Abstract
Caper (Capparis spinosa L.) is a xerophytic shrub cultivated for its flower buds and fruits, used as food and for their medicinal properties. Breeding programs and even proper taxonomic classification of the genus Capparis has been hampered so far by the lack of reliable genetic information and molecular markers. Here, we present the first genomic resource for C. spinosa, generated by transcriptomic approach and de novo assembly. The sequencing effort produced nearly 80 million clean reads assembled into 124,723 unitranscripts. Careful annotation and comparison with public databases revealed homologs to genes with a key role in important metabolic pathways linked to abiotic stress tolerance and bio-compounds production, such purine, thiamine and phenylpropanoid biosynthesis, α-linolenic acid and lipid metabolism. Additionally, a panel of genes involved in stomatal development/distribution and encoding for Stress Associated Proteins (SAPs) was also identified. We also used the transcriptomic data to uncover novel molecular markers for caper. Out of 50 SSRs tested, 14 proved polymorphic and represent the first set of SSR markers for the genus Capparis. This transcriptome will be an important contribution to future studies and breeding programs for this orphan crop, aiding to the development of improved varieties to sustain agriculture in arid conditions.
Similar content being viewed by others
Introduction
Global warming is changing Earth’s climate with possible negative effects on the growth and reproductive success of plants. Reduced plant productivity due to environmental changes1,2, such as high temperatures, heat waves and drought stress, might implicate incapacity to ensure global food security3,4. The Mediterranean region will be particularly affected by climate change, with increased aridity expected to occur (Intergovernmental Panel on Climate Change; http://www.ipcc.ch)5. Mediterranean agriculture will need to adapt to the new environmental conditions by growing drought tolerant crops. Selection and introduction of stress-tolerant cultivars of existing crops is a slow and costly process, requiring intensive research and field trials6. Another option is promoting alternative drought resistant crop species. Caper (Capparis spinosa L.) is a xerophilous crop showing a remarkable adaptability to harsh environments, and with promising potentialities for agrosystems under the threat of global warming7.
In the Mediterranean area, populations are generally grouped within one species, C. spinosa8,9,10,11,12,13, although the taxonomic classification of this species is controversial due to a large pattern of morphological and ecological variations14,15,16,17 and to the lack of specific molecular markers. Despite the high polymorphism, two main subspecies, which differ ecologically and morphologically11 are recognized in Europe: C. spinosa subsp. spinosa, showing derived characters and widespread from the Mediterranean to Central Asia, and C. spinosa subsp. rupestris (Sm.) Nyman, characterized by phenotypic features close to the tropical stock of the group, distributed in the Mediterranean Region and the Sahara13,17.
The fruits and flower buds of caper are utilized as food ingredient, generally in brine, and appreciated for their flavor and texture. Only locally, cultivation of capers is extensive and acquires economic relevance for farmers. In particular, the main current areas of production are localised in Morocco, Turkey, Spain and Italy, namely in the minor islands of Salina and Pantelleria. Being rich in bio-active compounds, capers have many important medicinal properties18,19,20,21,22,23,24,25,26,27,28. Moreover, like other Mediterranean species29,30,31, caper is also a source of natural compounds with allelopathic potential32,33. Therefore, the extracts from C. spinosa could also be used to develop natural products employable in an eco-friendly agriculture.
For its interest as gourmet food, its medicinal and allelopathic properties and the ability to thrive in arid conditions7, capers have great agricultural potential in areas with increasing drought conditions, such as the Mediterranean basin. The process of domestication of caper plants has been limited and cultivated varieties are still very similar to wild accessions34, leaving ample margins for enhancement of many traits, such as increased productivity, firmer buds, disease resistance and thornless habit. Breeding programs and an efficient exploitation of this orphan crop are hampered by confused taxonomy of the genus Capparis and the lack of genomic information. To date, only few sequences of the chloroplast35,36,37 and mitochondrial genome38 have been reported, with limited value for phylogenetic analyses and breeding programs. Currently, there are no nuclear Simple Sequence Repeat (SSR) markers described for the genus Capparis. Microsatellites or SSR are codominant and highly informative markers already broadly used to genotype a wide range of plant species39,40,41,42,43. Compared to other molecular markers, SSRs are abundant and uniformly distributed throughout plant genomes and show several advantages such as simplicity, high polymorphism, reproducibility, co-dominant inheritance and cross-species transferability44. For species with no genome annotated, as is the case of orphan crops, an effective strategy to uncover SSRs is to rely on transcriptomic sequences. In contrast to genomic SSRs, Expressed Sequence Tag (EST)-SSRs are located in the coding and untranslated regions and are highly transferable to related taxa45. Thus, EST-SSR markers can directly influence phenotype and can be considered efficient functional markers46.
The advent of Next Generation Sequencing (NGS) technologies combined with bioinformatics tools can generate extensive data on non-model species in a very cost-effective way47,48,49. Among NGS strategies, RNA Sequencing (RNA-Seq) approach50 is a high throughput technology that has great advantages in examining the fine structure of a transcriptome51,52 and provides an effective way to obtain large amounts of sequence data without prior genome information53,54,55. RNA-Seq has been widely used in many organisms to obtain mass sequence data for transcriptional analysis, gene discovery and molecular marker development52,54,55,56, showing a great potential as a tool for molecular breeding57.
Here, for the first time, we report the sequencing, de novo assembly, and annotation of the leaf transcriptome of C. spinosa subsp. rupestris, a primitive type closer to the tropical stock of the group13. In order to identify putative genes controlling the bioactive and high-value components production the assembly was functionally annotated using public databases. In addition, polymorphic EST-SSRs were identified in the leaf transcriptome, thereby obtaining the first set of co-dominant markers for the species.
This transcript dataset provides the most widespread resource currently available for gene discovery and markers development in C. spinosa. This resource will be instrumental for future breeding programs and phylogenetic studies of capers. In addition, the information now available will contribute to the sustainable adaptation of agricultural production in small islands and marginal areas of the Mediterranean region58 and in other regions affected by aridity and/or climate change.
Results
Sequencing, de novo assembly and functional annotation of C. spinosa leaf transcriptome
We performed RNA-Seq to assemble transcripts, identify genes and develop co-dominant markers for the first time in C. spinosa. Leaf transcriptome Illumina shotgun sequencing yielded nearly 80 million cleaned reads, de novo assembled into 208,677 transcripts with N50 length of 2,431 bp (mean length 1,493 bp) by Trinity (Table 1). To remove the redundant transcripts the clean reads were clustered by CD-HIT-EST generating 124,723 unigenes with N50 length of 2,380 bp (mean 1,417 bp) (Table 1). The quality of assembled unitranscripts was evaluated by comparing them to the set of Eudicotyledons genes using BUSCO quality assessment tool. Out of the 2,121 BUSCO groups searched, 87.8% (1,861 BUSCOs) were “complete” (i.e., 916 single-copy and 945 duplicated), 6.7% (142 BUSCOs) were “fragmented” and the remaining 5.5% (118 BUSCOs) were “missing”. In addition, one typical peak of GC content for plants, (around 50%) was found using QUAST59, underlining the absence of bacteria. In total we identify 0.40% of possible contaminations (e.g. bacteria and virus), representing only 1.78% of the ‘Other’ category of Fig. 1. Clustered transcripts were searched against the NCBI-nr databases revealing 89,670 (72%) transcripts whose translation was significantly similar to known proteins. In species distribution analysis, 47,749 (53%) transcripts showed homology (top blast hits) with Tarenaya hassleriana, followed by Eutrema salsugineum, Arabidopsis thaliana, Arabidopsis lyrata, and Camelina sativa with 4,704 (5%), 3,322 (4%), 3,183 (4%) and 2,519 (3%), respectively (Fig. 1). The assembled sequences were also queried against the Swiss UniprotKB database using BLASTx and BLASTp searches, respectively. Nearly 54% (66,902) unitranscripts had a blastx hit and 41% (51,048) of clustered transcripts with ORF ≥ 100 bp in length displayed significant homology for annotated protein sequences. When nucleotide and protein sequences were aligned against UniRef90, their homology increased to 85,294 (68%) and 62,339 (50%), respectively. Moreover, 46,099 (37%) unique Pfam protein motifs could be assigned and 3,341 (3%) protein sequences were predicted to have signal peptides (Table 2). The complete list of transcript annotations is shown in Supplementary Dataset S1.
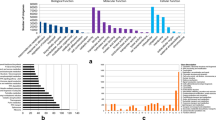
We extracted 27,035 non redundant GO terms from 51% transcripts and summarized them into 97 GOslim plant categories using CateGOrizer (Supplementary Dataset S2). The annotated clustered transcripts were grouped into the three main categories: most of the assignments (61%) belonged to the biological process (BP) category, while the remaining was shared between cellular component (CC) (14%) and molecular function (MF) classes (25%). Within BP, “cellular process”, “metabolic process”, “cellular component organization and biogenesis” were the main represented groups in a total of 44 level-2 categories. Within CC, 26 level-2 categories were identified. The top three groups were “cell”, “intracellular” and “cytoplasm”. Similarly, in the MF 24 level-2 GO terms were isolated and “catalytic”, “transferase” and “hydrolase activities” were the top three (Supplementary Fig. S1). In the KOG classification, 40,765 unitranscripts were classified into 24 KOG groups (Fig. 2). Among these, the cluster for “general function prediction only” (15%) represented the largest group, followed by “transcription” (10%), “replication, recombination and repair” (9%), “signal transduction mechanism” (8%). The “cell motility” was the smallest group, while no unigenes were classified as “extracellular structures” (Fig. 2).
EuKaryotic Orthologous Groups (KOG) in Capparis spinosa leaf transcriptome. The unigenes with significant homologies in the KOG database were grouped into 24 categories. The number of unigenes belonging to each category was reported in the y-axis, while the subgroups in the KOG classification were represented in the x-axis.
Biological pathway analyses in C. spinosa
To investigate functional biological pathways in C. spinosa, we exploited Transdecoder that assigns KO to unitranscripts (e-value ≤ 1*10−5). The unique KOs identified were mapped against the KEGG database to verify the correct sequencing of well represented pathways in C. spinosa. Among the 127 KEGG pathways identified (Supplementary Table S1), purine metabolism (669 sequences; 76 KOs, covering 37% of the pathway), was the most represented pathway as number of homologous leaf transcripts. Pyrimidine metabolism (504; 56, covering 57%), oxidative phosphorylation (414; 60, covering 28%), phenylpropanoid biosynthesis (316; 18, covering 49%), fatty acid metabolism (biosynthesis and degradation) (297; 22, covering 23%) and α-linolenic acid metabolism (163; 12) were also highly represented.
Because of their high representation and the known role of adenine, jasmonate, and flavonols in the abiotic stress tolerance60,61,62,63,64, we analyzed purine, thiamine and α-linolenic acid metabolism and phenylpropanoid biosynthesis in detail. In the C. spinosa leaf transcriptome, a high representation of purine metabolism was highlighted (Fig. 3A). Particularly, we found enzymes involved in the production of thiamine phosphates: thiamine-phosphate synthase (EC 2.5.1.3) catalyzing the reaction for thiamine phosphate synthesis, thiamine phosphatase (EC 3.6.1.15) converting thiamine di-phosphate in thiamine phosphate, thiamine di-phosphokinase (EC 2.7.6.2) and thiamine phosphate phosphatase that lead the conversion of thiamine to thiamine di-phosphate and thiamine phosphate in thiamine, respectively (Fig. 3B).
In the same way, α-linolenic acid metabolism (12 genes) is highly represented. In particular we identified jasmonate O-methyltransferase (EC 2.1.1.141) and acetyl-CoA C-acyltransferase (EC 2.3.1.16), involved in jasmonate biosynthesis (Fig. 4).
A large proportion of phenylpropanoid biosynthesis pathway was also reconstructed (18 genes), identifying some important enzymes, such as phenylalanine ammonia lyase (PAL) (EC 4.3.1.24), the first component in the phenylpropanoid pathway; 4-coumarate-CoA ligase (EC 6.2.1.12) andcinnamate-4-hydroxylase (C4H) (EC 1.14.13.1), that convert trans-cinnamic acid (CA) to p-coumaric acid (COA); 4-coumarate CoA ligase involved in p-coumaroyl-CoAsynthesis, an intermediate for hydroxycinnamic acids, flavonols and flavonol derivatives (Fig. 5A).
Considering the role of lipids as signaling in plant responses to abiotic stress, the unitranscripts were investigated for coding sequences of lipid metabolism. In this highly represented pathway, we found key enzymes of glycerolipid metabolism involved in the phosphatidic acid (PA) synthesis, such as 1-acyl-sn-glycerol-3-phosphate acyltransferase (EC 2.3.1.51) and diacylglycerol kinase (ATP) (EC 2.7.1.107), converting lysophosphatidic acid and L-1, 2-diacylglycerol, respectively, in PA; and in PA transformation (phosphatidate phosphatase; EC 3.1.3.4) (Fig. 5B). We also identified CDP-diacylglycerol-inositol 3-phosphatidyltransferase (EC 2.7.8.11) that catalyzes phosphatidylinositol (PI) synthesis (Fig. 5B).
To complete the analysis of molecules playing important role during environmental stress response, the occurrence of components of sphingolipid metabolism was also explored and 10 different enzymes could be retrieved. A large proportion of sphingolipid metabolism pathways could be reconstructed, including, among others, alkaline ceramidase (EC 3.5.1.23) involved in the synthesis of sphingosine, and serine palmitoyltransferase (EC 2.3.1.50) a key enzyme of sphingolipid metabolism required for the conversion of L-serine and palmitoyl-CoA into 3-Dehydrosphinganine (Supplementary Fig. S2A).
Additionally, we identified sequences mapped in different pathways involved in phytochemical biosynthesis, such as terpenoid metabolism (36), carotenoids (12 genes), glucosinolates (9), stilbenoids (4), and anthocyanins (2). Genes involved in oxidative phosphorylation (60) and photosynthesis (28), such as cytochrome c oxidase (EC 1.9.3.1) and photosystem I/II, respectively, were also detected and well represented (Supplementary Fig. S2B,C).
Seven genes, YODA (mitogen-activated protein kinase kinase kinase), ER (ERECTA), EPLF9/STOMAGEN (epidermal patterning factor-like protein 9), TMM (too many mouths) ERL1 (erecta-like1), GTL1 (GT2-like1) and FAMA (FMA/bHLH097), known to be involved in the modulation of stomatal development in response to drought, were also found (Supplementary Dataset S1). In addition, we identified transcripts with homology to Stress Associated Proteins (SAPs) genes that are potential candidates to improve abiotic stress tolerance in plants using biotechnological approaches65. We found 32 C. spinosa unitranscripts homologous to ten genes encoding for A. thaliana and O. sativa SAPs (Table 3; Supplementary Dataset S1). The average length of the transcripts is 1,607 bp, with values ranging between 249 bp (TRINITY_DN33098_c0_g1_i1; SAP10) and 4,447 bp (TRINITY_DN23049_c0_g1_i3; SAP1).
Simple sequence repeats isolation and validation
A total of 5,009 perfect simple sequence repeats (SSR) with repeat numbers ranging from 4 to 31 (from di- to hexa- nucleotide motifs) were identified using the MISA tool in the assembled uniscripts (Supplementary Table S2). Trinucleotide repeats were the most abundant (2,756, 55.0%), followed by hexanucleotide (1,115, 22.3%), tetranucleotide (566, 11.3%), dinucleotide (362, 7.2%) and pentanucleotide (210, 4.2%) (Table 4). The most common repeat number was 7, observed in 1,146 assemblies (22.9%), followed by 8 (854, 17.1%), 4 (828, 16.5%), and >10 (794, 15.9%) tandem repeats (Table 4). The most abundant motifs detected were TCT (320, 6.4%) and TTC (303, 6.1%), followed by GAA (272, 5.4%). More details about different repeat motif for the isolated EST-SSRs are listed in Supplementary Table S2.
Hundred-fifty primer pairs were designed using Primer3 (http://primer3.sourceforge.net/) and a first panel of 50 EST-SSRs was tested (Supplementary Table S3). The predicted SSRs were validated and evaluated for the polymorphism rate by using a set of 75 C. spinosa genotypes, collected across the distribution area of the species (Supplementary Table S4). Forty-one out of 50 tested EST-SSRs showed amplified fragments. Among them, 14 fragments fell outside the expected size range, and were not considered further. The other 27 EST-SSRs produced PCR fragments with the expected size, 14 of which were polymorphic with a number of alleles per locus ranging from 2 to 11 (mean 6), and values of He from 0.420 to 0.843 (mean 0.630) (Table 5), PIC from 0.332 to 0.826 (mean 0.583), Fis and Fst values from −0.058 to 0.830 (mean 0.062) and from 0.010 to 0.695 (mean 0.495), respectively (Table 5). The selected EST-SSRs showed strong discrimination power among the different taxa here considered. UPGMA phylogenetic tree and DAPC analysis based on SSR clearly discriminated the thorny group of C. spinosa subsp. spinosa from Italy, Creta and west Asia from the thornless group of C. spinosa subsp. rupestris from different regions and islands of Italy (hereinafter subsp. spinosa and rupestris, respectively) (Fig. 6). Among the group of subsp. spinosa, Sicilian populations differentiated from eastern Mediterranean and western Asia populations. The subsp. rupestris was more homogeneous, though samples from the small islands of Salina and, to a minor extent, Pantelleria and Ustica also skewed from the rest of the populations (Fig. 6).
Genetic relationships among genotypes belonging to Capparis spinosa collection sampled across the distribution area of the species. (A) Dendrogram generated by 14 polymorphic EST-SSR developed in the present study, using the UPGMA method and Bruvo’s distance. (B) DAPC analysis clustering of the eight populations studied using the first two principal components (Y-axis and X-axis, respectively). CC: C. spinosa subsp. spinosa; CR: C. spinosa subsp. rupestris. The samples used for the EST-SSR validation were gathered in 8 main groups: CC Sicily, CC world, CR Favignana, CR Italy, CR Pantelleria, CR Salina, CR Sicily and CR Ustica.
Discussion
Although C. spinosa is a rich source of bioactive compounds with important nutritional and medicinal values18,25,66,67,68, until now available genomics resources were limited. The lack of adequate molecular markers and genes identification are a limit for an efficient employment of this orphan crop, displaying agro-based potentialities and a high demand for exploitation7. In addition, the natural resistance to drought and harsh environmental conditions makes C. spinosa a potentially important resource in areas threatened by global warming and desertification. Therefore, the transcriptomic data generated in this study provide useful resources to support a full taxonomic revision of the genus Capparis (Capparaceae), and assist selection in the modern breeding programs in order to promote this crop, especially in the Mediterranean countries.
Illumina next-generation RNA-Seq was successfully used to develop a high-quality leaf transcriptome of C. spinosa subsp. rupestris, generating a number of transcripts, similar to other transcriptome studies on plants54,69,70,71. Although our work is focused only on one vegetative tissue (leaf), the first transcriptome profile of C. spinosa grown under natural conditions has been developed. About 72% of the unitranscripts were successfully assigned to genes in the NR database, and likewise a large number of unigenes (68%) and predicted proteins (50%) showed match by querying against UniRef90 database. In addition, species distribution analysis showed a high homology with Brassicaceae, a sister family to Cleomaceae, underlining the close evolutionary relationship of C. spinosa with this family72.
Biological pathways identification plays a crucial role to shed light into functional analysis and transcriptomic data. KEGG is an integrated database resource that integrates genomic, chemical and systemic functional information, a useful tool for the interpretation of transcriptomic data and widespread interrogation of an organism’s genome content73. Here, a number of pathways of C. spinosa were highly represented. Among these, we described in detail those involved in abiotic stress tolerance and bio-compounds production. We studied purine and thiamine metabolism, α-linolenic acid metabolism, phenylpropanoid biosynthesis, lipid metabolism, genes involved in stomatal development and distribution, and lastly the presence of SAPs.
Purine metabolism, particularly thiamine (vitamin B1) and related phosphate esters are involved as cofactors in response to abiotic and biotic stress74. Thiamine metabolism can be altered under environmental stress in Zea mays75, while in A. thaliana the abiotic defenses activation and stress tolerance were triggered by altered adenine metabolism61. Cellular adenine levels drive plant growth and biomass increase, playing a key role as signal in the response modulation to abiotic stress and acclimation61. Moreover, a recent study76 suggested a possible connection between purine catabolism and stress phyto-hormone homeostasis/signaling. Takagi et al.76 showed how allantoin, a metabolic intermediate of purine catabolism accumulates in plants under abiotic stress, activating the jasmonic acid responses via abscisic acid (ABA) and enhancing seedling tolerance to abiotic stress.
Several putative targets involved in plant abiotic stress response, belonging to α-linolenic acid metabolism, phenylpropanoid biosynthesis and lipid metabolism, were also found in this study. Comparing transcriptomic profiles of susceptible and tolerance rice varieties, α-linolenic acid metabolic pathway appears involved in the high drought tolerance77 and, recently, a link between α-linolenic acid and jasmonic acid biosynthesis with cold acclimation was uncovered in Camellia japonica through RNA-Seq analysis63. Phenylpropanoid pathway is responsible for the synthesis of a wide range of secondary metabolites in plant. As expected, the analysis revealed that the majority of the metabolic genes of this pathway are expressed in C. spinosa leaves. In particular we identified PAL and C4H, genes encoding enzymes that catalyze the first and second step of phenylpropanoid way, respectively, and responsible for biosynthesis of lignin. C4Hs have remained highly conserved across the plant kingdom and recent studies78,79 highlighted their key role in response to stresses (drought and cold) and as scavengers of Reactive Oxygen Species (ROS). In addition, genes linked to stress responses, including ethylene biosynthesis and signaling, showed altered expression levels in PAL knocked-down plants under non-challenging conditions80. PAL is also a biosynthetic source of salicylic acid (SA) in plants81, a master regulator in biotic and abiotic stress response in plants, including drought stress82,83.
Key enzymes of glycerolipid metabolism driving the PA synthesis were also detected. PA is a diacyl glycerophospholipid used as precursor for complex lipids biosynthesis and transiently generated in response to biotic and abiotic stress in plants. PA plays an essential role in ABA-induced production of ROS, osmotic changes and temperature stress response84,85,86,87. In the same way, since lipid-protein interactions are crucial for deciphering the signaling cascades, we studied and isolated phosphoinositides and sphingolipids, compounds belonging to the highly coordinated signaling network developed in plants, linked to acclimation or survival under abiotic stress88.
C. spinosa is drought tolerant and shows an efficient hydraulic conductivity due to the well-developed xylem vessels in stems7,89. Therefore, based on these evidences, we further assessed the presence of leaf transcripts homologous to genes involved in stomatal development and distribution that can be considered as key genes in the response to drought stress and water use efficiency (WUE). We found seven transcripts related to stomata. In particular, YODA is a MAPKK kinase gene and GTL1a transcriptional repressor of SDD1, a negative regulator of stomata development90 and density91. ERECTA has been the first identified major effector of WUE and a recent study92 demonstrated that the EDT1/HDG11-ERECTA-E2Fa genetic pathway reduced the stomatal density by increasing cell size, providing a new strategy to improve WUE in crops. The presence of YODA, ERECTA and GTL1 in the assembled unitranscripts might be associated to an adaptive response of C. spinosa to drought.
We also focused our attention to the presence of homologs encoding for SAPs. These A20/AN1 zinc-finger proteins have been shown to confer tolerance to multiple abiotic stresses in plants. In A. thaliana, AtSAP9 regulates abiotic/biotic stress responses probably via the ubiquitination/proteasome pathway93 and AtSAP13 is upregulated in response to Cd, ABA, and salt stresses94. In rice, SAP homologs are activated by multiple abiotic stresses (such as cold, salt, and dehydration)95. In Prunus, water retention and cell growth are regulated by a stress-associated protein (PpSAP1) through the TARGET OF RAPAMYCIN (TOR) pathway96. In poplar, the downregulation of PagSAP1 increases salt stress tolerance97. The finding of SAP homologs highlights the possible mechanisms involved in the adaptability of C. spinosa to a wide range of environmental conditions.
The production of secondary metabolites with medicinal properties could be reflected by the presence in our transcriptome of several genes involved in phytochemicals biosynthesis: prolycopene isomerase (EC 5.2.1.13) belonging to carotenoids and involved in lycopene biosynthesis; farnesyl diphosphate synthase (FPS) (EC 2.5.1.10), that catalyzes the synthesis of farnesyl diphosphate (FPP) in terpenoid metabolism; enzymes involved in chlorogenic acids (CGA) production, an important scavenging and antioxidant compound98; the anthocyanidin 3-O-glucoside 5-O-glucosyltransferase (EC 2.4.1.298) that converts pelargonidin 3-glucoside in pelargonin, compound with antioxidant activity; enzymes involved in glucobrassicin and glucoiberverin biosynthesis (glucosinolates) known as anti-cancer agents99; the MYB transcription factor Rosea1 (Ros1) that, together with Delila, enhances anthocyanin accumulation and abiotic stress tolerance in tobacco100.
Finally, we developed the first panel of co-dominant markers (EST-SSR) in caper. So far, genetic analysis of Capparis germplasm has largely relied on AFLP, RAPD, and ISSR markers34,101,102. The main reasons for using dominant markers were the lack of a genome sequence and/or transcriptome information for this species. Here, we identified 5,009 microsatellites from the assembled transcriptome in agreement with the frequencies reported in other studies52,103,104,105,106,107. When mono-nucleotide repeats were excluded, tri-nucleotide repeats were the most abundant class of SSRs, with TCT the most frequent motif in our dataset. This finding is consistent with results reported in other species, such as rice, wheat, barley107,108, cotton109 and asparagus110. To determine the level of polymorphism and discrimination power among this first set (50) of EST-SSRs, the markers were tested and validated using 75 selected samples. Nine primers pairs failed to produce amplicons, possibly due to primers spanning splicing sites, large introns, chimeric primer(s), or poor-quality sequences introns106,111. Fourteen primer pairs produced amplicons that deviated from expected size, which might have been produced by the presence of introns106,111, large insertions or repeat number variations, or a lack of specificity. Conversely, 27 EST-SSRs were validated, 14 of which (52%) were polymorphic. The polymorphism level was higher than the values reported in previous studies34,101,102, with genetic diversity values (He) > 0.5 for 11 out of 14 polymorphic EST-SSRs. These results suggest that the isolated sequences are suitable for the development of specific primers and confirmed the quality of the transcriptome assembled. Moreover, cluster and DAPC analysis highlighted the ability of selected EST-SSR to discriminate among taxa and origin of C. spinosa samples here analysed. In particular, for the subsp. spinosa the Sicilian plants grouped together and were separated by the rest of Mediterranean and Asian samples. In this regard, it is noteworthy that the plants from the Mediterranean island of Cyprus grouped together with Asian plants from Azerbaijan and China, rather than with Sicily. It is therefore tempting to speculate that a more extensive analysis of molecular polymorphism with samples collected worldwide with the SSR developed here might reveal unexpected scenarios of diffusion and evolution of caper. For the group of subsp. rupestris, the germplasm available was limited to Southern Italy, mainland Sicily and its minor islands (Supplementary Table S4). Consequently, due to the proximity of the sampling sites, the germplasm was more homogeneous respect to what observed for the subsp. spinosa (Fig. 6). Nevertheless, samples collected in the minor islands of Salina clearly formed a separate group. Caper plants from the islands of Pantelleria and Ustica also skewed from mainland Sicily and Italy, though less so. The minor island of Favignana, on the other hand, did not outgroup. Interestingly, caper cultivation is a major agricultural activity in the islands of Salina, Pantelleria and Ustica, but not in Favignana and in the rest of Sicily. It is therefore possible that attempts of selections by local farmers caused the observed deviation compared to wild type populations. Moreover, farmers in Salina traditionally propagate caper plants by clonal cuttings, as opposed to farmers in Pantelleria and Ustica who usually employ seeds112. This difference might justify the pronounced separation in the samples from Salina, since individual diversity in the selected plants is faithfully preserved and the cultivated germplasm does not mix any longer with the wild ancestors.
The novel molecular markers developed can be used in future studies assessing genetic diversity, phylogenetic analysis, marker assisted selection (MAS), mapping and association analysis in Capparis species worldwide. In addition, the newly designed EST-SSR primers for C. spinosa can also be tested in other species of the Capparaceae family currently lacking of their own genomic resources.
Methods
Plant material
We selected for RNA-Seq analysis three wild populations of C. spinosa subsp. rupestris to maximize the variety of genetic backgrounds and environmental conditions, in order to enrich the trancriptomic information (Supplementary Table S5). The three populations were used as biological replicates. For each population, mature leaves from three different specimens were collected at the same vegetative stage and packed in situ, then immediately frozen in liquid nitrogen and stored in the laboratory at −80 °C until use. Furthermore, a panel of 75 wild C. spinosa samples (Supplementary Table S4) were collected across the natural distribution area of the species for DNA extraction in order to validate the EST-SSR markers isolated.
RNA isolation and sequencing
RNA from each collected sample was extracted using a NucleoSpin RNA Plant (Macherey-Nagel GmbH & Co. KG, 52355 Düren, Germany) and treated with RNase-free DNase. RNA quality (RNA Integrity Number (RIN) > 8.0) was evaluated using an Agilent Bioanalyzer RNA nanochip (Agilent, Wilmington, DE). RNA-Seq libraries were independently prepared for three pools representing the three different populations. Each pool was composed by equal leaf amount of three specimens. Sequencing library was prepared using the Illumina TruSeq RNA Sample Preparation Kit v2 (Illumina, San Diego, CA, USA) according to manufacturer’s specifications; quality and insert size distribution were evaluated using Agilent Bioanalyzer DNA 1000 chip. Sequencing libraries were quantified using qPCR and sequenced in the same lane on an Illumina HiSeq. 1000 generating 2 × 100 nt paired-end reads.
De novo assembly and functional annotation of leaf transcriptome
Raw reads were adapter clipped and quality trimmed following recommendations from previous studies113,114. Adapter sequence contamination and low quality nucleotides (PHRED < 5) were removed using Trimmomatic version 0.33115. De novo transcriptome assembly of cleaned reads was carried out in Trinity (v.2.5.1)116 with default parameters. To generate transcriptome containing only unique transcripts for downstream analysis, CD-HIT-EST117 was used (identity cut-off ≥ 90%) by removing all repetitive, identical and near-identical transcripts. The quality and completeness of the de novo assembly were evaluated using BUSCO3 software v3118. This quality assessment tool provides high-resolution quantifications for genomes, gene sets, and transcriptomes and checks whether each of the BUSCO group is complete, duplicated, fragmented, or missing in the genome or transcriptome assembly. The unitranscripts were compared to the set of Eudicotyledons genes, which contains 2121 BUSCO groups from a total of 40 species in order to obtain a quantitative measure of the transcriptome completeness, based on evolutionarily informed expectations of gene content from near-universal single-copy orthologs. In addition, to evaluate potential contamination about bacteria and endophytes, QUAST software119 was used.
Functional annotation of unitranscripts was performed using the Trinotate pipeline (http://trinotate.sourceforge.net/) to identify open reading frames and assign best hits to UniprotKB (1*10−5), UniRef90 (1*10−5), PFAM-A (1*10−5), GO and KOG categories120. Transdecoder (v.3.1.0) (https://transdecoder.github.io/) was also used to de novo predict putative coding regions and protein sequences. Blastp search was carried out by using predicted Open Reading Frames (ORFs) as the query and the Swiss-Prot non-redundant database as the target. The HMMER package121 and Pfam databases122 were utilized to predict protein domains, while SignalP 4.1123 was used to predict the presence of signal peptides within the predicted ORFs. CateGOrizer124 was used to map GO terms to a parent plant to get a wide overview of the transcripts functional classification. Finally, KEGG Automatic Annotation Server (KAAS, http://www.genome.jp/kaas-bin/kaas_main?mode=est) was employed to map KEGG pathways of assigned caper orthologs125,126,127,128. All figures showing identified and highlighted pathways were developed through KEGG Mapper, Search Pathway using unique Kos (https://www.genome.jp/kegg/tool/map_pathway1.html). KO assignments were performed based on the bi-directional best hit of BLAST129.
Identification and validation of polymorphic EST-SSR markers
All clustered transcripts generated from de novo assembly were examined to identify new co-dominant molecular markers. SSRs based on short tandem repeats were identified through analyses of ESTs (EST–SSR) and carried out into gene-anchored marker loci130. SSR loci were detected using MicroSAtellite tool (MISA; http://pgrc.ipk-gatersleben.de/misa/misa.html)131. Di-, tri-, tetra-, penta-, and hexa-nucleotides were searched with a minimum of 20, 7, 5, 5, and 4 repeat units, respectively. A set of primer pairs (150) was designed using Primer3 software (http://primer3.sourceforge.net/)132 by imposing an amplicon size range of 100–400 bp, minimum and maximum GC contents 40 and 60%, and minimum and maximum melting temperature (Tm) values ranging from 58 to 60 °C, respectively (Supplementary Table S3). A first panel of 50 EST-SSR was tested and validated, verifying the primers specificity and amplicons size (Supplementary Table S3) on the above-mentioned panel of samples (Supplementary Table S4). Genomic DNA was extracted from leaves (200 mg) using the CTAB protocol133. DNA concentration and quality were checked with a Nanodrop ND1000 (Thermo Scientific). PCR amplifications were carried out in 20 µl reaction mixtures starting from 50 ng of DNA as previously described110. The fragments were analyzed on an ABI PRISM 3500 Genetic Analyzer (Applied Biosystems) and the alleles were sized by GENEMAPPER 4.0 (Applied Biosystems). Genetic diversity (He), mean allele number, fixation index (Fst), inbreeding coefficient (Fis) and Polymorphism Information Content (PIC) for each EST-SSR used were calculated by using PowerMarker v. 3.25134 and R/poppr135. Genetic relationships among studied genotypes were also investigated by cluster analysis and Discriminant Analysis of Principal Components (DAPC). The UPGMA (Unweighted Pair Group Method with Arithmetic Mean) phylogenetic tree was designed by using R/poppr135 with Bruvo’s distance136. The bootstrap analysis was performed based on 1,000 resamplings. DAPC, implemented in the R/adegenet137, was performed to infer population subdivision of the analysed collection, regardless of the geographic origin. Since only one sample (LAM01) belonging to the Lampedusa island population was available, this population has been excluded from DAPC analysis. In the output, samples were gathered in 8 main groups (Fig. 6B; Table S6). The number of principal components (PCs) retained was evaluated using the cross-validation procedure. We also used the K-means algorithm, ‘find.clusters’, to independently verify the assignment of individuals to clusters.
Data Availability
RNA-Seq generated for this project has been deposited in the NCBI BioProject (PRJNA311285) and NCBI SRA (SAMN04481463). The Transcriptome Shotgun Assembly project (submission number SUB5676995) has been deposited at DDBJ/EMBL/GenBank under the accession GHNF00000000. The version described in this paper is the first version, GHNF01000000. The assembled transcriptome can be accessed via NCBI (https://www.ncbi.nlm.nih.gov/genbank/tsa/). The microsatellite data for the population samples of C. spinosa are available in this article.
References
Yamori, W., Hikosaka, K. & Way, D. A. Temperature response of photosynthesis in C3, C4, and CAM plants: temperature acclimation and temperature adaptation. Photosynth. Res. 119, 101–117 (2014).
Xu, Z., Jiang, Y. & Zhou, G. Response and adaptation of photosynthesis, respiration, and antioxidant systems to elevated CO2 with environmental stress in plants. Front. Plant Sci. 6, 701, https://doi.org/10.3389/fpls.2015.00701 (2015).
Bita, C. E. & Gerats, T. Plant tolerance to high temperature in a changing environment: scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 4, 273, https://doi.org/10.3389/fpls.2013.00273 (2013).
Ray, D. K., Gerber, J. S., MacDonald, G. K. & West, P. C. Climate variation explains a third of global crop yield variability. Nat. Commun. 6, 5989, https://doi.org/10.1038/ncomms6989 (2015).
Pachauri, R. K. et al. “Climate change 2014: synthesis report,” in Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, eds Pachauri, R. and Meyer, L. (Geneva: IPCC), 151 (2014).
Thiry, A. A., Dulanto, P. N. C., Reynolds, M. P. & Davies, W. J. How can we improve crop genotypes to increase stress resilience and productivity in a future climate? A new crop screening method based on productivity and resistance to abiotic stress. J. Exp. Bot. 67, 5593–5603 (2016).
Chedraoui, S. et al. Capparis spinosa L. in A Systematic Review: A xerophilous species of multi values and promising potentialities for agrosystems under the threat of global warming. Front. Plant Sci. 8, 1845–1862 (2017).
Jacobs, M. The genus Capparis (Capparaceae) from the Indus to the Pacific. Blumea 12, 385–541 (1965).
Maire, R. Flore de l’Afrique du Nord. Paris: 12 Editions Paul Lechevalier (1965).
St. John, H. S. Revision of Capparis spinosa and its African, Asiatic and Pacific relatives. Micronesica 2, 25–44 (1965).
Higton, R. N. & Akeroyd, J. R. Variation in Capparis spinosa L. in Europe. Bot. J. Linn. Soc. 106, 104–112 (1991).
Heywood, V. H. “Capparis L.” in Flora Europaea, vol 1, ed. Cambridge University Press (Cambridge) 312 (1993).
Fici, S. Intraspecific variation and evolutionary trends in Capparis spinosa L. (Capparaceae). Plant Syst. Evol. 228, 123–141 (2001).
Zohary, M. The species of Capparis in the Mediterranean and the Near Eastern countries. B. Res. Coun. Israel 2, 49–64 (1960).
Inocencio, C., Rivera, D., Obón, C., Alcaraz, F. & Barrena, J. A systematic revision of Capparis section Capparis (Capparaceae). Ann. Mo. Bot. Gard. 93, 122–149 (2006).
Danin, A. Capparis in the East Mediterranean countries. Fl. Medit. 20, 179–185 (2010).
Fici, S. A taxonomic revision of the Capparis spinosa group (Capparaceae) from the Mediterranean to Central. Asia. Phytotaxa 174, 1–24 (2014).
Rivera, D., Inocencio, C., Obón, M. C. & Alcaraz, F. Review of food and medicinal uses of Capparis L. subgenus Capparis (Capparidaceae). Econom. Bot. 57, 515–534 (2003).
Eddouks, M., Lemhardi, A. & Michel, J. B. Hypolipidemic activity of aqueous extract of Capparis spinosa L. in normal and diabetic rats. J. Ethnopharmacol. 98, 345–350 (2005).
Lemhadri, A., Eddouks, M., Sulpice, T. & Burcelin, R. Anti-hyperglycaemic and anti-obesity effects of Capparis spinosa and Chamaemelum nobile aqueous extracts in HFD Mice. Am. J. Pharm. Toxicol. 2, 106–110 (2007).
Tesoriere, L., Butera, D., Gentile, C. & Livrea, M. A. Bioactive components of Caper (Capparis spinosa L.) from Sicily and antioxidant effects in a red meat simulated gastric digestion. J. Agr. Food. Chem. 55, 8465–8471 (2007).
Sze-Kwan, L. & Tzi-Bun, N. A protein with antiproliferative, antifungal and HIV-1 reverse transcriptase inhibitory activities from caper (Capparis spinosa) seeds. Phytomedicine 16, 444–450 (2009).
Haifeng, Z. et al. Anti inflammatory effects of caper (Capparis spinosa L.) fruit aqueous extract and the isolation of main phytochemicals. J. Agric. Food Chem. 58, 12717–12721 (2010).
Abraham, S. V. P. I., Palani, A., Ramaswamy, B. R., Shunmugiah, K. P. & Arumugam, V. R. Antiquorum sensing and antibiofilm potential of Capparis spinosa. Arch. Med. Res. 42, 658–668 (2011).
Tlili, N. et al. The caper (Capparis L.): ethnopharmacology, phytochemical and pharmacological properties. Fitoter. 82, 93–101 (2011).
Huseini, F. H. et al. Capparis spinosa L. (Caper) fruit extract in treatment of type 2 diabetic patients: a randomized double-blind placebo-controlled clinical trial. Complement. Ther. Med. 21, 447–452 (2013).
Hong-Juan, L., Tao, Y., Xue-Mei, C. & Chang-Hong, W. Comparative evaluation of anti-inflammatory and analgesic activities of various medicinal parts of Capparis spinosa: a consideration of ecological environment and resource conservation. Indian J. Med. Res. Pharm. Sci. 4, 53–59 (2014).
Yu, L. et al. Antioxidant and antitumor activities of Capparis spinosa L. and the related mechanisms. Oncol. Rep. 37, 357–367 (2017).
Mamoci, E. et al. Chemical composition and in vitro activity of plant extracts from Ferula communis and Dittrichia viscosa against postharvest fungi. Molecules 16, 2609–2625 (2011).
Araniti, F., Lupini, A., Mercati, F., Statti, G. A. & Abenavoli, M. R. Calamintha nepeta L. (Savi) as source of phytotoxic compounds: bio-guided fractionation in identifying biological active molecules. Acta Physiol. Plant. 35, 1979–1988 (2013).
Araniti, F. et al. Phytotoxic activity of Cachrys pungens Jan, a Mediterranean species: separation, identification and quantification of potential allelochemicals. Acta Physiol. Plant. 36, 1071–1083 (2014).
Caboni, P. et al. Nematicidal Activity of 2-Thiophenecarboxaldehyde and Methylisothiocyanate from Caper (Capparis spinosa) against Meloidogyne incognita. J. Agric. Food Chem. 60, 7345–7351 (2012).
Ladhari, A., Omezzine, F., Dellagreca, M., Zarrelli, A. & Haouala, R. Phytotoxic activity of Capparis spinosa L. and its discovered active compounds. Allelopathy J. 32, 175–190 (2013).
Gristina, A. S. et al. Hybridization in Capparis spinosa L.: Molecular and morphological evidence from a Mediterranean island complex. Flora 209, 733–741 (2014).
Hall, J. C. Systematics of Capparaceae and Cleomaceae: an evaluation of the generic delimitations of Capparis and Cleome using plastid DNA sequence data. Botany 86, 682–696 (2008).
Siragusa, M. & Carimi, F. Development of specific primers for cpSSR analysis in caper, olive and grapevine using consensus chloroplast primer pairs. Scie. Hortic. 120, 14–21 (2009).
Wang, Q., Zhang, M. L. & Yin, L. K. Phylogeographic structure of a tethyan relict Capparis spinosa (Capparaceae) traces Pleistocene geologic and climatic changes in the western Himalayas, Tianshan mountains, and adjacent desert regions. Biomed. Res. Int. 2016, 5792708, https://doi.org/10.1155/2016/5792708 (2016).
Grewe, F. et al. Comparative analysis of 11 Brassicales mitochondrial genomes and the mitochondrial transcriptome of Brassica oleracea. Mitochondrion 19, 135–143 (2014).
Jiao, Y. et al. Development of simple sequence repeat (SSR) markers from a genome survey of Chinese bayberry (Myrica rubra). BMC Genomics 13, 201, https://doi.org/10.1186/1471-2164-13-201 (2012).
Carimi, F. et al. Intra-varietal genetic diversity of the grapevine (Vitis vinifera L.) cultivar ‘Nero d’Avola’ as revealed by microsatellite markers. Genet. Res. Crop. Evol. 58, 967–975 (2011).
Mercati, F. et al. Genetic variation of an Italian long shelf-life tomato (Solanum lycopersicon L.) collection by using SSR and morphological fruit traits. Genet. Res. Crop. Evol. 62, 721–732 (2015).
Fu, Y. et al. Patterns of SSR variation in bread wheat (Triticum aestivum L.) seeds under ex situ gene-bank storage and accelerated ageing. Genet. Res. Crop. Evol. 64, 277–290 (2017).
Gristina, A. S. et al. Urgent need for preservation of grapevine (Vitis vinifera L. subsp. vinifera) germplasm from small circum-Sicilian islands as revealed by SSR markers and traditional use investigations. Genet. Res. Crop. Evol. 64, 1395–1415 (2017).
Morgante, M. & Olivieri, A. M. PCR-amplified microsatellites as markers in plant genetics. Plant J. 3, 175–182 (1993).
Zhao, Y., Williams, R., Prakash, C. S. & He, G. Identification and characterization of gene-based SSR markers in date palm (Phoenix dactylifera L.). BMC Plant Biol. 12, 237, https://doi.org/10.1186/1471-2229-12-237 (2013).
Li, Y. C., Korol, A. B., Fahima, T. & Nevo, E. Microsatellites within genes: structure, function, and evolution. Mol. Biol. Evol. 21, 991–1007 (2004).
Parchman, T. L., Geist, K. S., Grahnen, J. A., Benkman, C. W. & Buerkle, C. A. Transcriptome sequencing in an ecologically important tree species: assembly, annotation, and marker discovery. BMC Genomics 11, 180, https://doi.org/10.1186/1471-2164-11-180 (2010).
Ekblom, R. & Galindo, J. Applications of next generation sequencing in molecular ecology of non-model organisms. Heredity 107, 1–15 (2011).
Harkess, A. et al. The asparagus genome sheds light on the origin and evolution of a young Y chromosome. Nat. Commun. 8, 1279, https://doi.org/10.1038/s41467-017-01064-8 (2017).
Wang, Z., Gerstein, M. & Snyder, M. RNA-Seq: a revolutionary tool for transcriptomics. Nat. Rev. Genet. 10, 57–63 (2009).
Feng, C. et al. Transcriptomic analysis of Chinese bayberry (Myrica rubra) fruit development and ripening using RNA-Seq. BMC Genomics 13, 19, https://doi.org/10.1186/1471-2164-13-19 (2012).
Wei, L. et al. Transcriptome analysis of Houttuynia cordata Thunb. by Illumina Paired-End RNA Sequencing and SSR marker discovery. PLoS One 9, e84105, https://doi.org/10.1371/journal.pone.0084105 (2014).
Xia, Z. et al. RNA-Seq analysis and de novo transcriptome assembly of Hevea brasiliensis. Plant Mol. Biol. 77, 299–308 (2011).
Harkess, A. et al. J. Sex-biased gene expression in dioecious garden asparagus (Asparagus officinalis). New Phytol. 3, 883–892 (2015).
Evangelistella, C. et al. De novo assembly, functional annotation, and analysis of the giant reed (Arundo donax L.) leaf transcriptome provide tools for the development of a biofuel feedstock. BMC Biotechnol. Biofuels 10, 138, https://doi.org/10.1186/s13068-017-0828-7 (2017).
Brautigam, A., Mullick, T., Schliesky, S. & Weber, A. P. M. Critical assessment of assembly strategies for non-model species mRNA-Seq data and application of next-generation sequencing to the comparison of C3 and C4 species. J. Exp. Bot. 62, 3093–3102 (2011).
Martin, L. B., Fei, Z., Giovannoni, J. J. & Rose, J. K. Catalyzing plant science research with RNA-seq. Front. Plant Sci. 4, 66, https://doi.org/10.3389/fpls.2013.00066 (2013).
Carra, A. et al. In vitro plant regeneration of caper (Capparis spinosa L.) from floral explants and genetic stability of regenerants. Plant Cell Tiss. Org. Cult. 109, 373–381 (2012).
Singh, R., Ming, R. & Yu, Q. Comparative analysis of GC content variations in plant genomes. Tropical Plant Biol. 9, 136–140 (2016).
Tunc-Ozdemir, M. et al. Thiamin confers enhanced tolerance to oxidative stress in Arabidopsis. Plant Physiol. 151, 421–432 (2009).
Sukrong, S. et al. Improved growth and stress tolerance in the Arabidopsis oxt1 mutant triggered by altered adenine metabolism. Mol. Plant 5, 1310–1332 (2012).
Wasternack, C. Action of jasmonates in plant stress responses and development - Applied aspects. Biotechnol. Adv. 32, 31–39 (2014).
Li, Q. et al. RNA-seq based transcriptomic analysis uncovers α-linolenic acid and jasmonic acid biosynthesis pathways respond to cold acclimation in Camellia japonica. Sci. Rep. 6, 36463, https://doi.org/10.1038/srep36463 (2016).
Martinez, V. et al. Accumulation of flavonols over hydroxycinnamic acids favors oxidative damage protection protection under abiotic stress. Front. Plant Sci. 7, 838, https://doi.org/10.3389/fpls.2016.00838 (2016).
Giri, J. et al. SAPs as novel regulators of abiotic stress response in plants. BioEssays 35, 639–648 (2013).
Sozzi, G. O. & Vicente, A. Capers and caperberries. In: Handbook of Herbs and Spices. Ed. Peter, K. V. (Cambridge, Woodhead Publishing Limited, CRC Press) 3, 230–256 (2006).
Gull, T., Anwar, F., Sultana, B., Alcayde, C. A. M. & Nouman, W. Capparis species: a potential source of bioactives and high-value components: a review. Ind. Crops Prod. 67, 81–96 (2015).
Nabavi, S. F. et al. Pharmacological effects of Capparis spinosa L. Phytother. Res. 30, 1733–1744 (2016).
Yazawa, T., Kawahigashi, H., Matsumoto, T. & Mizuno, H. Simultaneous transcriptome analysis of Sorghum and Bipolaris sorghicola by using RNA-seq in combination with de novo transcriptome assembly. PLoS One 8, e62460, https://doi.org/10.1371/journal.pone.0062460 (2013).
Han, S. et al. Differential gene expression in leaf tissues between mutant and wild-type genotypes response to late leaf spot in peanut (Arachis hypogaea L.). PLoS One 12, e0183428, https://doi.org/10.1371/journal.pone.0183428 (2017).
Ponniah, S. K., Thimmapuram, J., Bhide, K., Kalavacharla, V. & Manoharan, M. Comparative analysis of the root transcriptomes of cultivated sweetpotato (Ipomoea batatas [L.] Lam) and its wild ancestor (Ipomoea trifida [Kunth] G. Don). BMC Plant Biol. 17, 9, https://doi.org/10.1186/s12870-016-0950-x (2017).
Iltis, H. H., Hall, J. C., Cochrane, T. S. & Sytsma, K. J. Studies in the Cleomaceae I. On the separate recognition of Capparaceae, Cleomaceae, and Brassicaceae. Ann. Mo. Bot. Gard. 98, 28–36 (2011).
Kanehisa, M. & Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 28, 27–30, https://doi.org/10.1093/nar/28.1.27 (2000).
Goyer, A. Thiamine in plants: aspects of its metabolism and functions. Phytochemistry 71, 1615–1624 (2010).
Rapala-Kozik, M., Kowalska, E. & Ostrowska, K. Modulation of thiamine metabolism in Zea mays seedlings under conditions of abiotic stress. J. Exp. Bot. 59, 4133–4143 (2008).
Takagi, H. et al. Allantoin, a stress-related purine metabolite, can activate jasmonate signaling in a MYC2-regulated and abscisic acid-dependent manner. J. Exp. Bot. 67, 2519–2532 (2016).
Lenka, S. K., Katiyar, A., Chinnusamy, V. & Bansal, K. C. Comparative analysis of drought-responsive transcriptome in Indica rice genotypes with contrasting drought tolerance. Plant Biotechnol. J. 9, 315–327 (2011).
Wang, A. et al. A sweet potato cinnamate 4-hydroxylase gene, IbC4H, increases phenolics content and enhances drought tolerance in tobacco. Acta Physiol. Plant. 39, 276, https://doi.org/10.1007/s11738-017-2551-1 (2017).
Cheng, S. et al. Characterization and expression patterns of a cinnamate-4-hydroxylase gene involved in lignin biosynthesis and in response to various stresses and hormonal treatments in Ginkgo biloba. Acta Physiol. Plant. 40, 7, https://doi.org/10.1007/s11738-017-2585-4 (2018).
Cass, C. L. et al. Effects of PHENYLALANINE AMMONIA LYASE (PAL) knockdown on cell wall composition, biomass digestibility, and biotic and abiotic stress responses in. Brachypodium. J. Exp. Bot. 66, 4317–4335 (2015).
Chen, Z., Zheng, Z., Huang, J., Lai, Z. & Fan, B. Biosynthesis of salicylic acid in plants. Plant Signal. Behav. 4, 493–496 (2009).
Kumar, D. Salicylic acid signaling in disease resistance. Plant Sci. 228, 127–134 (2014).
Khan, M. I., Fatma, M., Per, T. S., Anjum, N. A. & Khan, N. A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 6, 462, https://doi.org/10.3389/fpls.2015.00462 (2015).
Hong, Y., Zheng, S. & Wang, X. Dual functions of phospholipase Dα1 in plant response to drought. Mol. Plant 1, 262–269 (2008).
Arisz, S. A., Testerink, C., Munnik, T. & Plant, P. A. signaling via diacylglycerol kinase. Biochim. Biophys. Acta 1791, 869–875 (2009).
Zhang, Y. et al. Phospholipase Dα1 and phosphatidic acid regulate NADPH oxidase activity and production of reactive oxygen species in ABA-mediated stomatal closure in Arabidopsis. Plant Cell 21, 2357–2377 (2009).
Yu, L. et al. Phosphatidic acid mediates salt stress response by regulation of MPK6 in Arabidopsis thaliana. New Phytol. 188, 762–773 (2010).
Hou, Q., Ufer, G. & Bartels, D. Lipid signalling in plant responses to abiotic stress. Plant Cell Environ. 39, 1029–1048 (2016).
Levizou, E., Drilias, P. & Kyparissis, A. Exceptional photosynthetic performance of Capparis spinosa L. under adverse conditions of Mediterranean summer. Photosynthetica 42, 229–235 (2004).
Meng, L. S. & Yao, S. Q. Transcription co‐activator Arabidopsis ANGUSTIFOLIA3 (AN3) regulates water‐use efficiency and drought tolerance by modulating stomatal density and improving root architecture by the transrepression of YODA (YDA). Plant Biotechnol. J. 13, 893–902 (2015).
Yoo, C. Y., Hasegawa, P. M. & Mickelbart, M. V. Regulation of stomatal density by the GTL1 transcription factor for improving water use efficiency. Plant Signal. Behav. 6, 1069–1071 (2011).
Xiang, C. et al. A genetic pathway composed of EDT1/HDG11, ERECTA, and E2Fa loci regulates water use efficiency by modulating stomatal density. BioRxiv 2017, 232801, https://doi.org/10.1101/232801 (2017).
Kang, M. et al. Arabidopsis stress associated protein 9 mediates biotic and abiotic stress responsive ABA signaling via the proteasome pathway. Plant Cell Environ. 40, 702–716 (2017).
Dixit, A. et al. A stress‐associated protein, AtSAP13, from Arabidopsis thaliana provides tolerance to multiple abiotic stresses. Plant Cell Environ. 41, 1171–1185 (2018).
Vij, S. & Tyagi, A. K. Genome-wide analysis of the stress associated protein (SAP) gene family containing A20/AN1 zinc-finger (s) in rice and their phylogenetic relationship with Arabidopsis. Mol. Gen. Genom. 276, 565–575 (2006).
Lloret, A. et al. Dual regulation of water retention and cell growth by a stress-associated protein (SAP) gene in Prunus. Sci. Rep. 7, 332, https://doi.org/10.1038/s41598-017-00471-7 (2017).
Yoon, S. K. et al. Downregulation of stress-associated protein 1 (PagSAP1) increases salt stress tolerance in poplar (Populus alba × P. glandulosa). Trees 32, 823–833 (2018).
Jeszka-Skowron, M., Sentkowska, A. & Pyrzynska, K. & Paz De Peña, M. Chlorogenic acids, caffeine content and antioxidant properties of green coffee extracts - influence of green coffee bean preparation. Eur. Food Res. Technol. 242, 1403–1409 (2016).
Mithen, R. F., Dekker, M., Verkerk, R., Rabot, S. & Johnson, I. T. The nutritional significance, biosynthesis and bioavailability of glucosinolates in human foods. J. Sci. Food Agr. 80, 967–984 (2000).
Naing, A. H. et al. Overexpression of snapdragon Delila (Del) gene in tobacco enhances anthocyanin accumulation and abiotic stress tolerance. BMC Plant Biol. 17, 65, https://doi.org/10.1186/s12870-017-1015-5 (2017).
Inocencio, C., Cowan, R. S., Alcaraz, F., Rivera, D. & Fay, M. F. AFLP fingerprinting in Capparis subgenus Capparis related to the commercial sources of capers. Gen. Res. Crop. Evol. 52, 137–144 (2005).
Özbek, Ö. & Kara, A. Genetic variation in natural populations of Capparis from Turkey, as revealed by RAPD analysis. Plant Syst. Evol. 299, 1911–1933 (2013).
Wei, W. et al. Characterization of the sesame (Sesamum indicum L.) global transcriptome using Illumina paired-end sequencing and development of EST-SSR markers. BMC Genomics 12, 451, https://doi.org/10.1186/1471-2164-12-451 (2011).
Jiang, B., Xie, D., Liu, W., Peng, Q. & He, X. De Novo assembly and characterization of the transcriptome, and development of SSR Markers in Wax Gourd (Benincasa hispida). PLoS One 8, e71054, https://doi.org/10.1371/journal.pone.0071054 (2013).
Hu, L. et al. De Novo assembly and characterization of fruit transcriptome in black pepper (Piper nigrum). PLoS One 10, e0129822, https://doi.org/10.1371/journal.pone.0129822 (2015).
Varshney, R. K., Graner, A. & Sorrells, M. E. Genic microsatellite markers in plants: features and applications. Trends Biotechnol. 23, 48–55 (2005).
Ramsay, L. et al. Intimate association of microsatellite repeats with retrotransposons and other dispersed repetitive elements in barley. Plant J. 17, 415–425 (1999).
La Rota, M., Kantety, R. S., Yu, J. K. & Sorrells, M. E. Nonrandom distribution and frequencies of genomic and EST-derived microsatellite markers in rice, wheat, and barley. BMC Genomics 6, 23, https://doi.org/10.1186/1471-2164-6-23 (2005).
Xie, F., Sun, G., Stiller, J. W. & Zhang, B. Genome-wide functional analysis of the cotton transcriptome by creating an integrated EST database. PLoS One 6, e26980, https://doi.org/10.1371/journal.pone.0026980 (2011).
Mercati, F. et al. Single nucleotide polymorphism isolated from a novel EST dataset in garden asparagus (Asparagus officinalis L.). Plant Sci. 203, 115–123 (2013).
Zeng, S. et al. Development of a EST dataset and characterization of EST-SSRs in a traditional Chinese medicinal plant, Epimedium sagittatum (Sieb. Et Zucc.) Maxim. BMC Genomics 11, 94, https://doi.org/10.1186/1471-2164-11-94 (2010).
Barbera, G., Di Lorenzo, R. & Barone, E. Observations on Capparis populations cultivated in Sicily and on their vegetative and productive behaviour. Agric. Mediterr. 121, 32–39 (1991).
MacManes, M. D. On the optimal trimming of high-throughput mRNA sequence data. Front. Genet. 5, 13, https://doi.org/10.3389/fgene.2014.00013 (2014).
Mbandi, S. K., Hesse, U., Rees, D. J. G. & Christoffels, A. A glance at quality score: implication for de novo transcriptome reconstruction of Illumina reads. Front. Genet. 5, 17, https://doi.org/10.3389/fgene.2014.00017 (2014).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: A flexible trimmer for Illumina Sequence Data. Bioinformatics 30, 2114–2120 (2014).
Grabherr, M. G. et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotech. 29, 644–652 (2011).
Huang, Y., Niu, B., Gao, Y., Fu, L. & Li, W. CD-HIT Suite: a web server for clustering and comparing biological sequences. Bioinformatics 26, 680–682 (2010).
Simão, F. A., Waterhouse, R. M., Ioannidis, P., Kriventseva, E. V. & Zdobnov, E. M. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 31, 3210–3212 (2015).
Gurevich, A., Saveliev, V., Vyahhi, N. & Tesler, G. QUAST: quality assessment tool for genome assemblies. Bioinformatics 29, 1072–1075 (2013).
Bryant, D. M. et al. A tissue-mapped axolotl de novo transcriptome enables identification of limb regeneration factors. Cell Rep. 18, 762–776 (2017).
Finn, R. D., Clements, J. & Eddy, S. R. HMMER web server: interactive sequence similarity searching. Nucleic Acids Res. 39, W29–W37, https://doi.org/10.1093/nar/gkr367 (2011).
Punta, M. et al. The Pfam protein families database. Nucleic Acids Res. 40 (Database issue), D290–D301; https://doi.org/10.1093/nar/gkr1065 (2012).
Petersen, T. N., Brunak, S., von Heijne, G. & Nielsen, H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat. Methods. 8, 785–786 (2011).
Hu, Z. L., Bao, J. & Reecy, J. M. CateGOrizer: A Web-Based Program to Batch Analyze Gene Ontology Classification Categories. Online J. Bioinform. 9, 108–112 (2008).
Moriya, Y., Itoh, M., Okuda, S., Yoshizawa, A. C. & Kanehisa, M. KAAS: an automatic genome annotation and pathway reconstruction server. Nucleic Acids Res. 35, W182–W185, https://doi.org/10.1093/nar/gkm321 (2007).
Okuda, S. et al. KEGG Atlas mapping for global analysis of metabolic pathways. Nucleic Acids Res. 36, W423–W426, https://doi.org/10.1093/nar/gkn282 (2008).
Kanehisa, M. et al. New approach for understanding genome variations in KEGG. Nucleic Acids Res. 47, D590–D595, https://doi.org/10.1093/nar/gky962 (2019).
Kanehisa, M. et al. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 45, D353–D361, https://doi.org/10.1093/nar/gkw1092 (2017).
Altschul, S. F., Gish, W., Miller, W., Myers, E. W. & Lipman, D. J. Basic local alignment search tool. J. Mol. Biol. 215, 403–410 (1990).
Ellis, J. R. & Burke, J. M. EST-SSRs as a resource for population genetic analyses. Heredity 99, 125–132 (2007).
Beier, S., Thiel, T., Münch, T., Scholz, U. & Mascher, M. MISA-web: a web server for microsatellite prediction. Bioinformatics 33, 2583–2585 (2017).
Untergasser, A. et al. Primer3–new capabilities and interfaces. Nucleic Acids Res. 40, e115, https://doi.org/10.1093/nar/gks596 (2012).
Lodhi, M. A., Ye, G., Weeden, N. F. & Reisch, B. I. A simple and efficient method for DNA extraction from grapevine cultivars and Vitis species. Mol. Biol. Rep. 12, 6–13 (1994).
Liu, K. & Muse, S. V. PowerMarker: an integrated analysis environment for genetic marker analysis. Bioinformatics 21, 2128–2129 (2005).
Kamvar, Z. N., Tabima, J. F. & Grünwald, N. J. Poppr: an R package for genetic analysis of populations with clonal, partially clonal, and/or sexual reproduction. PeerJ 2, e281, https://doi.org/10.7717/peerj.281 (2014).
Bruvo, R., Michiels, N. K., D’Souza, T. G. & Schulenburg, H. A simple method for the calculation of microsatellite genotype distances irrespective of ploidy level. Mol. Ecol. 13, 2101–2106 (2004).
Jombart, T. & Ahmed, I. Adegenet 1.3-1: new tools for the analysis of genome-wide SNP data. Bioinformatics 27, 3070–3071 (2011).
Acknowledgements
The authors would like to thank Dr. Alberto Ferrarini and Dr. Andrea Minio (Università degli Studi di Verona) for their technical support during the samples sequencing.
Author information
Authors and Affiliations
Contributions
F.M., I.F., A.S.G. and F.C. conceived the study and designed the experiments. A.S.G., F.C., S.F. and F.M. collected the samples. F.M., A.M. and M.E.N. performed the experiments. I.F. analysed the NGS data. F.M., R.D.M, I.F., A.S.G. and F.C. prepared the manuscript. All the authors critically read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mercati, F., Fontana, I., Gristina, A.S. et al. Transcriptome analysis and codominant markers development in caper, a drought tolerant orphan crop with medicinal value. Sci Rep 9, 10411 (2019). https://doi.org/10.1038/s41598-019-46613-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-46613-x
This article is cited by
-
The genus Capparis L. (Capparaceae) in Laos and Cambodia
Kew Bulletin (2023)
-
Transcriptomic responses to drought stress in Polygonatum kingianum tuber
BMC Plant Biology (2021)
-
Genome-wide simple sequence repeats (SSR) markers discovered from whole-genome sequence comparisons of multiple spinach accessions
Scientific Reports (2021)
-
The genetic legacy of fragmentation and overexploitation in the threatened medicinal African pepper-bark tree, Warburgia salutaris
Scientific Reports (2020)
-
Characterization of Sicilian rosemary (Rosmarinus officinalis L.) germplasm through a multidisciplinary approach
Planta (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.