation) and pupal survival were similar between the insecticide exposed and control individuals (β22; β23). However, insecticide exposed individuals were more likely to survive as adults (from adult emergence to 10 days) when compared to the control group (β24; Fig. 1). The odds for survival are between 0.98 and 17.99 times higher in the insecticide exposed group with 95% probability, and the probability for survival to be larger than 1 is high.
Paternal effects, as measured in a subset of the males of the study, did not affect offspring survival and had a very minor effect on offspring body mass (Supplementary Table 1). Therefore, we focus on maternal effects and pesticide treatments as the major sources of variation in the study.
Within-generational insecticide effect on development time and adult body mass in the first generation
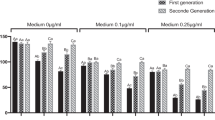
Development time (i.e. time in days from egg hatching to adult emergence and it is log-transformed in the model; Supp. Fig. 1a,b) was similar between insecticide exposed and control individuals (α21). Males developed around half a day faster than females (α31; Supp. Fig. 1a,b). Adult emergence body mass was similar between insecticide exposed and control individuals (α22; Supp. Fig. 2a,b). However, within-generational insecticide exposure had a positive effect on 10-day body mass (α23; Fig. 2a,b). Insecticide exposed females and males were on average 10 and 2 mg heavier than control females and males (Fig. 2a,b). Females were on average 18.4 mg heavier than males at emergence (i.e. 0-day) (α32; Supp. Fig. 2a,b) and on average 31.8 mg heavier at 10 days (α33; Fig. 2a,b).
Body mass for (a) female and (b) male Colorado potato beetles in the first generation measured at the age of 10 days. Body mass for (c) female and (d) male beetles in the second generation measured at the age of 7 days. Control: Control- offspring control: maternal control (within-generational treatment: transgenerational treatment).
Within-and transgenerational insecticide effects on survival in the second generation
After the short term exposure, the 24 h larval survival was lower in the insecticide exposure group than in the control group (δ21; Fig. 3) but was not strongly associated with descendants from the previous generations´ (i.e. maternal) insecticide exposure (δ31). 24 h larval survival in the second generation after 24 h insecticide exposure was on average 0.13 times lower when compared to the control group (δ21; Fig. 3). There could also be a small positive interaction effect between the within- and transgenerational insecticide exposure on the 24 h larval survival (δ51). In other words when exposed to insecticide, the offspring of insecticide treated mothers had better survival than the offspring of control mothers.
Within- and transgenerational insecticide stress effects on survival (%) between different life stages in the second generation Colorado potato beetles. Within-generational insecticide stress exposure decreases larval and pupal survival within the 24 h when compared to control group. Transgenerational insecticide treatment decreases larval mortality when compared to larvae descending from control mothers. Insecticide: Control within-generational treatment: transgenerational treatment means that within generational treatment was insecticide stress and transgenerational treatment was control.
Total larval survival was higher for larvae that descended from the insecticide exposed mothers than for those descending from the control mothers (δ32). The within-generational insecticide seems to slightly reduce survival of the offspring of control mothers (δ22) but not the survival of the offspring of the insecticide treated mothers (δ52). Total larval survival was on average 1.75 times higher for beetles descending from the insecticide-exposed mothers when compared to those produced by the control mothers (Fig. 3).
Survival in the pupal stage was not affected by the within- (δ23) or by the within- and transgenerational insecticide exposure interaction (δ53). However, transgenerational insecticide exposure (δ33) increased pupal survival on average 1.79 times (δ33) when compared to those in the control group. Adult survival between 0 and 14 days was not so clearly affected by the within- (δ24) or transgenerational insecticide exposure (δ34).
Within- and transgenerational insecticide effects on the development time and body mass in the second generation
Development time (i.e. days, from egg date until adult emergence; log-transformed in the model) in the second generation was not affected by the within- (γ21) or transgenerational insecticide exposure (γ51). Males developed faster than females (γ31; Supp. Fig. 3a,b). There was no interaction effect on development time between the within- and transgenerational insecticide exposure (γ61). Within- generational insecticide exposure had no clear effect on the emergence body mass (γ22) or on the body mass at age of 7 (γ23) or 14 days (γ24). Transgenerational insecticide exposure, however, had a positive effect on emergence body mass (γ52), and on body mass at age of 7 (γ53; Fig. 2c,d) and 14 days (γ54; Supp. Fig. 4a–d). At the age of 14 days, the transgenerational insecticide exposure had a positive effect with a large variance. No clear interaction effects were observed between the within- and transgenerational insecticide exposure on the emergence body mass (γ62) or on the body mass at age of 7 (γ63) or 14 days (γ64). There was an indication of a sex and transgenerational treatment interaction effect on the body mass at the age of 7 days (γ43), suggesting that the increase in body mass is larger in females. Females were heavier than males on the emergence day (γ32) at the age of 7 (γ33; Fig. 2c,d) and 14 days (γ34; Supp. Fig. 4a–d).
Within- and transgenerational effects on relative lipid content (%), water content (%), and dry mass (%) in the second generation
Relative lipid content did not differ between within- (γ25) or transgenerational insecticide exposure and control groups (γ45). No within- and transgenerational treatment interaction was found either (γ55). However, relative lipid content was higher for males than for females (γ35; Supp. Fig. 5a,b).
Water content did not differ between within- (γ26) or transgenerational insecticide exposure and control groups (γ46). No within- and transgenerational treatment interaction was found either (γ56). Water content did not differ between males and females (γ36).
Dry mass did not differ between within- (γ27) or transgenerational insecticide exposure and control groups (γ47). No within- and transgenerational treatment interaction was found either (γ57). Dry mass did not differ between males and females (γ37).
Discussion
Invasive pest species are often repeatedly controlled by pesticides. Whereas exposure to high pesticide doses are in general lethal and form a strong selection pressure, exposure to mild, sublethal doses may lead to within- and transgenerational stress effects on survival and fitness-related traits. These effects, in turn, may contribute to the persistence of populations under stressful environments3,32. More importantly, higher stress tolerance or organismal flexibility of invasive species could facilitate invasions and contribute to population dynamics3. Here we show that exposure to sublethal pyrethroid insecticide stress can induce both positive within- and transgenerational effects manifested as higher survival and higher adult body mass of the Colorado potato beetle, which may have implications for the invasion success of the species.
Our results show that within-generational exposure to sublethal insecticide stress as larvae resulted in higher adult survival in the first generation beetles (Fig. 2). The higher adult survival of stress-exposed beetles could derive from hormetic effects. These hormetic effects might derive either from direct stimulatory responses14,33,34 or as an initial disruption of homeostasis, which is followed by an over-compensation response35. Here, the latter response pattern is more likely since the positive effect on survival was detectable in the adult stage. This indicates that exposure to stress during early stages of development can have long-lasting hormetic effects and may even increase stress resistance by contributing to survival in the adult stage. Similarly, several other studies have suggested that high stress resistance can be associated with increased longevity or survival36,37. Overall, high adult survival may contribute to invasion success because, in the field conditions, adults have been shown to engage in long-distance seasonal migration, which is followed by reproduction38, and thus insecticide-stressed individuals might be more successful. Therefore sub-lethal insecticide exposure, being a stressor, could promote invasiveness if invasive populations originate from high-stress-environments. This has been previously shown in the fresh water copepod (Eurytemora affinis), where invasive populations originate from more stressful environments39 and thus insecticide exposure could stimulate similar stress effects.
We found that stress-exposed beetles had higher 10-day body mass than control individuals in the first generation. Higher body mass could be a result of an organism trying to cope with energetic losses that derive from the insecticide detoxification which are forced to upregulate the feeding or assimilation of food40,41. In general, higher body mass is related to higher fitness in insects31. For instance, a high female body mass is often correlated with high mating success, reproduction probability, fecundity, and offspring quality as well as with high overwintering survival42,43,44,45. We did not explicitly measure egg laying success and thus exposure to insecticide stress could have negative effect on reproduction. However, our data shows that exposure to insecticide stress leads to higher survival and female body mass, and thus could also lead to higher reproduction rate. For example, exposure to sublethal doses of pyrethroid insecticide increased the net reproductive rate in the maize weevil (Sitophilus zeamais)15. Furthermore, if individuals from the first generation engage in the long-distance seasonal migration which typically is followed by reproduction in the Colorado potato beetle38. The increase in their body mass due to stress can further increase the invasion success as larger individuals could be more fertile and successful at dispersing. For example, in butterflies, body size is related to dispersal ability46. Overall, as both survival and body mass are very relevant fitness-related traits the observed positive within-generational stress effects on adult survival and body mass may facilitate the invasion of the beetle into novel or stressful environments.
Females in the second generation were more sensitive to stress exposure than males. This was manifested as a higher adult body mass in the females descending from insecticide-stressed mothers compared to the females descending from control mothers, whereas smaller differences in body mass were observed among males (Fig. 2b,d). Sex-specific stress effects have been shown by other studies, and they often suggest that females are more sensitive to stress than males16,35,47,48. Sex-specific differences in sensitivity may be due to sexual size dimorphism49, sex-linked pyrethroid resistance mechanism50 or hormetic effects that are induced by partly different mechanisms in males and females48.
We expected insecticide stress to have positive transgenerational effects and indeed, we found that maternal insecticide stress exposure resulted in around 17% higher larval survival (Fig. 3) and in higher female adult body mass (Fig. 2c). Positive transgenerational (hormetic) effects can be mediated via epigenetic effects. Epigenetic modifications can lead to changes in the DNA methylation patterns, can suppress or increase gene expression levels and thus affect the resistance levels to insecticides51. For example, Kishimoto et al.52 has shown that parental hormetic responses are transmitted to their offspring via epigenetic memory that is maintained through histone modifications. A future study could perform a genome-wide methylation profiling of possible DNA methylation polymorphisms between insecticide exposed group and control group53. Adaptive maternal effects are important in evolutionary dynamics because they may facilitate immediate phenotypic plasticity and/or impact both the direction as well as the rate of genetic change in response to the selection, and therefore may generate rapid phenotypic change within a population32. We show also that the maternal effect on the survival of their offspring is present in the larval and pupal stage and possibly also in the adult stage. Also, the higher body mass is visible already at the emergence day, which reflects the body mass of the larval period. Since we did not measure the body mass during the larval period, we can only speculate that the higher survival during the larval period results from the higher larval body mass. However, higher body mass during the larval stage can be especially important when managing invasive pest species, as insecticide applications commonly target the larval stage to minimize the crop losses.
We observed a small positive interaction effect between the within- and transgenerational insecticide exposure on larval survival after 24 hours. Although the variance of the estimated interaction effect was high, this suggests that the insecticide exposed mothers produced offspring with higher stress tolerance as their offspring survived insecticide exposure better than the offspring from the control mothers. Previously Uller et al.54 have found only weak evidence for anticipatory parental effects, particularly when the maternal environment is poor, and suggested that it might be quite rare in natural systems. However, it is also possible that our result is due to the selection for higher resistance to pyrethroids, although we used only a sublethal dosage.
Contrary to our expectation, within- or transgenerational insecticide stress did not result in higher lipid content of adult beetles. Even though the Folch method is a commonly used protocol to estimate relative lipid content, it can overestimate the lipid content55. Thus, small stress-induced differences in the relative lipid content might become less visible. It is also possible that there are no differences in the relative lipid content but that there could be differences in qualitative lipid composition (e.g. due to differences in lipid classes) or fatty acid profiles. Since it is known that stressed individuals can have elevated metabolic rates, and in turn increase their energy demand56, this might lead to differences in lipid composition.
Conclusions
This study shows that even minute sublethal insecticide stress exposure can induce both within- and transgenerational positive effects. Thus, sublethal insecticide stress exposure can have long lasting non-desired adaptive effects, as this could lead to higher adult survival and higher body mass compared to non-exposed individuals. However, these exposed individuals will then produce offspring with higher larval and pupal survival and even higher adult body mass. Both higher larval survival and higher body mass may increase invasion potential and exacerbate management problems. It is therefore important to take into account potential performance-enhancing sub-lethal insecticide stress effects when developing pest management strategies.
Material and Methods
Study animal and insecticide exposure
The first generation Colorado potato beetles used in the study were the fourth generation descendants of beetles collected in potato fields in Vermont (USA; 44° 43′ N, 73° 20′ E) in 2010. Field collected beetles were mated in the laboratory and the beetles of the next generation were overwintered individually in plastic jars (100 ml, containing 60 ml of peat) in controlled climate cabinets (Type B3100; WeissTechnic, Reiskirchen-Lindenstruth, Germany) at 5 °C. Third generation adults were mated and reared at 23 °C under a long day regime of 18 h light (16 h light with 2 h of dim light to imitate sunrise and sunset, 6 h dark) to induce reproduction57. Beetles were fed ad libitum with fresh leaves and stems of potato (van Gogh variety). Oviposition was monitored, eggs collected and hatching checked daily.
When larvae reached the second instar (n = 245), they were randomly divided into control and insecticide treatments. Larvae were weighed (AM 100, Mettler, Columbus, OH, USA) before the treatment application. Larvae were moved onto a Petri dish (9 cm in diameter) containing a filter paper, and 1 ml of 1.59 mg/l deltamethrin solution (Trademark Decis, Aventis CropScience, Copenhagen, Denmark) was pipetted on the filter paper. The insecticide dose was chosen based on preliminary bioassays that showed around 10% mortality at the applied dose (Margus A, unpublished). In the control group, 1 ml of water was pipetted on the filter paper. After two hours a potato leaflet was supplied. Larvae were exposed to the respective treatments for 24 hours after which alive larvae were transferred onto new Petri dishes, reared individually and fed ad libitum with fresh potato leaves until pupation. Mortality was checked and recorded daily. Survival across the whole larval development is named total larval survival. Last instar larvae were placed individually in soil jars filled with peat for pupation. Adults were weighed (n = 140; ±0.1 mg; AM100, Mettler, Columbus, OH, USA) on the day of emergence and again when 10 days old. Mortality at different life stages (larva, pupa, adult) was recorded daily. Development time in days from egg hatching to adult emergence was counted.
To investigate transgenerational (i.e. maternal exposure to insecticide) stress effects on offspring performance and whether their tolerance to insecticide stress is influenced by transgenerational stress, each control male (n = 14) from the first generation was mated with 4 unrelated females, two of which were control females and the other two were insecticide-treated as larvae. So, in total we had 56 families. Each male was swapped among females every second day so that each male was mated with each of the four females at least three times. Rearing and insecticide treatment of larvae (second generation, n = 842) were conducted as described above and larvae were randomly divided into control and insecticide treatments. After emergence, second generation adults were sexed, weighed (at days 0, 7 and 14) and thereafter reared for 14 days at a constant temperature of 23 °C under a short day of 12 h light (10 h light with 2 h of dim light, 12 h dark). Mortality at different stages (larva, pupa, and adult) was recorded daily. Egg-to-adult development time was counted as above. After 14 days beetles were snap-frozen in liquid nitrogen and stored at −80 °C until lipid content analysis.
Relative lipid content measurement
Total lipid content of the second generation adult beetles (n = 391) was measured from 14-day old adult beetles to investigate whether the within- and/or transgenerational insecticide stress exposure affects the size of energy reserves. At that age, beetles are ready to enter diapause58,59. The majority of lipids are located in the fat body, which is an important tissue involved in many metabolic functions and is the major energy storage in insects60. Total lipid content was estimated by using a modified Folch method57,61. Beetles were first weighed (fresh weight), then dried for 72 h at 55 °C and reweighed (dry weight). Lipids were extracted by placing beetles into small glass vials (20 ml) filled with 10 ml of chloroform: methanol solution (2:1) for 72 h at 20 °C and afterward dried for another 72 h at 55 °C. Thereafter beetles were again weighed (lean weight). Relative lipid content (%) is calculated by subtracting lean weight from dry weight and dividing by fresh weight. Water content (%) is calculated by subtracting fresh weight from lean weight and dividing by fresh weight. Dry mass (%) is calculated by dividing dry weight by fresh weight.
Statistical analysis
A Bayesian approach was chosen for the modelling task because it allows the posterior distribution of the first generation to be used as a prior for the second generation. A single Bayesian model was constructed for the whole experiment. This enables the flow of information between the generations for missing data imputation. The design of the experiment together with the associated causal assumptions and missing data mechanism are depicted in Fig. 4. Bayesian inference begins with the assumption that all of the model parameters are random variables and thus the task is to estimate their posterior distribution given some prior information about the parameters and additional evidence in the form of collected data62.
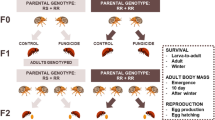
Experimental design to test the within- and transgenerational effects of sublethal insecticide exposure on survival and body mass in the Colorado potato beetle. Here the progress of the study is visualized by the ordering of the nodes. The vertical axis describes the observational time and different generations and the horizontal axis describes the causal order of events. Here the dashed arrows correspond to transgenerational (TG) causal relationships. For example, the treatment of the first generation parents has an effect on body mass of the offspring in the second generation. Open circles denote unobserved variables. Filled circles denote variables that have been measured from the sample. Similarly, diamonds denote variables that have been determined by the researcher, such as the assigned treatments or mating of each generation in this case. Our graphical presentation is a simplified version of66.
The effect of insecticide stress exposure in both generations and all development stages was investigated with a logistic regression model, where the survival (alive, dead) in different life stages was the response variable and within-generational treatment and transgenerational (i.e. maternal insecticide exposure) treatment were regarded as explanatory variables. Survival was analysed at each developmental stage separately because the life-cycle is partitioned into distinct stages (e.g. larva, pupa and adult). Survival probability in later life stages was modelled as conditional on having survived through the previous life stages. Thus, individuals that had died in previous stages were excluded from models for later stages in both generations. Development time (i.e. from egg hatching and hatching date to adult emergence day, in days), body mass (mg), relative lipid content (%), water content (%), and dry mass (%) were analysed with a linear model, where transgenerational treatment, within-generational treatment, and sex were considered as explanatory factors. Development time was log-transformed to better approximate it with a normal distribution. Relative lipid content (%), water content (%), and dry mass (%) were analysed with a beta model, with a logistic link for the expectation. First, a full model was fitted with parameters corresponding to interactions. If the posterior 95% credible intervals contained 0, the interactions were excluded from the final models with the exception of treatment interactions, which were kept in the model as they are one of the primary interests. Credible interaval is a interval of the shortest interval of the posterior density that contains 95% of the probability mass. In the next section, the reported intervals explicitly refer to the corresponding posterior distribution of the model parameter associated with the explanatory variable in question. The parametric forms of the final model equations are given in Supplementary Material 1.
The analysis was carried out using R63 and JAGS64 with the addition of the R-package rjags65. A posterior sample of size 10000 was drawn from single Markov chain with a burn-in period of two million iterations. The chain ran for an additional 5 million iterations and every 500th draw was accepted into the final sample. 10 data samples were generated using the posterior distribution to compare against the real data.
The validity of the model was checked by comparing posterior predictive distributions against the real data. The Markov chain by studying the trace plots was diagnosed and autocorrelations in addition to the 1 and 2-dimensional marginal distributions of the posterior predictive comparisons and Markov Chain Monte Carlo diagnostics are depicted in Supplementary Table 2. The estimated parameters for the statistical model are presented in Supplementary Table 3.
References
Mack, R. N. et al. Biotic invasions: causes, epidemiology, global consequences, and control. Ecol. Appl. 10, 689–710 (2000).
Carroll, S. P. et al. Applying evolutionary biology to address global challenges. Science 346, 1245993 (2014).
Hufbauer, R. A. et al. Anthropogenically induced adaptation to invade (AIAI): contemporary adaptation to human-altered habitats within the native range can promote invasions. Evol. Appl. 5, 89–101 (2012).
Lee, C. E. & Gelembiuk, G. W. Evolutionary origins of invasive populations. Evol. Appl. 1, 427–448 (2008).
Ghalambor, C. K., McKay, J. K., Carroll, S. P. & Reznick, D. N. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol. 21, 394–407 (2007).
Randall, D., Burggren, W., French, K. & Eckert, R. Eckert animal physiology: mechanisms and adaptations. Macmillan (2002).
Badyaev, A. V. Stress-induced variation in evolution: from behavioural plasticity to genetic assimilation. Proc. R Soc. Lond. B Biol. Sci. 272, 877–886 (2005).
Hoffmann, A. A. & Hercus, M. J. Environmental Stress as an Evolutionary Force. Bioscience 50, 217–226 (2000).
Desneux, N. et al. Diaeretiella rapae limits Myzus persicae populations after applications of deltamethrin in oilseed rape. J. Econ. Entomol. 98, 9–17 (2005).
Guedes, R. N. C., Walse, S. S. & Throne, J. E. Sublethal exposure, insecticide resistance, and community stress. Curr. Opin, Insect Sci. 21, 47–53 (2017).
Costa, V. & Moradas-Ferreira, P. Oxidative stress and signal transduction in Saccharomyces cerevisiae: insights into ageing, apoptosis and diseases. Mol. Aspects Med. 22, 217–246 (2001).
Piiroinen, S. et al. Pre-invasion history and demography shape the genetic variation in the insecticide resistance-related acetylcholinesterase 2 gene in the invasive Colorado potato beetle. BMC Evol. Biol. 13, 1–13 (2013).
Piiroinen, S., Boman, S., Lyytinen, A., Mappes, J. & Lindström, L. Sublethal effects of deltamethrin exposure of parental generations on physiological traits and overwintering in Leptinotarsa decemlineata. J. Appl. Entomol. 138, 149–158 (2014).
Guedes, R. N. C. & Cutler, G. C. Insecticide-induced hormesis and arthropod pest management. Pest. Manag. Sci. 70, 690–697 (2014).
Guedes, N., Tolledo, J., Corrêa, A. S. & Guedes, R. Insecticide‐induced hormesis in an insecticide‐resistant strain of the maize weevil, Sitophilus zeamais. J. Appl. Entomol. 134, 142–148 (2010).
Piiroinen, S., Lyytinen, A. & Lindström, L. Stress for invasion success? Temperature stress of preceding generations modifies the response to insecticide stress in an invasive pest insect. Evol. Appl. 6, 313–323 (2013).
Nestler, E. J. Transgenerational Epigenetic Contributions to Stress Responses: Fact or Fiction? PLOS Biol. 14, e1002426 (2016).
Mousseau, T. A. & Fox, C. W. eds. Maternal effects as adaptations. Oxford University Press (1998).
Bonduriansky, R. & Day, T. Nongenetic Inheritance and Its Evolutionary Implications. Annu. Rev. Ecol. Evol. Syst. 40, 103–125 (2009).
Shikano, I., Oak, M. C., Halpert-Scanderbeg, O. & Cory, J. S. Trade-offs between transgenerational transfer of nutritional stress tolerance and immune priming. Funct. Ecol. 29, 1156–1164 (2015).
Nystrand, M. & Dowling, D. K. Dose-dependent effects of an immune challenge at both ultimate and proximate levels in Drosophila melanogaster. J. Evol. Biol. 27, 876–888 (2014).
Grindstaff, J. L., Brodie, E. D. & Ketterson, E. D. Immune function across generations: integrating mechanism and evolutionary process in maternal antibody transmission. Proc. R. Soc. Lond. B. Biol. Sci. 270, 2309–2319 (2003).
Day, T. & Bonduriansky, R. A Unified Approach to the Evolutionary Consequences of Genetic and Nongenetic Inheritance. Am. Nat. 178, E36 (2011).
EPPO. Distribution Maps of Quarantine Pests for Europe, Leptinotarsa decemlineata. ADAS, Nottingham, UK (2006).
Alyokhin, A. Colorado potato beetle management on potatoes: current challenges and future prospects. Fruit. Veg. Cereal Sci. Biotech. 3, 10–19 (2009).
Alyokhin, A., Vincent, C. & Giordanengo, P. Eds Insect pests of potato: global perspectives on biology and management. Academic Press (2012).
Bebber, D. P., Ramotowski, M. A. & Gurr, S. J. Crop pests and pathogens move polewards in a warming world. Nat. Clim. Change 3, 985–988 (2013).
Alyokhin, A., Baker, M., Mota-Sanchez, D., Dively, G. & Grafius, E. Colorado potato beetle resistance to insecticides. American Journal of Potato Research 85, 395–413 (2008).
Alyokhin, A., Vincent, C. & Giordanengo, P. Eds Insect Pests of Potato. Global Perspectives on Biology and Management. Academic Press, Oxford, UK (2013).
Alyokhin, A. et al. The Red Queen in a potato field: integrated pest management versus chemical dependency in Colorado potato beetle control. Pest Manag. Sci. 71, 343–356 (2015).
Lyytinen, A., Lindström, L. & Mappes, J. Genetic variation in growth and development time under two selection regimes in Leptinotarsa decemlineata. Entomol. Exp. Appl. 127, 157–167 (2008).
Räsänen, K. & Kruuk, L. Maternal effects and evolution at ecological time‐scales. Funct. Ecol. 21, 408–421 (2007).
Guedes, R., Smagghe, G., Stark, J. D. & Desneux, N. Pesticide-induced stress in arthropod pests for optimized integrated pest management programs. Annu. Rev. Entomol. 61, 43–62 (2016).
Calabrese, E. J. & Blain, R. The occurrence of hormetic dose responses in the toxicological literature, the hormesis database: an overview. Toxicol. Appl. Pharmacol. 202, 289–301 (2005).
Tejeda, M. T. et al. Effects of size, sex and teneral resources on the resistance to hydric stress in the tephritid fruit fly Anastrepha ludens. J. Insect Physiol. 70, 73–80 (2014).
Kirkwood, T. B. & Austad, S. N. Why do we age? Nature 408, 233–238 (2000).
Vermeulen, C. J. & Loeschcke, V. Longevity and the stress response in Drosophila. Exp. Gerontol. 42, 153–159 (2007).
Voss, R. H. & Ferro, D. N. Phenology of flight and walking by Colorado potato beetle (Coleoptera: Chrysomelidae) adults in western Massachusetts. Environ. Entomol. 19, 117–122 (1990).
Lee, C. E. Rapid and repeated invasions of fresh water by the copepod Eurytemora affinis. Evolution 53, 1423–1434 (1999).
Jager, T., Barsi, A. & Ducrot, V. Hormesis on life-history traits: is there such thing as a free lunch? Ecotoxicology 22, 263–270 (2013).
Campero, M., Slos, S., Ollevier, F. & Stoks, R. Sublethal pesticide concentrations and predation jointly shape life history: behavioral and physiological mechanisms. Ecol. Appl. 17, 2111–2122 (2007).
Honěk, A. Intraspecific variation in body size and fecundity in insects: a general relationship. Oikos, 483–492 (1993).
Stearns, S. C. In The evolution of life histories (Oxford University press, 1992).
Kingsolver, J. G. & Huey, R. B. Size, temperature, and fitness: three rules. Evol. Ecol. Res. 10, 251–268 (2008).
Kingsolver, J. G., Pfennig, D. W. & Phillips, P. Individual-level selection as a cause of Cope’s rule of phyletic size increase. Evolution 58, 1608–1612 (2004).
Stevens, V. M. et al. How is dispersal integrated in life histories: a quantitative analysis using butterflies. Ecol. lett. 15, 74–86 (2012).
Sørensen, J. G., Kristensen, T. N., Kristensen, K. V. & Loeschcke, V. Sex specific effects of heat induced hormesis in Hsf-deficient Drosophila melanogaster. Exp. Gerontol. 42, 1123–1129 (2007).
Hercus, M. J., Loeschcke, V. & Rattan, S. I. Lifespan extension of Drosophila melanogaster through hormesis by repeated mild heat stress. Biogerontology 4, 149–156 (2003).
Blanckenhorn, W. U. Behavioral causes and consequences of sexual size dimorphism. Ethology 111, 977–1016 (2005).
Argentine, J. A., Lee, S. H., Sos, M. A., Barry, S. R. & Clark, J. M. Permethrin resistance in a near isogenic strain of Colorado potato beetle. Pestic. Biochem. Physiol. 53, 97–115 (1995).
Brevik, K., Lindström, L., McKay, S. D. & Chen, Y. H. Transgenerational effects of insecticides—implications for rapid pest evolution in agroecosystems. Curr. Opin Insect Sci. 26, 34–40 (2018).
Kishimoto, S., Uno, M., Okabe, E., Nono, M. & Nishida, E. Environmental stresses induce transgenerationally inheritable survival advantages via germline-to-soma communication in Caenorhabditis elegans. Nat. Commun. 8, 14031 (2017).
Ffrench-Constant, R. H. The molecular genetics of insecticide resistance. Genetics 194, 807–815 (2013).
Uller, T., Nakagawa, S. & English, S. Weak evidence for anticipatory parental effects in plants and animals. J. Evol. Biol. 26, 2161–2170 (2013).
Newman, H., Gordon, E. A., Heggen, D. W. & Keller, M. D. Rapid extraction of triglycerides from human adipose tissue with petroleum ether. Clin. Chem. 18, 290–292 (1972).
Dingha, B. N., Moar, W. J. & Appel, A. G. Effects of Bacillus thuringiensis Cry1C toxin on the metabolic rate of Cry1C resistant and susceptible Spodoptera exigua (Lepidoptera: Noctuidae). Physiol. Entomol. 29, 409–418 (2004).
Lehmann, P., Lyytinen, A., Sinisalo, T. & Lindström, L. Population dependent effects of photoperiod on diapause related physiological traits in an invasive beetle (Leptinotarsa decemlineata). J. Insect Physiol. 58, 1146–1158 (2012).
Piiroinen, S. Range expansion to novel environments: evolutionary physiology and genetics in Leptinotarsa decemlineata. Jyväskylä studies in biological and environmental science 209 (2010).
Lehmann, P., Lyytinen, A., Piiroinen, S. & Lindström, L. Latitudinal differences in diapause related photoperiodic responses of European Colorado potato beetles (Leptinotarsa decemlineata). Evol. Ecol. 29, 269–282 (2015).
Arrese, E. L. & Soulages, J. L. Insect fat body: energy, metabolism, and regulation. Annu. Rev. Entomol. 55, 207–225 (2010).
Folch, J., Lees, M. & Sloane Stanley, G. H. A simple method for the isolation and purification of total lipides from animal tissures. J Biol Chem, 497–509 (1957).
Gelman, A., Carlin, J. B., Stern, H. S. & Rubin, D. B. In Bayesian data analysis. Chapman & Hall/CRC Boca Raton, FL, USA (2014).
Team, R. C. No title. R: a language and environment for statistical computing. Vienna, Austria (2015).
Plummer, M. rjags: Bayesian graphical models using MCMC. R package version 3 (2013).
Plummer, M., Stukalov, A., Denwood, M. & Plummer, M. M. Package ‘rjags’. Vienna, Austria (2016).
Karvanen, J. Study design in causal models. Scandinavian Journal of Statistics 42, 361–377 (2015).
Acknowledgements
Authors would like to thank Kati Kivisaari and Joel Rahkonen for their assistance with rearing the beetles. This study was funded by the Academy of Finland (250248) and Centre of Excellence in Biological Interactions Research (210000284414). Since Colorado potato beetle in a quarantine species in Finland the experiment was performed under the licence (8787/0614/2011).
Author information
Authors and Affiliations
Contributions
L.L., S.P., P.L. and A.M. conceived the experiment. S.P., A.M., P.L. and L.L. conducted the experiment. S.T., J.K. and A.M. analysed the results. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Margus, A., Piiroinen, S., Lehmann, P. et al. Sublethal Pyrethroid Insecticide Exposure Carries Positive Fitness Effects Over Generations in a Pest Insect. Sci Rep 9, 11320 (2019). https://doi.org/10.1038/s41598-019-47473-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-47473-1
This article is cited by
-
Behavioral responses and life history traits of Taiwanese and Indonesian populations of Aedes aegypti surviving deltamethrin–clothianidin treatment
Parasites & Vectors (2024)
-
Distribution of invasive versus native whitefly species and their pyrethroid knock-down resistance allele in a context of interspecific hybridization
Scientific Reports (2022)
-
Gregarines modulate insect responses to sublethal insecticide residues
Oecologia (2022)
-
Sublethal effects of fenvalerate on biological performance and life table parameters of the grass-lawn armyworm, Spodoptera cilium (Lepidoptera: Noctuidae)
International Journal of Tropical Insect Science (2022)
-
Possible enzymatic mechanism underlying chemical tolerance and characteristics of tolerant population in Scapholeberis kingi
Environmental Science and Pollution Research (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.