Abstract
The Thaumastocoris peregrinus spread to eucalyptus plantations in many countries. Chemical control is a questionable measure, mainly due to the environmental impact, high cost and moreover has the use restricted by the forest certifications. Bio-insecticides may have similar efficiency to chemical products to control T. peregrinus. The chemical thiamethoxam, thiamethoxam + lambda-cyhalothrin, acephate and the microbial Beauveria bassiana and Metarhizium anisopliae insecticides were tested at different doses to manage T. peregrinus. The products were sprayed on eucalyptus plants using aircraft and populations of this insect were counted before application and at 1, 14 and 21 days afterwards (DAA). Ten eucalyptus trees were evaluated per plot, with the collection of ten leaves from the middle third of the crown of each tree, and the number of T. peregrinus nymphs and adults obtained per leaf was determined. All the chemical insecticides had similar control at 1 DAA for T. peregrinus nymphs and adults. At 14 DAA, the number of T. peregrinus nymphs and adults on eucalyptus leaves was similar for the chemical and microbial insecticide treatments. At 21 DAA the control efficiency of T. peregrinus nymphs and adults was higher than 80% with all insecticides. The entomopathogenic insecticides have potential for aerial application to control T. peregrinus nymphs and adults and provide viable and environmentally-friendly alternative to manage this pest.
Similar content being viewed by others
Introduction
Eucalyptus (Myrtales: Myrtaceae), a native plant mainly from Australia, was introduced to Brazil and planted in genetically homogeneous and continuous areas to produce raw material for the forestry industry1,2. Eucalyptus plantations occupy 5.7 million hectares, representing 72% of the total planted trees in this country3. Homogeneous forests may be more susceptible to pests4,5,6 reducing productivity of Eucalyptus plants7,8,9.
Thaumastocoris peregrinus Carpintero & Dellapé 2006 (Hemiptera: Thaumastocoridae) is a serious pest with a rapid dispersal rate in eucalyptus species and hybrids due to high reproductive capacity, rapid colonization and broad infestation10,11. An Australian native, this pest spread to South Africa, Zimbabwe, Malawi, Kenya12 and to countries such as Argentina13, Brazil14,15 Chile16, Italy17, New Zealand18, Portugal19, Uruguay20 and Mexico21. In Brazil, this insect was detected in 2008 in Rio Grande do Sul and São Paulo states and later in Minas Gerais, Espírito Santo, Rio de Janeiro, Mato Grosso do Sul15, Goiás22, Paraná14, Santa Catarina23 and Sergipe24 states.
The short life cycle and high reproductive potential facilitate the rapid population growth of T. peregrinus in the field10,11,25,26, reducing photosynthetic apparatus an thus tree growth15 and productivity27. The analysis of the ecophysiological variables allows evaluating damages to the photosynthetic ability of E. camaldulensis by the bronze bug attack27. Sap sucking by T. peregrinus nymphs and adults causes chlorotic spots, leaf fall and decreases photosynthetic area12,14,17, which can lead to plant death15. Leaves damaged by T. peregrinus are initially silver, subsequently turning brown and red, which gives the tree a bronzed appearance, justifying its common name as bronze bug15. Eucalyptus species planted in Brazil includes Eucalyptus camaldulensis, Eucalyputus grandis and Eucalyptus urophylla and hybrids adequate for the T. peregrinus development and reproduction26.
Chemical control is, usually, used in insect population outbreaks and T. peregrinus was managed in urban areas with the systemic imidacloprid insecticide28. In Brazil the pyrethroid Capture 400 EC (FMC Agricultural Solutions) is the only product registered to control T. peregrinus. However, chemical control can cause environmental impact including reduction of natural enemies, intoxication of users and environmental contamination by the use of these products in extensive areas and moreover they have high cost and are restricted by the forest certification bodies29,30,31. Aerial spraying with insecticides may impact wildlife and beneficial insects32. The issue with aerial applications of neonicotinoids is related to its drift, gradual accumulation in target crop and non-crop vegetation, phloem-mediated translocation to nectar or pollen, the subsequent lethal and sub-lethal impacts on herbivores and higher trophic levels (including birds and arthropod natural enemies)32,33. Thus it is necessary to propose alternative control which are efficient, cost-effective and environmentally sound34,35.
Efficient strategies to manage the T. peregrinus in commercial plantations in Brazil are unavailable, thus, biological control is the viable option against this pest30,36. The Cleruchoides noackae Lin and Huber (Hymenoptera: Mymaridae)12, Hemerobius bolivari Banks (Neuroptera: Hemerobiidae)19, Chrysoperla externa (Hagen) (Neuroptera: Chrysopidae)15 and predatory stinkbug have been reported as natural enemies of T. peregrinus19,37,38. In Brazil, microbial control is a viable alternative due to favorable environmental conditions. Entomopathogenic fungi are used against agricultural insect pests, because they are natural to the environment. Beauveria bassiana (Bals.) Vuillemin and Metarhyzium anisopliae (Metsch.) Sorokin have wide host range39. They are used via inoculative, conservative, incremental or inundative applications and penetrate host integument40. Metarhizium anisopliae and B. bassiana are effective against forest pests41,42,43. However, it is important to determine the concentrations of mycoinsecticide to overcome natural host defense mechanism barriers and to cause host death40,44. The importance of the forestry sector to the Brazilian economy and the introduction of T. peregrinus into Brazil make it necessary to reduce population outbreaks of this pest.
The objective of this study was to investigate the efficiency of entomopathogenic fungi compared to chemical insecticides to control T. peregrinus.
Results
The number of T. peregrinus nymphs and adults per eucalyptus leaf, before application, was similar between treatments (Table 1).
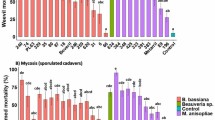
The number of T. peregrinus nymphs and adults per leaf was lower 1 day after the application of the insecticides thiamethoxam (Actara), thiamethoxam + lambda cyhalothrin (Engeo Pleno) and acephate (Orthene) (Table 1), observing greater efficiency with the second product (Fig. 1). The control efficiency was 73; 81; 88; 90 and 95% for the acephate (Orthene), thiamethoxam (Actara) and Engeo Pleno at the rates of 0.15, 0.2 and 0.1 kg/ha, respectively (Fig. 1).
Efficiency (%) of the insecticides Boveril (BIT 0.5 = BIT 0.5 kg/ha, BIT 1.0 = BIT 1.0 kg/ha, BIT 1.5 = BIT 1.5 kg/ha), Metarril (MIT 0.25 = MIT 0.25 kg/ha, MIT 0.50 = MIT 0.50 kg/ha, MIT 1.0 = MIT 1.0 kg/ha), Actara 0.1 = Actara (thiamethoxam 0.1 kg/ha), Actara 0.15 = Actara (thiamethoxam) 0.15 kg/ha, Actara 0.2 = Actara (thiamethoxam) 0.2 kg/ha, Engeo Pleno = Engeo Pleno (lambda cialothrin + thiamethoxam) 0.2 l/ha e Orthene = Orthene (acephate) 0.5 kg/ha to control Thaumastocoris peregrinus (Hemiptera: Thaumastocoridae) nymphs and adults in the first (1 day after application = DAA), second (14 DAA) and third (21 DAA) evaluations (Henderson-Tilton formula).
Fourteen days after the application of the chemical and biological insecticides, the number of T. peregrinus nymphs and adults were similar (Table 1). The efficiency of thiamethoxam + lambda-cyhalothrin (0.2 L/ha), M. anisopliae (1 kg/ha), thiamethoxam (0.1 kg/ha) and B. bassiana (0.5 and 1 Kg/ha) was 81, 83, 91, 92 and 94%, respectively (Fig. 1).
The number of T. peregrinus nymphs and adults at 21 days after application was lower for B. bassiana with the lowest doses and similar to the treatments with Engeo Pleno, Orthene and Actara (0.15 Kg/ha) (Table 1). The control was above 80% in all treatments, with greater efficiency for B. bassiana 0.5 kg/ha (99%) and acephate 0.5 kg/ha (97%) (Fig. 1).
Discussion
Biological insecticides, such as entomopathogenic fungi, are safer and have lower health risks than chemicals in pest control45,46. The bronze bug mortality by B. bassiana and M. anisopliae in the field is poorly studied but microbial products may have high efficiency in the integrated management of this pest in forest crops. Temperatures of 27, 28 and 29 °C and precipitation of 20; 25 and 63 mm in August, September and October were adequate for sporulation and favored the control efficiency of T. peregrinus by B. bassiana and M. anisopliae, as observed in other works46,47. These entomopathogens have a wide host range, but their germination, conidia persistence, host mortality and sporulation depend on adequate environmental conditions such as temperature and humidity48,49. Beauvaria bassiana and M. anisopliae can grow between 5 to 30 and 5 to 40 °C respectively, but showing optimal growth at temperatures of 25 and 30 °C50, determining their efficacy in biological control. This makes necessary an adequacy between the temperature and humidity for the efficiency of these fungi. Entomopathogen applications in the field are preferable in the late afternoon to avoid the negative impact of abrupt changes in temperature51. The low precipitation level may initially compromise the spore penetration and survival even at adequate temperatures. However, increased precipitation favored conidiogenesis in the first dead individuals, with horizontal transmission and dissemination of the disease throughout the populations. This is important because the potential for fungus conidiogenesis is determinant in the pathogen spread among pest individuals.
The higher efficiency of the chemical insecticides, 1 day after application, shows its faster impact due to the pyrethroid and neonicotinoid knock-down effect. This is similar to other neonicotinoids and pyrethroids such as imidacloprid and lambda-cyhalothrin against Bucephalogonia xanthophis (Berg 1879) (Hemiptera: Cicadellidae) with mortality above 90% in 24 h and near 100% within 48 h52. The neonicotinoid-pyrethroid mixture (thiamethoxam + lambda-cyhalothrin) increased the control efficiency of T. peregrinus with results similar to that observed for Myzus persicae nicotianae Blackman 1987 (Hemiptera: Aphididae) up to 70 DAA in tobacco crop53. Imidacloprid and thiamethoxam are systemic insecticides of the neonicotinoid group that act as an acetylcholine agonist in the synapses of the central nervous system54. The pyrethroid lambda-cyhalothrin is a sodium channel modulator, causing repetitive and uncontrolled impulses, hyperexcitation and death55. However, these insecticides have rapid action and often are toxic to beneficial organisms. Bees may come into direct contact during pollen and nectar collection or through contaminated water32,33. Thiamethoxam is toxic to parasitoids and predators in forest environments and agricultural crops56. In addition, airborne application with chemical insecticides may aggravate the situation by contaminating nearby wild and cultivated plants33.
The similar control efficiency of B. bassiana and M. anisopliae entomopathogens and chemicals for the T. peregrinus nymphs and adults after 14 DAA suggests efficiency of the microbial control. The horizontal transmission and dissemination of diseases in pest populations determine the pathogen efficiency being favored by greater humidities57. Delayed effects via horizontal transmission for entomopathogens have been reported for M. anisopliae on Oncometopia facialis (Signoret) (Hemiptera: Cicadellidae)58 and B. bassiana in Bemisia tabaci (Gennadius, 1889) (Hemiptera: Aleyrodidae)45. Epizootic occurrences of Entomophtorales fungi were reported for T. peregrinus nymphs and adults in a Eucalyptus plantation in São Paulo state, Brazil44. Entomopathogen use in integrated pest management is a viable, low-risk technique and has the capacity to exploit a wide host range through different action modes46. Fungi cause death by penetrating and destroying the external arthropod cuticle59,60. The fungi B. bassiana and M. anisopliae have rapid dispersion in the field with potential to control forest insect outbreaks40,41, and sucking insects such as Nilaparvata lugens Stål (Hemiptera: Delphacidae)61 and Diaphorina citri Kuwayama (Hemiptera: Liviidae)62. The gregarious behavior of T. peregrinus25,28,63 may facilitate entomopathogenic fungal epizootics in the field. The entomopathogenic fungi such as M. anisopliae and B. bassiana are effective against pest insects but they can affect natural enemies. This suggests a careful criterion in using these fungi to maintain the effectiveness of the control exerted by both.
The control efficiency of T. peregrinus, at 21 days, with the entomopathogen products, mainly for B. bassiana, shows residual effects and the horizontal dispersion capacity of this fungus as reported against Bucephalogonia xanthophis (Berg) (Hemiptera: Cicadellidae)45. Entomopathogenic fungi are slower acting and need higher relative humidity and/or rainy periods. Additionally, they require longer periods to cause mortality compared to synthetic chemical products64, but side-effects in infected insects reduces feeding and damage65. The pathogenicity and virulence of the mycoinsecticides indicate that the fungi overcome the physical barriers such as insect sclerotic exoskeleton as found for the natural occurrence of a fungus from the order Entomophthtorales on T. peregrinus in São Paulo state, Brazil44. The tegument may act as a physical barrier to the penetration and the germinative tube or may have chemical properties inhibiting conidia germination. Thaumastocoris peregrinus control over 80% at 21 DAA using entomopathogenic fungi indicates the delayed effect of this product as found for B. bassiana surviving and colonizing foliar tissues 30 days after inoculation without damaging plants66.
Beauveria bassiana and M. anisopliae have with potential to control T. peregrinus as found against Hemiptera pests such as aphid67, Riptortus pedestris (Fabricius, 1775)68, Diaphorina citri Kuwayama69, Bemisia tabaci70. Certified forest companies seek practices that conserve the environment, such as integrated pest management, giving preference to biological, cultural control and the use of less toxic products. Biological control is the only viable option to manage T. peregrinus in commercial eucalyptus plantations reducing the toxicity drift caused by the pyrethroid and the neocotinoid in aerial applications. The compatibility of chemicals with microbial agents and the effect of these products on natural enemies need better studies for integrated pest management.
The T. peregrinus control was similar with entomopathogens and chemical insecticides. The efficiency of the fungi B. bassiana and M. anisopliae at lower concentrations and its high residual period shows the potential of these products to control T. peregrinus nymphs and adults in eucalyptus plantations with low impact on other organisms such as parasitoids and predators. The adoption of control measures may be part of integrated management programs, where other control strategies can be used in a joint manner.
Methods
Obtaining fungal spores
The fungus Beauveria bassiana (isolated ESALQ PL63- obtained from Atta spp. in Piracicaba, São Paulo, Brazil) was the active ingredient of the product Boveril and Metarhizium anisopliae (ESALQ E9 isolate - obtained from Mahanarva posticata in Boca da Mata, Alagoas, Brazil) that of the product Metarril. Both are deposited in the Bank of the Laboratory of Pathology and Microbial Control of Insects of ESALQ/USP Piracicaba, São Paulo, Brazil. These microorganisms were cultured by solid fermentation in rice and their conidia were dried and extracted for the assays. Spore production followed a methodology described71, with modifications. This methodology includes pre-baking the rice, packing it in polypropylene bags, closing the bags and sterilizing them for 20 minutes in an autoclave at 121 °C. After cooling the rice, the substrate is inoculated with microorganism strains, homogenized by manual shaking and stored in air-conditioned rooms with a controlled temperature of 25.5 ± 1.0 °C and 12 hour photoperiod and placed on shelves for four days. After this time, the rice with mycelium was spread in trays for another eight days until the conidia sporulation. After this process, the solid fermentation product is dried for three days under the same conditions of controlled temperature and photoperiod and sieved to extract the pure conidia (Personal communication, Luciano Koppert).
The pure spores of the entomopathogenic fungi were used in the same proportion of the active ingredient used in the commercial product Boveril and Metarril corresponding to 2.5 × 109 spores/ha and 6.9 × 109 spores/ha respectively.
Conducting the experiment
The experiment was carried out in Pompéu, Minas Gerais state, Brazil in areas of the Vallourec & Mannesmann Florestal (V&M) with a randomized complete block design. The 12 treatments were conducted with chemical and biological insecticides with different concentrations (Table 2) with four replications and 48 plots, each 40 m wide and 500 m long, equivalent to 2 ha. The evaluation was done in the central area (2 ha) of each plot to avoid drift contamination between treatments. The clones VM01 (hybrids of E. urophylla and E. camaldulensis) with approximately 12 to 16 months old and spaced 2 × 3 m were used.
The products and water (control) were sprayed using an agricultural aircraft model Ipanema with Micronair AU 5000 rotary spray nozzles with electronic beacon DGPS in a round-trip evolution system with a diameter of 200 micrometer drops. After each spraying with the respective treatments, the tank was cleaned with 100 liters of water, with the aid of a tank kite. The temperature and humidity conditions in the field (Table 2) were adequate for the B. bassiana and M. anisopliae survival and development.
Thaumastocoris peregrinus nymphs and adults were collected before and at 1, 14 and 21 days after spraying. Microbial insecticides were not evaluated 1 day after application due to their slower action.
Ten trees were evaluated per plot with the collection of ten leaves from the middle third of the crown of each one43. The leaves were removed from the plant and packed in sealed paper bags which were transported to the FCA/UNESP Biological Control of Forest Pests Laboratory in Botucatu, São Paulo state, Brazil, where live insects were counted.
The mean numbers of T. peregrinus nymphs and adults per eucalyptus leaf were submitted to variance analysis and compared using Tukey test (p < 0.05). The control efficiency of the products was corrected by the Henderson-Tilton’s formula72, adequate to evaluate the number of live insects in a non-uniform population: efficiency (%) = [(numbers in the control before application x numbers in the treatment after application)/(number in the control after application x numbers in the treatment before application)] × 100}.
References
Dos Santos, A. et al. Multispectral characterization, prediction and mapping of Thaumastocoris peregrinus (Hemiptera: Thaumascoridae) attack in Eucalyptus plantations using remote sensing. J Spat Sci 62, 127–137 (2017).
Miranda, M. A. S., Ribeiro, G. B. D., Valverde, S. R. & Isbaex, C. Eucalyptus sp. woodchip potential for industrial thermal energy production. Rer Arvore 41, e410604 (2017).
IBÁ – Instituto Brasileiro de Árvores. Relatório 2017. São Paulo: IBÁ (2017).
Zanuncio, J. C., Alves, J. B., Santos, G. P. & Campos, W. O. Monitoring and population-dynamics of Lepidoptera associated with eucalyptus. VI- Belo Oriente Region, Minas Gerais, Brazil. Pesqui Agropecu Bras 28, 1121–1127 (1993).
Zanuncio, T. V., Zanuncio, J. C., Miranda, M. M. M. & de Barros Medeiros, A. G. Effect of plantation age on diversity and population fluctuation of Lepidoptera collected in Eucalyptus plantations in Brazil. Forest Ecol Manag 108, 91–98 (1998).
Zanuncio, J. C., Lacerda, M. C., Zanuncio, T. V., Silva, A. M. C. & Espindula, M. C. Fertility table and rate of population growth of the predator Supputius cincticeps (Heteroptera: Pentatomidae) on one plants of Eucalyptus cloeziana in the field. Ann Appl Biol 144, 357–361 (2004a).
Santos, A., Zanetti, R., Almado, R. P. & Zanuncio, J. C. Cerambycidae associated with hybrid Eucalyptus urograndis and native vegetation in Carbonita, Minas Gerais State, Brazil. Fla Entomol 97, 523–527 (2014).
Zanuncio, J. C., Lopes, E. T., Leite, H. G. & Fialho, M. C. Q. Sampling methods for monitoring the number and area of colonies of leaf cutting ants (Hymenoptera: Formicidae) in Eucalyptus plantations in Brazil. Sociobiology 44, 337–344 (2004b).
Zanuncio, J. C., Tavares, W. S., Ramalho, F. S., Leite, G. L. D. & Serrão, J. E. Sarsina violascens spatial and temporal distributions affected by native vegetation strips in eucalyptus plantations. Pesqui Agropecu Bras 6, 703 (2016).
Nadel., R. L. & Noack, A. E. Current understanding of the biology of Thaumastocoris peregrinus in the quest for a management strategy. Int J Pest Manage 58, 257–266 (2012).
Souza, G. K. et al. Reproductive tract histology of Thaumastocoris peregrinus (Hemiptera: Thaumastocoridae). Ann Entomol Soc Am 107, 853–857 (2014).
Nadel, R. L. et al. Population dynamics of Thaumastocoris peregrinus in Eucalyptus plantations of South Africa. J Pest Sci 88, 97–106 (2015).
Carpintero, D. L. & Dellape, P. M. A new species of Thaumastocoris Kirkaldy from Argentina (Heteroptera: Thaumastocoridae: Thaumastocorinae). Zootaxa 1228, 61–68 (2006).
Barbosa, L. R., Santos, F., Wilcken, C. F. & Soliman, E. P. Registro de Thaumastocoris peregrinus (Hemiptera: Thaumastocoridae) no estado do Paraná. Pesqui Flor Bras 30, 75–77 (2010).
Wilcken, C. F. et al. Bronze bug Thaumastocoris peregrinus Carpintero and Dellapé (Hemiptera: Thaumastocoridae) on Eucalyptus in Brazil and its distribution. J Plant Prot Res 50, 201–205 (2010).
Ide, S., Ruiz, C., Sandoval, A. & Valenzuela, J. Detección de Thaumastocoris peregrinus (Hemiptera: Thaumastocoridae) asociado a Eucalyptus spp. en Chile. Bosque 32, 309–313 (2011).
Laudonia, S. & Sasso, R. The bronze bug Thaumastocoris peregrinus: a new insect recorded in Italy, damaging to Eucalyptus trees. B Insectol 65, 89–93 (2012).
Sopow, S., George, S. & Ward, N. Bronze bug, Thaumastocoris peregrinus: a new Eucalyptus pest in New Zealand. Surveillance 39, 43–46 (2012).
Garcia, A., Figueiredo, E., Valente, C., Montserrat, V. J. & Branco, M. First record of Thaumastocoris peregrinus in Portugal and of the neotropical predator Hemerobius bolivari in. Europe. B Insectol 66(251), 256 (2013).
Martínez, G. & Bianchi, M. Primer registro para Uruguay de la chinche del eucalipto, Thaumastocoris peregrinus Carpintero y Dellappé, 2006 (Heteroptera: Thaumastocoridae). Agrociência 14, 15–18 (2010).
Jiménez-Quiroz, E., Vanegas-Rico, J. M., Morales-Martínez, O., Lomeli-Flores, J. R. & Rodríguez-Leyva, E. First Record of the Bronze Bug, Thaumastocoris peregrinus Carpintero & Dellapé 2006 (Hemiptera: Thaumastocoridae), in Mexico. J Agr Urban Entomol 32, 35–39 (2016).
Pereira, J. M., Melo, A. P. C., Fernandes, P. M. & Soliman, E. P. Ocorrência de Thaumastocoris peregrinus Carpintero & Dellapé (Hemiptera: Thaumastocoridae) no Estado do Goiás. Cienc Rural 43, 254–257 (2013).
Savaris, M., Lampert, S., Pereira, P. R. V. S. & Salvadori, J. R. Primeiro registro de Thaumastocoris peregrinus para o estado de Santa Catarina, e novas áreas de ocorrência para o Rio Grande do Sul, Brasil. Cienc Rural 41, 1874–1876 (2011).
Ribeiro, G. T. et al. First report of Thaumastocoris peregrinus in eucalyptus plantations in the State of Sergipe, Brazil (Hemiptera: Thaumastocoridae). Entomol Am 121, 23–26 (2015).
Noack, A. E. & Rose, H. A. Life-history of Thaumastocoris peregrinus and Thaumastocoris sp. in the laboratory with some observations on behaviour. Gen Appl Entomol 36, 27–33 (2007).
Soliman, E. P. et al. Biology of Thaumastocoris peregrinus Carpintero & Dellapé (Hemiptera: Thaumastocoridae) in different eucalyptus species and hybrids. Phytoparasitica 40, 223–230 (2012).
Almeida, K. E. C. D., Silva, J. G. S. D., Silva, I. M. D. A., Costa, A. L. D. & Laia, M. L. D. Ecophysiological analysis of Eucalyptus camaldulensis (Dehnh) submitted to attack from Thaumastocoris peregrinus (Carpintero & Dellape). Rev Arvore 42, e420120 (2018).
Noack, A. E., Kaapro, J., Bartimote-Aufflick, K., Mansfield, S. & Rose, H. Efficacy of Imidacloprid in the control of Thaumastocoris peregrinus on Eucalyptus scoparia in Sydney, Australia. Arboric Urban For 35, 192–196 (2009).
Zanetti, R. et al. An overview of integrated management of leaf-cutting ants (Hymenoptera: Formicidae) in Brazilian forest plantations. Forests 5, 439–455 (2014).
Zanuncio, J. C. et al. The impact of the Forest Stewardship Council (FSC) pesticide policy on the management of leaf-cutting ants and termites in certified forests in Brazil. Ann Forest Sci 73, 205–214 (2016).
Lemes, P. G., Zanuncio, J. C., Serrão, J. E. & Lawson, S. A. Forest stewardship council (FSC) pesticide policy and integrated pest management in certified tropical plantations. Environ Sci Pollut R 24, 1283–1295 (2017).
Auteri, D. et al. Neonicotinoids and bees: The case of the European regulatory risk assessment. Sci Total Environ 579, 966–971 (2016).
Wood, T. J. & Goulson, D. The environmental risks of neonicotinoid pesticides: a review of the evidence post 2013. Environ Sci Pollut R 24, 17285–17325 (2017).
Bragança, M., De Souza, O. & Zanuncio, J. C. Environmental heterogeneity as a strategy for pest management in Eucalyptus plantations. Forest Ecol Manag 102, 9–12 (1998).
Fialho, M. C. et al. Prey digestion in the midgut of the predatory bug Podisus nigrispinus (Hemiptera: Pentatomidae). J Insect Physiol 58, 850–856 (2012).
Mewes, W. L. C., Teixeira, M. M., Fernandes, H. C., Zanuncio, J. C. & Tiburcio, R. A. S. Parâmetros característicos da pulverização pneumática em copas de árvores de eucalipto. Rev Arvore 39, 635–640 (2015).
Souza, G. K. et al. First record of a native heteropteran preying on the introduced eucalyptus pest, Thaumastocoris peregrinus (Hemiptera: Thaumastocoridae), in Brazil. Fla Entomol 95, 517–520 (2012).
Dias, T. K. R. et al. Predation of Thaumastocoris peregrinus (Hemiptera: Thaumastocoridae) by Atopozelus opsimus (Hemiptera: Reduviidae) in Brazil. ISJ- Invert Surviv J 11, 224–227 (2014).
Lord, J. C. From Metchnikoff to Monsanto and beyond: The path of microbial control. J Invertebr Pathol 89, 19–29 (2005).
Shah, P. A. & Pell, J. K. Entomopathogenic fungi as biological control agents. Appl Microbiol Biot 61, 413–423 (2003).
Draganova, S. et al. Fungal pathogens on some lepidopteran forest pests in Bulgaria. Acta Zool Bulg 65, 179–186 (2013).
Williams, C. D. et al. Control of a major pest of forestry, Hylobius abietis, with entomopathogenic nematodes and fungi using eradicant and prophylactic strategies. For Ecol Manag 305, 212–222 (2013).
Lima, A. C. V., Wilcken, C. F., Ferreira-Filho, P. J., Serrão, J. E. & Zanuncio, J. C. Intra plant spatial distribution of Thaumastocoris peregrinus Carpintero & Dellapé (Hemiptera: Thaumastocoridae) on Eucalyptus grandis plants. Phytoparasitica 44, 411–418 (2016).
Mascarin, G. M., Durate, V. S., Branbao, M. M. & Juinor, D. I. Natural occurrence of Zoophthora radicans (Entomophthorales: Entomophthoraceae) on Thaumastocoris peregrinus (Heteroptera: Thaumastocoridae), an invasive pest recently found in Brazil. J Invertebr Pathol 110, 401–404 (2012).
Espinel, C., Torres, L., Grijalba, E., Villamizar, L. & Cotes, A. M. Preformulados para control de la mosca blanca Bemisia tabaci (Hemiptera: Aleyrodidae) en condiciones de laboratório. Rev Colomb Entomol 34, 22–27 (2008).
Hussain, A., Rizwan-Ul-Haq, M., Al-Ayedh, H. & Al-Jabr, A. M. Mycoinsecticides: potential and future perspective. Recent Pat Food Nutr Agric 6, 45–53 (2014).
Tumuhaise, V. et al. Temperature-dependent growth and virulence, and mass production potential of two candidate isolates of Metarhizium anisopliae (Metschnikoff) Sorokin for managing Maruca vitrata Fabricius (Lepidoptera: Crambidae) on cowpea. Afr Entomol 26, 73–83 (2018).
Ekesi, S., Maniania, N. K. & Ampong-Nyarko, K. Effect of temperature on germination, radial growth and virulence of Metarhizium anisopliae and Beauveria bassiana on Megalurothrips sjostedti. Biocontrol Sci Techn 9, 177–185 (1999).
Mwamburi, L. A., Laing, M. D. & Miller, R. M. Effect of surfactants and temperature on germination and vegetative growth of Beauveria bassiana. Braz J Microbiol 46, 67–74 (2015).
Hallsworth, J. E. & Magan, N. Water and temperature relations of growth of the entomogenous fungi Beauveria bassiana, Metarhizium anisopliae, and Paecilomyces farinosus. J Invertebr Pathol 74, 261–266 (1999).
Lecuona, R. E., Rodriguez, J. & La Rossa, F. R. Effect of constant and cyclical temperatures on the mortality of Triatoma Infestans (Klug) (Hemiptera: Reduviidae) treated with Beauvaria bassiana (Bals.) Vuill. (Hyphomycetes). Neotrop Entomol 34, 675–679 (2005).
Bezerra-Silva, G. C. D., Silva, M. A., Miranda, M. P. & Lopes, J. R. S. Effect of contact and systemic insecticides on the sharpshooter Bucephalogonia xanthophis (Hemiptera: Cicadellidae), a vector of Xylella fastidiosa in citrus. Fla Entomol 95, 854–861 (2012).
Fuentes-Contreras, E. et al. Evaluación de la eficacia, efecto residual y de volteo de aplicaciones en pretransplante de insecticidas nicotinoides y mezclas de nicotinoide-piretroide para el control de Myzus persicae nicotianae (Hemiptera: Aphididae) en Tabaco. Agric Tec 67, 16–22 (2007).
Matsuda, K. et al. Neonicotinoids: insecticides acting on insect acetylcholine receptors. Trends Pharmacol Sci 22, 573–580 (2001).
Soderlund, D. M. & Knipple, D. C. The molecular biology of knockdown resistance to Pyrethroid insecticides. Insect Biochem Mol Biol 33, 563–577 (2003).
Preetha, G., Stanley, J., Suresh, S., Kuttalam, S. & Samiyappan, R. Toxicity of selected insecticides to Trichogramma chilonis: assessing their safety in the rice ecosystem. Phytoparasitica 37, 209–215 (2009).
Cheraghi, A., Habibpour, B., Mossadegh, M. S. & Sharififard, M. Horizontal transmission of the entomopathogen fungus Metarhizium anisopliae in Microcerotermes diversus groups. Insects 3, 709–718 (2012).
Pria Júnior, W. D., Lacava, P. T., Messias, C. L., Azevedo, J. A. & Lacava, P. M. Bioassay assessment of Metarhizium anisopliae (Metchnikoff) Sorokin (Deuteromycota: Hyphomycetes) against Oncometopia facialis (Signoret) (Hemiptera: Cicadellidae). Braz J Microbiol 39, 128–132 (2008).
Hegedus, D. & Khachatourians, G. The impact of biotechnology on hyphomycetous fungal insect biocontrol agents. Biotechnol Adv 13, 455–490 (1995).
Beemelmanns, C., Guo, H., Rischer, M. & Poulsen, M. Natural products from microbes associated with insects. Beilstein J Org Chem 12, 314–327 (2016).
Li, M. et al. Selection of Beauveria isolates pathogenic to adults of Nilaparvata lugens. J Insect Sci 14, 1–12 (2014).
Orduño-Cruz, N. et al. In vivo selection of entomopathogenic fungal isolates for control of Diaphorina citri (Hemiptera: Liviidae). Biol Control 90, 1–5 (2015).
Jacobs, D. H. & Neser, S. Thaumastocoris australicus Kirkaldy (Heteroptera: Thaumastocoridae): a new insect arrival in South Africa, damaging to Eucalyptus trees: research in action. Afr J Sci 101, 233–236 (2005).
Lomer, C. J., Bateman, R. P., Johnson, D. L., Langewald, J. & Thomas, M. Biological control of locust and grasshoppers. Annu Rev Entomol 44, 667–702 (2001).
Pelizza, S. A., Mariottini, Y., Russo, M. L., Cabello, M. N. & Lange, C. E. Survival and fecundity of Dichroplus maculipennis and Ronderosia bergi (Orthoptera: Acrididae: Melanoplinae) following infection by Beauveria bassiana (Ascomycota: Hypocreales) under laboratory conditions. Biocontrol Sci Techn 23, 701–710 (2013).
Gómez-Vidal, S., Lopez-Llorca, L. V., Jansson, H. B. & Salinas, J. Endophytic colonization of date palm (Phoenix dactylifera L.) leaves by entomopathogenic fungi. Micron 37, 624–632 (2006).
Mweke, A. et al. Evaluation of the entomopathogenic fungi Metarhizium anisopliae, Beauveria bassiana and Isaria sp. for the management of Aphis craccivora (Hemiptera: Aphididdae). J Econ Entomol 111, 1587–1594 (2018).
Lee, S. J., Kim, S., Skinner, M., Parker, B. L. & Kim, J. S. Screen bag formulation of Beauveria and Metarhizium granules to manage Riptortus pedestris (Hemiptera: Alydidae). J Asia-Pac Entomol 19, 887–892 (2016).
Ayala-Zermeño, M. A. et al. Characterisation of entomopathogenic fungi used in the biological control programme of Diaphorina citri in Mexico. Biocontrol Sci Techn 25, 1192–1207 (2015).
Zafar, J., Freed, S., Khan, B. A. & Farooq, M. Effectiveness of Beauveria bassiana against cotton whitefly, Bemisia tabaci (Gennadius) (Aleyrodidae: Homoptera) on different host plants. Pak J Zool 48, 91–99 (2016).
Leite, L. G., Batista Filho, A., Almeida, J. E. M. & Alves, S. B. Produção de fungos entomopatogênicos. Ribeirão Preto: AS, 92p (2003).
Henderson, C. F. & Tilton, E. W. Tests with acaricides against the brown wheat mite. J Econ Entomol 48, 157–161 (1955).
Acknowledgements
We acknowledge the financial support from “Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) and “Programa Cooperativo sobre Proteção Florestal (PROTEF) do Instituto de Pesquisas e Estudos Florestais (IPEF)”. Dr. Phillip John Villani (The University of Melbourne, Australia) revised and corrected the English language used in this manuscript.
Author information
Authors and Affiliations
Contributions
M.H.F.d.A.D.P., C.F.W., A.C.V.L., E.P.S., B.V.F., I.M.S. and J.C.Z. designed the research; A.C.V.L., E.P.S., L.R.B. and B.V.F. performed the experiments; M.H.F.d.A.D.P., A.J.V.Z., C.F.W., A.C.V.L., E.P.S., B.V.F., I.M.S., L.R.B. and J.C.Z. analyzed the data, participated in writing and approved the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wilcken, C.F., Dal Pogetto, M.H.F.d.A., Lima, A.C.V. et al. Chemical vs entomopathogenic control of Thaumastocoris peregrinus (Hemiptera: Thaumastocoridae) via aerial application in eucalyptus plantations. Sci Rep 9, 9416 (2019). https://doi.org/10.1038/s41598-019-45802-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-45802-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.