Abstract
The Metabolic Cost of Walking (MCoW) is an important variable of daily life that has been studied extensively. Several studies suggest that MCoW is higher in Older Adults (OA) than in Young Adults (YA). However, it is difficult to compare values across studies due to differences in the way MCoW was expressed, the units in which it was reported and the walking speed at which it was measured. To provide an overview of MCoW in OA and YA and to investigate the quantitative effect of age on MCoW, we have conducted a literature review and performed two meta-analyses. We extracted data on MCoW in healthy YA (18–41 years old) and healthy OA (≥59 years old) and calculated, if not already reported, the Gross (GCoW) and Net MCoW (NCoW) in J/kg/m. If studies reported MCoW measured at multiple speeds, we selected those values for YA and OA at which MCoW was minimal. All studies directly comparing YA and OA were selected for meta-analyses. From all studies reviewed, the average GCoW in YA was 3.4 ± 0.4 J/kg/m and 3.8 ± 0.4 J/kg/m in OA (~12% more in OA), and the average NCoW in YA was 2.4 ± 0.4 J/kg/m and 2.8 ± 0.5 J/kg/m in OA (~17% more in OA). Our meta-analyses indicated a statistically significant elevation of both GCoW and NCoW (p < 0.001) for OA. In terms of GCoW, OA expended about 0.3 J/kg/m more metabolic energy than YA and about 0.4 J/kg/m more metabolic energy than YA in terms of NCoW. Our study showed a statistically significant elevation in MCoW of OA over YA. However, from the literature it is unclear if this elevation is directly caused by age or due to an interaction between age and methodology. We recommend further research comparing MCoW in healthy OA and YA during “natural” over-ground walking and treadmill walking, after sufficient familiarization time.
Similar content being viewed by others
Introduction
Glossary
-
a.
WS in [m/s]: the measured walking speed either over-ground or on a treadmill.
-
b.
\(\dot{V}{O}_{2}\) in [ml/kg/min]: the rate of oxygen consumption.
-
c.
EEq of \(\dot{V}{O}_{2}\) in [J/kg/min]: Energetic Equivalent of the oxygen consumed during walking.
-
d.
RMR in [J/kg/min]: the rate of metabolic energy consumption during quiet standing or sitting (Resting Metabolic Rate).
-
e.
MCoW in [J/kg/m]: metabolic energy consumed, per kilogram of body mass per meter travelled, typically expressed as gross or net values (Metabolic Cost of Walking).
-
f.
GCoW in [J/kg/m]: EEq/(WS ×60) (Gross Cost of Walking).
-
g.
NCoW in [J/kg/m]: (EEq – RMR)/(WS ×60) (Net Cost of Walking).
-
h.
RER [dimensioneless]: the amount of carbon dioxide produced divided by the amount of oxygen consumed (Respiratory Exchange Ratio).
-
i.
OA, YA: (Old Adults, mean age ≥ 59 years) and (Young Adults, mean age 18–41 years).
Walking is the most prevalent physical activity for humans and may account for up to thirty percent (30%) of an adult’s daily energy expenditure1. Hence, the Metabolic Cost of Walking (MCoW), defined as metabolic energy expended per meter travelled, is an important variable in daily life and has been studied extensively. It is generally presupposed that humans, under normal circumstances, select their gait parameters (like step frequency and walking velocity) to minimize MCoW e.g.2,3,4. MCoW has been studied in healthy children, teenagers, young and elderly adults, at preferred or fixed walking speeds and during both over-ground and treadmill walking. One major finding that has been reported by several studies is that MCoW is higher in healthy Older Adults e.g.5,6,7 (OA; to be consistent with the analyzed literature, defined here as mean age ≥ 59 years) than in Young Adults (YA; here defined as mean age from 18–41 years), although a recent extensive study has found contradictory results8. An elevated MCoW may cause OA to become fatigued more quickly and walk less, which in turn may lead to functional limitations and reduced societal participations. Currently, no quantitative analyses are present in the literature that directly compare the results across studies on MCoW in YA and OA, or on the differences between these two groups. Such analyses may be important, for example to decide whether the elevation in MCoW of OA over YA is clinically relevant.
Directly comparing the results in the literature across different studies on MCoW is not without problems. A first difficulty comparing quantitative measures across studies arises from the ways in which MCoW was expressed. Several studies expressed MCoW only in terms of Gross Cost of Walking (GCoW) e.g.9,10 which is the total amount of metabolic energy consumed per kilogram of body mass per meter travelled. Other studies expressed MCoW only in terms of Net Cost of Walking (NCoW) e.g.5,7,11 which is assumed to be the total amount of metabolic energy expended due to walking per se (e.g., due to cross-bridge cycling and the Ca2+ pump in the muscle) calculated by subtracting first the Resting Metabolic Rate (RMR) from Energetic Equivalent (EEq) of the oxygen consumed and then divided by the Walking Speed (WS). GCoW and NCoW are different in nature and quantity, making it difficult to interpret results across studies that only reported either one of these. For example, an increase in NCoW does not necessarily mean an increase in GCoW (and vice-versa) as there is evidence in literature that RMR may be different between YA and OA11,12. In addition, some of these studies do not report RMR values making it impossible to convert GCoW to NCoW and vice versa. A second difficulty is that MCoW and (rate of) energy expenditure have been reported in various units such as ml of O2/kg/m9, J/kg/m11 or W/kg13 and it is not trivial to convert one to another. For example, to convert from ml of O2/kg/m to J/kg/m, Respiratory Exchange Ratio (RER) is required, which typically differs among subjects and is often not reported. A third problem arises from differences in walking speed at which MCoW was measured. For example, Stoquart et al.14 reported MCoW in YA at a walking speed of 1.1 m/s, whereas Davies and Dalsky15 reported MCoW in OA at a speed of 1.5 m/s. This is a problem because it is known that MCoW depends on walking speed e.g.4,16,17. Some studies used an identical fixed speed for YA and OA, but the values at which speed was fixed varied across studies, (e.g., 1.0 m/s in5 and 1.3 m/s in18). All of these difficulties mentioned above hamper the direct comparison of the results across different studies and therewith the observation of the actual values of MCoW in YA and OA.
Recently, Aboutorabi et al.19 performed a literature review on effects of aging on the temporo-spatial (gait) parameters, kinematics, kinetics and energetics of gait. They concluded that aging led to an increase in MCoW. However, their list of included studies was not exhaustive, they did not address the difficulties described above and, more importantly, they did not quantify the elevation identified.
The primary aims of the present study were to provide an overview of the literature on MCoW in YA and OA and to investigate quantitatively the elevation of MCoW related to aging on the basis of the published literature. To do so, we first carefully searched the literature on MCoW in both YA and OA and selected all relevant scientific articles using the Preferred Reporting Items for Systematic Review and Meta Analyses (PRISMA) method. We then statistically analyzed group differences, and then selected studies in which both YA and OA were measured in the same experimental setup. To estimate the effect size of MCoW between YA and OA we subsequently performed two meta-analyses, one on GCoW and the other on NCoW on these selected studies, that directly compared YA and OA.
Methods
Inclusion and exclusion criteria
We considered only studies published in peer reviewed journals that reported MCoW in healthy humans. In this study, we focused on healthy young and elderly adults and excluded all studies involving subjects with disabilities, comorbidities and a history of falling. We noted that the study of Malatesta et al.6 did not mention whether the value for MCoW was gross or net. Based on the reported values, we are fairly confident that they were GCoW values and have included the study in our analyses; the values for the two OA groups (G80 and G65) were averaged and used for our meta-analyses. We furthermore specifically comment on the exclusion of two studies from our analyses. First, Thomas et al.20 decided to pool the data of MCoW in their analysis as they did not find any significant difference in the Preferred Walking Speed (PWS) of healthy young and elderly women. Second, Pearce et al.21 also pooled MCoW for both YA and OA as they were only interested in the effect of walking surface on MCoW. The results of these studies are obviously unsuitable for our purpose of investigating an age-related elevation in MCoW.
Search strategy
A review protocol was developed based on the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA)-statement (see: www.prisma-statement.org)22. A comprehensive search was performed in the bibliographic databases PubMed, Embase.com, Ebsco/SPORTDiscus and Wiley/Cochrane Library in collaboration with a medical librarian. Databases were searched from inception up to 14 Aug 2018. The following terms were used (including synonyms and closely related words) as index terms or free-text words: “Aged”, “Elderly”, “Gait”, “Walking”, “Energy metabolism”. The search was performed without date, language or publication status restriction. The full search strategies for all databases can be found in the Supplementary Information (see: Appendix D). The initial list of the papers was selected through evaluation of the Title, Keywords and Abstract. Then, a further selection based on screening of the Methodology, Protocol and the Aims of the studies was conducted by all authors independently. A flow-diagram for PRISMA method is shown in Fig. 1. Screening was done simultaneously using a manual search process undertaken in EndNote (Clarivate Analytics) and online by the open-source web application Rayyan QCRI (Qatar Foundation & Qatar Computing Research Institute).
Definition of age group
In our analyses we followed the definition of age group (OA vs YA) used by the authors of the individual studies. This resulted in a somewhat arbitrary overall definition of OA group of 59 years and older. To test for robustness against definition of the age group OA, we also performed statistical tests following the definition by the United Nations (UN) of elderly, being of the age of 65 and older, which showed only a marginal influence of the difference in definition of OA. The only study in the meta-analyses that had OA below 65 was that of Jones et al.23.
Overview of analyses
From the selected studies, we extracted all data on MCoW and calculated, if not already reported, GCoW and NCoW. If the unit in which MCoW was reported was not in the SI unit of J/kg/m, we converted the reported values accordingly. Studies typically either report MCoW for self-selected or preferred walking speed. If studies reported MCoW measured at multiple speeds, we selected MCoW values for both YA and OA at the speeds at which MCoW values were minimal as it is known that under normal circumstances humans tend to self-select walking speeds in close proximity of the minimal MCoW speed. Using the selected data, we first reported MCoW in both YA and OA and statistically analyzed group differences in walking speed (since the selected studies included both the experimentally fixed and preferred walking speeds) and MCoW between YA and OA. We then performed two meta-analyses, one on GCoW and one on NCoW, on the selected studies that directly compared YA and OA. We did this in order to account for the heterogeneity across the studies and differences in the experimental sample size and to calculate the effect size of MCoW between YA and OA.
Calculations of MCoW
Not all of the included studies reported all the variables of interest (being GCoW, NCoW, RMR and RER). If any of these variables was not reported, we attempted to calculate its mean and standard deviation from the other variables reported. In general, calculating means is straightforward, but calculating standard deviations is not possible unless the individual sample data are known, which was generally not the case. Whenever standard deviations were not given, we estimated them (see Appendix A for detailed overview of the calculated variables of interest and Discussion). In general, we used the following two equations to estimate standard deviations. For addition and subtraction of two quantities (say z = a + b or z = a − b), the estimated standard deviation of z (\({{\rm{\sigma }}}_{{{\rm{z}}}_{{\rm{est}}}}\)) was calculated as:
with \({{\rm{\sigma }}}_{a}\) and \({{\rm{\sigma }}}_{b}\) the known standard deviations of a and b. For multiplication and division of two quantities (say z = ab or z = a/b), the estimated standard deviation was calculated as:
Finally, we note here that in some studies RMR was measured while sitting, whereas in other studies it was measured while standing. RMR is close to 14% lower during sitting than during standing24 and this may influence the values obtained for either GCoW or NCoW when only one of these was reported.
Statistical analysis
We checked for normality of the data using a Shapiro-Wilk hypothesis test along with violinplots and QQ plots. The result of the test and the visual inspection of the plots showed that the data was non-normally distributed. Additionally, Levene’s test was used to check for the equality of variances and no violation of the equality was observed in the data. Based on these outcomes we chose to use a non-parametric Wilcoxon rank sum test.
We analyzed whether walking speeds at which MCoW (for both GCoW and NCoW) was measured differed between YA and OA using an independent two-sided non-parametric Wilcoxon rank sum test. After that, we used an independent one-sided non-parametric Wilcoxon rank sum test to test for an elevation of GCoW and NCoW of OA over YA. The open-source software JASP (version 0.9.0.1) was used for these statistical tests. However, from these analyses it is not possible to draw a definitive conclusion on the effect size of GCoW and NCoW between YA and OA. This is because not all selected studies directly compared YA and OA, and across the selected studies there were considerable differences in sample size, experimental methodology and various other factors like equipment, ethnicity and gender of subjects, etc. To be able to calculate the (pooled) effect size of MCoW between YA and OA, we selected only those studies that directly compared YA and OA, 19 in total (see Fig. 1) and performed meta-analyses on them. We ended up with 13 studies for GCoW and 18 studies for NCoW (both being a large-scale meta-analysis25), because in 5 studies RMR was not mentioned and only GCoW or NCoW was reported. If some studies did not report RMR it became impossible to calculate GCoW/NCoW when only one was reported. Some of these studies measured MCoW at the same fixed walking speed in YA and OA, while others measured MCoW at individually preferred walking speeds, that differed between YA and OA.
To account for the possible heterogeneity across studies, we used a continuous random-effects model. The studies were weighed according to their respective sample sizes and standard deviations of their group mean values. The meta-analyses were performed using the open-source software packages Open Meta[Analyst] (Brown University of Public Health, Providence, USA) and JAMOVI (version 0.9.1.12). The two-arm metric of mean differences of individual studies was used to calculate the pooled effect size, the 95% confidence intervals and the 95% prediction intervals. Pooled mean differences were calculated as MCoW for OA minus MCoW for YA for both GCoW and NCoW. In addition, we calculated for both GCoW and NCoW the pooled standardized mean difference, which is the (pooled) mean difference normalized relative to pooled standard deviation computed using Hedge’s G.
Validation and sensitivity tests
To assess the validity of the a-priori assumption of heterogeneity in the meta-analyses, we used the three standard tests of heterogeneity (Cochran’s Q, tau2 and I2). A sensitivity analysis was performed on each of the studies to check for the robustness of the meta-analysis using the Leave-One-Out method.
Risk of bias assessment
The appraisal tool for cross-sectional studies (AXIS26) was used as a tool to conduct the risk of bias assessment for the individual studies included in our meta-analyses. In short, all the studies were judged systematically on each of the specific sections of the articles starting from Introduction, Methods, Results to Discussion and other relevant parameters. Twenty specific questions were used to evaluate each of these sections of the articles with the option of answering them by a ‘YES’, ‘NO’ or ‘DO NOT KNOW’. Additionally there is an option to leave specific comments explaining the answers provided for each of the questions.
Results
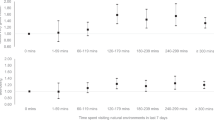
Figure 2A,B depict the mean GCoW and NCoW values as function of mean age of a group of studies reporting values for GCoW and/or NCoW. The data were then grouped into either YA (black dots) or OA (red dots) as was done in the selected studies. The mean walking speeds were found to be not statistically different between these groups (1.2 ± 0.2 m/s in YA vs 1.2 ± 0.2 m/s in OA, mean ± SD, p = 0.58). It was found that the average GCoW was 12% higher in OA (3.8 ± 0.4 J/kg/m) than in YA (3.4 ± 0.4 J/kg/m) and that this elevation was statistically significant (p < 0.001). The average NCoW was 17% higher in OA (2.8 ± 0.5 J/kg/m) than in YA (2.4 ± 0.4 J/kg/m); this elevation was also statistically significant (p < 0.001). These results indicate an elevation in GCoW and NCoW in OA compared to YA.
Metabolic Cost of Walking across the mean age in each study. (A) Gross Cost of Walking across age. (B) Net Cost of Walking across age. Black solid circles represent YA and the red solid circles OA. Each of the circles is a mean GCoW value or a mean NCoW value from a selected study. The dashed straight lines (black and red) show the mean metabolic cost of a particular age-group for both GCoW and NCoW40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56.
Overview of the results of the meta-analysis using a random effects model on the differences in Gross Metabolic Cost of Walking between YA and OA. A positive Mean Difference (MD) means that OA have higher GCoW than YA. The position of the red squares corresponds to the MD value per study and the horizontal black line to the 95% CI. The size of the square is proportional to the relative weight of that study w(%) to compute the overall MD (blue diamond and vertical black dashed line). The width of the diamond represents the 95% CI of the overall MD and the blue arrow represents the 95% PI of the overall MD. If a 95% CI spans zero (indicated by the red vertical dashed line), that study has found no significant elevation in GCoW. This meta-analysis shows a significant overall increase in GCoW of OA over YA (p < 0.001). Note that the p-value mentioned in the forest plot is the p-value for the Cochran’s Q heterogeneity test.
After this, a selection of studies was made from the studies in Fig. 2, which directly compared YA and OA. No statistically significant differences (p = 0.59) were found in the overall walking speeds between YA (1.2 ± 0.2 m/s) and OA (1.2 ± 0.1 m/s) of these studies. We then used these studies to perform two meta-analyses to calculate mean difference in MCoW between OA and YA. Figures 3 and 4 depict forest plots for GCoW and NCoW, respectively, showing for each of the individual studies the mean difference (red squares) and 95% Confidence Interval (95% CI, black lines), as well as the estimated pooled mean difference (center of blue diamond and dashed black summary line), 95% CI (width of blue diamond) and 95% Prediction Interval (PI, blue arrow). The size of the relative weight (w) of a particular study (indicated by the size of the squares in Figs 3 and 4) was dependent on the study sample size and standard deviation of the study sample mean (see27). Both for GCoW and NCoW we found a pooled mean difference that was statistically significant (0.31 J/kg/m and 0.38 J/kg/m, respectively, both p < 0.001). Note that the pooled mean difference of NCoW was 23% higher than that of GCoW; this indicates a difference in RMR between YA and OA, which has been reported before in the literature11,12. The 95% CI estimates the uncertainty of capturing the mean difference. By taking into account the heterogeneity across studies, the 95% PI estimates the interval in which an effect size from a future study assessing the same outcome will lie28.
Overview of the results of the meta-analysis using a random effects model on the differences in Net Metabolic Cost of Walking between YA and OA. Symbols and conventions are the same as in Fig. 3. This meta-analysis shows a significant overall increase in NCoW of OA over YA (p < 0.001). Note again that the p-value mentioned in the forest plot is the p-value for Cochran’s Q heterogeneity test.
In order to assess the magnitude of the (pooled) effect size, we calculated the pooled standardized mean difference (Hedge’s G), which was 0.65 for GCoW and 1.00 for NCoW. Quantitatively, according to general guidelines29, the effect size was in the range of medium to large for GCoW and large for NCoW.
Validation and sensitivity tests
In order to assess the validity of our a-priori assumption of heterogeneity, we performed three standard statistical tests for heterogeneity. Firstly, we computed Cochran’s Q, which is the weighted sum of squared differences between the individual study effects and the pooled effect across studies. Cochran’s Q was 55.7 (p < 0.001) and 53.2 (p < 0.001) for GCoW and NCoW, respectively, indicating a statistically significant heterogeneity. Since Cochran’s Q test has limited power to detect heterogeneity when using a small number of studies30, we secondly calculated I2, which is a measure of variation in the pooled effect size proportional to the total amount of variance and is independent of the number of studies. The calculated values of I2 were 78% and 68% for respectively GCoW and NCoW (0% meaning complete homogeneity). Even though there are no known concrete thresholds of I2, these values indicate substantial heterogeneity (see30). Thirdly, we calculated tau2 values, which show the variance among the studies; these were 0.043 (with Standard error = 0.028) for GCoW and 0.013 (with Standard error = 0.01) for NCoW (a tau2 value of greater than one indicates a meta-analysis should not be performed on the selected studies). The non-zero values of tau2 again indicate that there was heterogeneity across studies. The overall results support our choice of using a random effects model for our meta-analyses.
Finally, we performed sensitivity analyses of the meta-analyses using the Leave-One-Out method, which involves repeating the meta-analyses leaving out one of the studies in turn (see Figs 5 and 6). Each red diamond in Figs 5 and 6 represents the estimated pooled mean difference (middle of diamond) and 95% CI (the width of the diamond) while leaving out the indicated study from the meta-analyses. The red arrow indicates the 95% PI. These figures show that leaving out any one of the studies would not have affected the statistical significance of the overall results of the meta-analyses (p < 0.001 for both GCoW and NCoW). These figures furthermore show that the choice of definition of OA group did not influence our conclusions; by leaving out23, the only study which defined OA to be below 65 years old (being >59), pooled MD only marginally changed. Note, however, that the 95% PI for leaving out8 in the meta-analysis for GCoW does change; the interval does not span zero. This is because this study found a higher mean value for YA than OA.
Leave-one-out plot of the meta-analysis on GCoW. The blue diamond now represents the pooled MD with its 95% CI, and the red diamonds represent the pooled MD along with its 95% CI when leaving out the study indicated. The arrows represent the 95% PI for the overall pooled MD. This figure shows that leaving out any of the studies does not cause the 95% CI to span zero for any of the red diamonds. Thus, the statistical significance of the elevation of GCoW in OA is not affected by leaving out any one of the studies.
Leave-one-out plot of the meta-analysis on NCoW. Symbols and conventions are the same as in Fig. 5. As with GCoW, the statistical significance of the elevation of NCoW in OA is not affected by leaving out any one of the studies.
Risk of bias assessment
The risk of bias assessment was done using the AXIS tool as mentioned in the Methods section. To summarize, none of the studies reported an a-priori statistical power calculation and few of the studies had a possible selection bias in recruiting human subjects for the experiments. Overall, the studies were found to be of good quality. For a detailed description of the assessment please see Appendix B.
Discussion
We set out to report and pictorially represent MCoW in healthy YA and OA and to investigate the quantitative effect of age on MCoW through a literature review and meta-analyses. We extracted all data on MCoW in healthy YA and healthy OA and calculated, if not already reported, GCoW and NCoW in units of J/kg/m. If studies reported MCoW measured at multiple speeds, we selected the speed and MCoW values for YA and OA at which MCoW was minimal. An analysis of the compilation of GCoW and NCoW values from the literature as a function of age (see Fig. 2) showed a statistically significant elevation for OA over YA. To calculate the pooled effect size of MCoW between YA and OA, we performed two meta-analyses on the selected studies that directly compared YA and OA. In terms of GCoW, OA expended about 0.31 J/kg/m more than YA at similar walking speeds (walking speeds included in the meta-analyses were not significantly different between OA and YA). In terms of NCoW, OA expended about 0.38 J/kg/m more than YA. There was (~23%) more elevation for NCoW than GCoW for OA over YA. These results were found to be robust against choices made in statistical models and parameters. Figures 5 and 6 furthermore showed that leaving out any of the studies would not have affected the statistical significance of the overall results of the meta-analyses (in terms of pooled MD and 95% CI). These figures also show that the choice of definition of OA group did not influence our conclusions; even leaving out23, the only study in the meta-analyses that had OA below 65 years of age, only marginally changed the results. Although it is difficult to directly assess the clinical relevance from statistical results, the standardized effect sizes were medium to large, indicating that these differences are indeed clinically relevant31.
For our analyses, we have expressed MCoW in terms of both GCoW and NCoW and in units of J/kg/m. Since not all studies used in our analyses reported the variables of interest, or reported them in different units, we attempted to determine these from the other variables that were reported. In general, as stated in the Methods, calculating means is straightforward. However, calculating standard deviations is not possible unless the individual sample data are known, which were typically not reported. Since standard deviations are required to perform statistical tests we had to resort to estimating them. We estimated standard deviations conservatively (see: Calculations of MCoW in Methods and in Appendix A), which decreased the likelihood of finding a statistically significant elevation. Nevertheless, our meta-analyses showed a significant overall elevation in both GCoW and NCoW of OA over YA and thus our results were not critically influenced by the estimation of the standard deviations.
In our analyses, we combined studies that measured MCoW at different walking speeds (e.g. at a range of fixed values versus self-selected/preferred walking speed). As stated before, when several fixed speeds were used in a particular study, we selected the speed accompanied by the lowest MCoW. Typically, PWS is measured in advance of the actual experiment and this speed is then also fixed during the experiment itself. Importantly, before conducting the analyses on MCoW we analyzed group differences in walking speed and showed that the preferred walking speeds were not statistically significantly different, and in fact were very similar. All in all, we can say that it was safe to combine ‘self-paced’ and ‘fixed-speed’ conditions studies.
A general concern with meta-analyses is the risk of positive publication bias32, also known as the ‘file-drawer’ phenomenon. The general way of assessing a positive publication bias, is to make use of a Funnel plot and to test for asymmetry. In a Funnel plot, standard error is shown against mean difference (effect size). Based on funnel plots, there is no reason to assume the presence of positive publication bias for GCoW (see: Appendix C for details). For NCoW this is less clear as we found a statistically significant asymmetry in the Funnel plot, which can be caused by publication bias. However, the majority of the papers in the Funnel plot for NCoW are also in the Funnel plot for GCoW. It is therefore less likely, albeit not impossible, that a positive selection bias is present for NCoW only. In addition, several other factors also introduce asymmetry (e.g. heterogeneity within and among the selected studies and differences in methodologies of studies performed33). Unfortunately, there is no decisive way to test for the presence of positive publication bias.
Having established that there is a difference in MCoW between YA and OA, a question arises whether the elevated MCoW in healthy OA is a direct effect of age, or is due to age-related confounders? There is indeed some evidence in the literature that points to a possible effect of age on MCoW. Malatesta et al.6 measured MCoW in ten YA and two groups of OA (ten subjects from 60–69 years old and ten subjects from 77–86 years old) and found that for all the five speeds measured, significant differences in MCoW were found between YA and the oldest OA group, and only for the two highest speeds between YA and youngest OA group. In addition, in a large study, Gaesser et al.8 measured MCoW in 96 healthy YA and in 94 healthy OA that were divided into three age sub-groups: 31 subjects from 60–64 years old, 34 from 65–69 years old and 29 from >69 years old. As stated before, they found no statistically significant differences in GCoW between YA and any of OA groups (actually, the mean GCoW in YA was higher). However, for NCoW they reported a significantly elevated cost only for the oldest group (>69 years old, p = 0.04). Be that as it may, such an age related influence does not explain the contradictory results of Gaesser et al.8 with our meta-analyses. Since we have grouped age ≥59 as OA, our mean difference should be, if anything, lower than the mean difference between YA and the oldest OA group from8, which is clearly not the case. Thus, based on the literature, it cannot be determined whether the elevation found in our study is directly caused by age, or whether age-related confounders play a role.
A possible confounder is that OA need more familiarization time than YA in experimental settings. For example, it has been shown that OA need substantially more time than YA to find their natural gait on a treadmill34. Wass et al.35 showed that OA require at least 15 minutes of familiarization time on a treadmill to approximate natural over-ground gait kinematics for a single walking speed at level grade of treadmill. Most of the studies in our meta-analyses were conducted on a treadmill and, unfortunately, often did not provide details about the familiarization time, rendering a correlational analysis between familiarization time and MCoW impossible. Based on reported total familiarization time across conditions, the average familiarization time per walking condition ranged from 1.2 minutes e.g.36 to 10 minutes e.g.12 which may not have been enough to arrive at normal gait and MCoW34. Gaesser et al.8 performed their study on a treadmill at a fixed speed (1.34 m/s) and did not find an elevation of MCoW in OA over YA. Interestingly, in that study though no details on the familiarization protocol were given in the article, but ample familiarization time was provided (personal communication). To conclude, at this point it is unclear if treadmill walking in combination with insufficient familiarization time is a confounding factor in the elevation in MCoW in OA.
If insufficient familiarization time in treadmill experiments is the true reason for elevation in MCoW of OA over YA, one would expect that the elevation in MCoW would not be present when measured during normal over-ground walking. In the two studies in our meta-analyses that measured MCoW during over-ground walking37,38, MCoW was elevated in OA over YA, just as in the treadmill studies; (first two studies listed in Fig. 4). However, the preferred walking speed of OA group was significantly lower than that of YA group, which, as suggested by the authors, was the likely cause of the found elevation in MCoW of OA over YA.
Other possible confounders are differences in fitness level, activity level and anthropometrics. It is quite possible that the normal daily physical activity level differs between YA and OA and that this may influence the elevation in MCoW found in OA. Unfortunately, details about the daily physical activity level of participants are often not reported; typically the articles included in the meta-analyses report only that their subjects are all “fit and healthy” for both YA and OA. In addition, the body mass and body composition may also confound the results. Some of the studies in our meta-analyses statistically analyzed the differences in body mass and Body Mass Index (BMI), and found statistical differences between YA and OA. In our analyses, we used reported mass-normalized values of MCoW for both YA and OA, assuming that all participants have the same proportion of muscle mass to body mass, which may thus well not be the case and may influence the found results (see39) of our study. Since the studies analyzed here did not report individual data, we were not able to control for the effect of body composition on MCoW using an Analysis of Covariance (ANCOVA)39.
To conclude, our analyses show a robust and statistically significant elevation in MCoW of OA over YA when walking at comparable speeds. However, it cannot be determined on the basis of the literature whether this elevation is caused directly by age or is caused by age-related confounders. This merits further research comparing MCoW in healthy, active and anthropometrically matched OA and YA during both “natural” over-ground walking and treadmill walking at fixed walking speeds with sufficient familiarization time.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
McArdle, W. D., Katch, F. I. & Katch, V. L. Exercise Physiology. Philadelphia, PA: Lea & Febiger (1986).
Donelan, J. M., Kram, R. & Kuo, A. D. Mechanical and metabolic determinants of the preferred step width in human walking. Proc. R. Soc. B Biol. Sci. 268, 1985–1992 (2001).
Bertram, J. E. A. & Ruina, A. Multiple walking speed-frequency relations are predicted by constrained optimization. J. Theor. Biol. 209, 445–453 (2001).
Zarrugh, M. Y., Todd, F. N. & Ralston, H. J. Optimization of energy expenditure during level walking. Eur. J. Appl. Physiol. Occup. Physiol. 33, 293–306 (1974).
Hortobagyi, T., Finch, A., Solnik, S., Rider, P. & DeVita, P. Association between muscle activation and metabolic cost of walking in young and old adults. J Gerontol A Biol Sci Med Sci 66, 541–547 (2011).
Malatesta, D. et al. Energy cost of walking and gait instability in healthy 65- and 80-yr-olds. J. Appl. Physiol. 95, 2248–2256 (2003).
Mian, O. S., Thom, J. M., Ardigo, L. P., Narici, M. V. & Minetti, A. E. Metabolic cost, mechanical work, and efficiency during walking in young and older men. Acta Physiol. 186, 127–139 (2006).
Gaesser, G. A., Tucker, W. J., Sawyer, B. J., Bhammar, D. M. & Angadi, S. S. Cycling efficiency and energy cost of walking in young and older adults. J. Appl. Physiol. 124, 414–420 (2018).
Horiuchi, M., Endo, J., Horiuchi, Y. & Abe, D. Comparisons of energy cost and economical walking speed at various gradients in healthy, active younger and older adults. J. Exerc. Sci. Fit. 13, 79–85 (2015).
Shimada, H. et al. Relationship between whole body oxygen consumption and skeletal muscle glucose metabolism during walking in older adults: FDG PET study. Aging Clin Exp Res 23, 175–182 (2011).
Peterson, D. S. & Martin, P. E. Effects of age and walking speed on coactivation and cost of walking in healthy adults. Gait Posture 31, 355–359 (2010).
Ortega, J. D. & Farley, C. T. Effects of aging on mechanical efficiency and muscle activation during level and uphill walking. J. Electromyogr. Kinesiol. 25, 193–198 (2015).
Ortega, J. D. & Farley, C. T. Individual limb work does not explain the greater metabolic cost of walking in elderly adults. J. Appl. Physiol. 102, 2266–2273 (2007).
Stoquart, G., Detrembleur, C. & Lejeune, T. Effect of speed on kinematic, kinetic, electromyographic and energetic reference values during treadmill walking. Neurophysiol. Clin. 38, 105–116 (2008).
Davies, M. J. & Dalsky, G. P. Economy of mobility in older adults. J. Orthop. Sports Phys. Ther. 26, 69–72 (1997).
Ralston, H. J. Comparison of energy expenditure during treadmill walking and floor walking. J. Appl. Physiol. 15, 1156 (1960).
Waters, R. L. & Mulroy, S. The energy expenditure of normal and pathologic gait. Gait Posture 9, 207–231 (1999).
Ortega, J. D., Fehlman, L. A. & Farley, C. T. Effects of aging and arm swing on the metabolic cost of stability in human walking. J. Biomech. 41, 3303–3308 (2008).
Aboutorabi, A., Arazpour, M., Bahramizadeh, M., Hutchins, S. W. & Fadayevatan, R. The effect of aging on gait parameters in able-bodied older subjects: a literature review. Aging Clin. Exp. Res. 28, 393–405 (2016).
Thomas, E. E., De Vito, G. & Macaluso, A. Physiological costs and temporo-spatial parameters of walking on a treadmill vary with body weight unloading and speedin both healthy young and older women. Eur. J. Appl. Physiol. 100, 293–299 (2007).
Pearce, M. E. et al. Energy cost of treadmill and floor walking at self-selected paces. Eur. J. Appl. Physiol. Occup. Physiol. 52, 115–9 (1983).
Liberati, A. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339 (2009).
Jones, L. M., Waters, D. L. & Legge, M. Walking speed at self-selected exercise pace is lower but energy cost higher in older versus younger women. J Phys Act Heal. 6, 327–332 (2009).
Weyand, P. G., Smith, B. R. & Sandell, R. F. Assessing the metabolic cost of walking: the influence of baseline subtractions. 31st Annual International Conference of the IEEE EMBS Minneapolis, Minnesota, USA, September 2–6, 6878–6881 (2009).
Mathes, T. & Kuss, O. A comparison of methods for meta-analysis of a small number of studies with binary outcomes. Res. Synth. Methods 9, 366–381 (2018).
Downes, M. J., Brennan, M. L., Williams, H. C. & Dean, R. S. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ Open 6, 1–7 (2016).
Cumming G. Understanding the New Statistics: Effect Sizes, Confidence Intervals, and Meta-analysis. Routledge (2012).
Borenstein, M., Hedges, L. V., Higgins, J. P. T. & Rothstein, H. R. Introduction to Meta-Analysis. (Chichester (UK): Wiley, 2009).
Cohen, J. Statistical Power Analysis for the Behavioral Sciences. (NY: Academic Press, 1969).
Higgins, J. P. T., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. BMJ Br. Med. J. 327, 557–560 (2003).
Page, P. Beyond statistical significance: clinical interpretation of rehabilitation research literature. Int. J. Sports Phys. Ther. 9, 726–36 (2014).
Begg, C. B. & Berlin, J. A. Publication Bias: A Problem in Interpreting Medical Data. Wiley for the Royal Statistical Society Stable 151, 419–463 (2016).
Egger, M., Davey Smith, G., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–34 (1997).
Schellenbach, M., Lövdén, M., Verrel, J., Krüger, A. & Lindenberger, U. Adult age differences in familiarization to treadmill walking within virtual environments. Gait Posture 31, 295–299 (2010).
Wass, E., Taylor, N. F. & Matsas, A. Familiarisation to treadmill walking in unimpaired older people. Gait Posture 21, 72–79 (2005).
Pincheira, P. A., Stenroth, L., Avela, J. & Cronin, N. J. Individual Leg Muscle Contributions to the Cost of Walking: Effects of Age and Walking Speed. J. Aging Phys. Act. 25, 295–304 (2017).
Waters, R. L., Hislop, H. J., Perry, J., Thomas, L. & Campbell, J. Comparative cost of walking in young and old adults. J. Orthop. Res. 1, 73–6 (1983).
Waters, R. L., Lunsford, B. R., Perry, J. & Byrd, R. Energy-speed relationship of walking: Standard tables. J. Orthop. Res. 6, 215–222 (1988).
Packard, G. C. & Boardman, T. J. The use of percentages and size-specific indices to normalize physiological data for variation in body size: Wasted time, wasted effort? Comp. Biochem. Physiol. - A Mol. Integr. Physiol. 122, 37–44 (1999).
Martin, P. E., Rothstein, D. E. & Larish, D. D. Effects of age and physical activity status on the speed-aerobic demand relationship of walking. J. Appl. Physiol. 73, 200–206 (1992).
Weyand, P. G., Smith, B. R., Puyau, M. R. & Butte, N. F. The mass-specific energy cost of human walking is set by stature. J. Exp. Biol. 213, 3972–3979 (2010).
Silder, A., Besier, T. & Delp, S. L. Predicting the metabolic cost of incline walking from muscle activity and walking mechanics. J. Biomech. 45, 1842–1849 (2012).
Ortega, J. D. Minimizing center of mass vertical movement increases metabolic cost in walking. J. Appl. Physiol. 99, 2099–2107 (2005).
Valenti, G., Bonomi, A. G. & Westerterp, K. R. Body acceleration as indicator for walking economy in an ageing population. PLoS One 10, 1–10 (2015).
Vannetti, F., Pasquini, G., Vitiello, N. & Molino-Lova, R. Effects of lower limb length and body proportions on the energy cost of overground walking in older persons. ScientificWorldJournal. 2014, 318204 (2014).
Ortega, J. D., Beck, O. N., Roby, J. M., Turney, A. L. & Kram, R. Running for exercise mitigates age-related deterioration of walking economy. PLoS One 9 (2014).
Schuch, C. P., Balbinot, G., Boos, M., Peyré-Tartaruga, L. A. & Susta, D. The role of anthropometric changes due to aging on human walking: Mechanical work, pendulum and efficiency. Biol. Sport 28, 165–170 (2011).
Malatesta, D. et al. Effect of an overground walking training on gait performance in healthy 65- to 80-year-olds. Exp. Gerontol. 45, 427–434 (2010).
Fiser, W. M. et al. Energetics of walking in elderly people: factors related to gait speed. J Gerontol A Biol Sci Med Sci 65, 1332–1337 (2010).
Terracciano, A. et al. Personality, Metabolic Rate and Aerobic Capacity. PLoS One 8, e54746 (2013).
Thomas, E. E., De Vito, G. & Macaluso, A. Speed training with body weight unloading improves walking energy cost and maximal speed in 75- to 85-year-old healthy women. J. Appl. Physiol. 103, 1598–603 (2007).
Mian, O. S. et al. Effect of a 12-month physical conditioning programme on the metabolic cost of walking in healthy older adults. Eur. J. Appl. Physiol. 100, 499–505 (2007).
Dean, J. C., Alexander, N. B. & Kuo, A. D. The Effect of Lateral Stabilization on Walking in Young and Old Adults. IEEE Trans. Biomed. Eng. 54, 1919–1926 (2007).
Haveman-Nies, A., Van Iperen, C. & Deurenberg, P. Energy expenditure at rest and during activities: A comparison between young and elderly women. Am. J. Hum. Biol. 8, 383–388 (1996).
McCann, D. J. & Adams, W. C. A dimensional paradigm for identifying the size-independent cost of walking. Paradigme dimensionnel pour l’ identification de la depense energetique produite par la locomotion, independamment de la taille. Med. Sci. Sport. Exerc. 34, 1009–1017 (2002).
Floreani, M. et al. Effects of 14 days of bed rest and following physical training on metabolic cost, mechanical work, and efficiency during walking in older and young healthy males. PLoS One 13, e0194291 (2018).
Acknowledgements
The authors would like to thank Drs Ralph De Vries of the Vrije Universiteit Amsterdam University Library for his help and contribution to the literature search for this article. This is an independent study funded by the European Commission under the MOVE-AGE Erasmus Mundus Joint Doctorate Scheme (Grant No. 2015-1614) and carried out at the Vrije Universiteit, Amsterdam. D.A. Kistemaker was supported by European Union (EU) Horizon 2020 Program H2020-MSCA-IF-665457.
Author information
Authors and Affiliations
Contributions
S.D.G. – Performed the literature search, did the data analysis, drafted the manuscript and revised it. S.D.G. & D.A.K. – Made the figures, drafted and revised the manuscript. S.D.G., M.F.B. & D.A.K. – Conceptualized and designed the study, was involved in the manuscript development and revision.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Das Gupta, S., Bobbert, M.F. & Kistemaker, D.A. The Metabolic Cost of Walking in healthy young and older adults – A Systematic Review and Meta Analysis. Sci Rep 9, 9956 (2019). https://doi.org/10.1038/s41598-019-45602-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-45602-4
This article is cited by
-
Optimizing exoskeleton assistance to improve walking speed and energy economy for older adults
Journal of NeuroEngineering and Rehabilitation (2024)
-
The elevated metabolic cost of walking at preferred speeds of healthy elderly on treadmills compared to overground is not related to increased self-reported anxiety
European Journal of Applied Physiology (2023)
-
Can humans perceive the metabolic benefit provided by augmentative exoskeletons?
Journal of NeuroEngineering and Rehabilitation (2022)
-
How fiber dynamics of plantarflexor and dorsiflexor muscles based on EMG-driven approach can explain the metabolic cost at different gait speeds
European Journal of Applied Physiology (2022)
-
Walking cadence (steps/min) and intensity in 61–85-year-old adults: the CADENCE-Adults study
International Journal of Behavioral Nutrition and Physical Activity (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.