Abstract
Landscape management affects species interactions and can have notable effects on food web structure. Local parasitoid populations in greenhouses usually migrate from outside crops; biological control of greenhouse aphids may be thus highly dependent on the composition of surrounding landscape. However, it is less clear how surrounding landscape composition affects primary-hyperparasitoid food webs and pest control services in greenhouses. We investigated the food web of parasitoids on melon-cotton aphid (Aphis gossypii Glover) in watermelon greenhouses in two suburban Beijing counties over two years. We used the quantitative food web metrics (generality, vulnerability, link density, and interaction evenness) to assess the effects of landscape composition on primary-hyperparasitoid food web structure. We found that landscape with more cropland within 1–3 km tended to have more primary parasitoids per hyperparasitoid species (generality). Higher proportions of woodland at the 0.5 km scale were negatively correlated with the mean numbers of hyperparasitoid per primary parasitoid species (vulnerability), as well as with hyperparasitism rate and hyperparasitoid richness. Link density, interaction evenness and aphid mortality caused by parasitoids (parasitism rate) were not affected by landscape factors. However, active primary parasitism (biocontrol potential) increased with the proportion of woodland. This suggested that the bottom-up effect induced by primary parasitoids might benefit hyperparasitoids, thus exerting little influence of primary parasitoids on pest control. The top-down effect of hyperparasitoids may reduce with increasing woodland proportion. To enhance the effects of primary parasitoids, landscape management programs should also target, and thus limit the impact of hyperparasitoids.
Similar content being viewed by others
Introduction
In landscape ecology and biological pest control, the effects of landscape composition on species interactions and ecological functions are important topics1,2,3. Empirical work demonstrates that changes in landscape structure can alter host-parasitoid dynamics, affecting populations at a large scale4. Food web, which depicts network of trophic relationships in ecological community, has become a major concern in many studies. The diversity and complexity of food webs are considered to be important factors determining ecosystem function and stability5. The quantitative food web metrics can give a robust description of community structure, providing insights into the dynamic processes that structure ecological communities6. In agricultural landscape, agricultural intensification and the loss of semi-natural habitats can reduce biodiversity and associated biological control services7,8,9. Empirical study on the food-webs of cereal aphids across a landscape complexity gradient has shown that aphid-parasitoid food webs are less complex, with higher rates of parasitism in complex landscapes10. Understanding of food web structures and their responses to environmental factors can have implication for population management of pest and natural enemy11,12.
In most studies with parasitoids of agricultural pests, parasitoid species richness and/or parasitism rates in the crop habitat increases with landscape complexity (13,14,15,16, but see17,18). The percentage of arable land (or non-crop habitat) in a landscape is commonly used to indicate the landscape complexity15,19. Natural enemies in crop fields often benefit from the presence of non-crop habitats in landscapes, as these provide key resources such as alternative hosts, food resources, shelter and/or overwintering habitats20,21. Hyperparasitoids should be considered as a new target in biological control22. They disrupt biological control of aphids mediated by primary parasitoids23,24, and also may take advantage of non-crop habitats in a complex landscape15,22. That makes the effects of landscape context on biological control efficiency uncertain25,26. It is suggested that community and interaction structures might not be detected in studies that focus simply on species richness and abundance27. Thus assessing the landscape effects on community level is necessary. Food web interactions have previously been studied for aphid–parasitoid systems in the open field landscape context (e.g.10), but aphid-parasitoid interactions in greenhouses have not been explicitly explored, especially with respect to hyperparasitoid ecology.
As greenhouse crops are grown under controlled condition, greenhouse ecosystems are often very simple with low biodiversity. Stable environmental conditions are apparently conducive to some pests and diseases28. The general experience is that infestation by several small pest species cannot be avoided29. Greenhouses have very diversified structures, including sheet glass, fiberglass or plastic. Compared to glasshouses which are rather closed units, plastic greenhouses are more openly structured creating a constant interchange of pests and beneficials between the greenhouse crops and the neighboring outdoor crops and weeds30. Plastic greenhouses are common in temperate zones, e.g. China. Polyphagous pests which can feed on many crop plants and wild plants quickly become prevalent in plastic greenhouses. A polyphagous aphid, Aphis gossypii Glover, damages cucurbits and hundreds of other crops31. Therefore, it can move from surrounding plants to the greenhouse crops. Previous study showed that A. gossypii could colonize several different hosts successively with seasonal change32. The immigration of aphids into the greenhouse usually causes sudden and largely unpredictable density increases. The parasitoids can also move from surroundings to greenhouses, so the parasitoids in greenhouses have a certain degree of species diversity33. Therefore, biological control programs should consider not only releasing natural enemies in the greenhouses, but also attempting to conserve local populations in the surrounding areas28.
Little is known about how surrounding landscape composition affects food webs of (hyper)parasitoids in greenhouses. To address this knowledge gap, we investigated the primary parasitoids and hyperparasitoids of melon-cotton aphid (A. gossypii) in watermelon greenhouses located on the suburban plain of Beijing, China. A. gossypii is the only aphid species infesting watermelon in the Beijing area32,34. In this area, the management of A. gossypii under greenhouses conditions is based on chemical control. Nowadays, several biological control programs are under development and these include the identification of potential natural enemies. We calculated the food web metrics of primary-hyperparasitoid communities to test the following hypotheses: (i) the migration of parasitoids from surrounding croplands into greenhouses increases the food web complexity; (ii) landscape composition affects the primary parasitoids and hyperparasitoids differently; (iii) aphid control efficiency is related to food web structure.
Materials and Methods
Study sites and sampling
Sampling was conducted in Daxing and Shunyi counties, suburban Beijing, northern China. These two counties, which are 70 km apart, are located on plains that consist primarily of cultivated fields. There were six different sampling sites in Daxing and five in Shunyi: each site was in a different village. In general, sites were >3.0 km apart; one pair of sites was 1.2 km apart. Sampling sites varied slightly between 2015 (four in Daxing and five in Shunyi) and 2016 (five in Daxing and four in Shunyi). At each site, we sampled three to five neighboring watermelon greenhouses in the center of the landscape. Plastic greenhouses prevailed in our study area. These relatively open structures allowed pests and natural enemies to move between greenhouse crops and field crops.
At each site, we collected mummified aphids from as many watermelon plants as possible. Sampling was conducted twice between May and June of 2015, and three times between May and June of 2016. During each sampling event, we collect leaves with aphids and mummified aphids for 1 h per site. The GPS coordinates of each site were recorded using a Magellan handheld GPS receiver, a latitude-longitude projection. Sampling locations were confirmed by consulting topographical maps of the study area and Google Earth.
After collection, watermelon leaves were transported to our laboratory. Leaves were placed in fine-mesh cages and maintained at 25 ± 1 °C, with 70% relative humidity and a 14:10 light:dark photoperiod. Leaves were checked daily for parasitoid emergence. All of the mummies were removed from leaves and kept in gelatine capsules for the later identification of emerging adult parasitoids. As the different genera of primary parasitoids can be distinguished by mummy characters35, it was possible to identify the relationships between primary and hyperparasitoids. Emerging parasitoids were promptly placed in 95% ethanol and stored at −20 °C. Specimens were identified to species by comparison with materials that had been previously authoritatively identified and with the aid of taxonomic keys36,37,38,39. Morphological identification of specimens was performed by the Laboratory of Zoological Systematics and Evolution (IZCAS: Institute of Zoology, Chinese Academy of Sciences, Beijing). We used DNA barcoding to confirm morphological identifications33. We used the parasitism rate to reflect parasitoid-associated aphid mortality rate which was calculated as proportion of all parasitoids from all aphids (including mummies). In addition, we used the active primary parasitism to reflect the biocontrol potential which was calculated as the ratio of primary parasitoids (nonparasite) to all aphids. Hyperparasitism rates were calculated as the proportion of hyperparasitoid mummies from all mummies (including primary and hyperparasitoids).
Quantitative food web structure
We pooled the data by site in each year, and analyzed the quantitative structures of primary-hyperparasitoid food web for each site. We calculated four food web metrics namely generality, vulnerability, link density and interaction evenness (for detailed formulae see12,40. Generality is the weighted mean number of host species (primary parasitoid) per consumer species (hyperparasitoid). Vulnerability is the weighted mean number of consumers (hyperparasitoid) per host species (primary parasitoid). Link density is the weighted mean number of links per specie which represents the ratio of the number of trophic interactions to the number of species. Interaction evenness are analogous to Shannon evenness, but with trophic interaction instead of species as the base unit41. These metrics are often used as measures of food web complexity.
Landscape analysis
Land cover maps of sampling sites were obtained from a digital map provided by the ZiYuan-3 survey satellite (ZY-3) with 5-m resolution. Landscape structure was analyzed using ArcGIS 10 (ESRI, Redlands, CA, USA). Land covers were classified into seven general categories, including: cropland, vegetable, orchard, grassland, woodland, water, and urban. Cropland was mainly in cultivation with crops such as winter wheat and soybean. Vegetable contained protected fields, e.g. greenhouses in which vegetable were usually planted. The orchard had perennial crops, such as orchard trees. The grassland was grass dominated land. Woodland included all forest and shrub landing excluding the trees in residential sites. Water represented the wetland and open water. Urban represented human architecture and roads etc. For each of the sampling sites, landscape composition was estimated in four circular sectors (0.5, 1, 2 and 3 km radii), representing a nested set of landscape sectors at four spatial scales. The percentage of each category was measured within each landscape circle. At the 0.5 km scale, percentage cropland was negatively correlated with percentage woodland and percentage vegetable, while at 1 km scale, percentage cropland was only negatively correlated with percentage vegetable. At 2 km and 3 km scales, percentage cropland was strongly positively correlated with percentage grassland (see supplementary material Table S2).
Statistical analysis
All analyses were performed in the statistical program R version 3.4.342. We firstly analyzed the influence of year and county on each food web metrics, richness of primary parasitoid, richness of hyperparasitoid and (hyper)parasitism rates by using one-way analysis of covariance (ANCOVA). Then we analyzed the landscape effects on food web metrics as well as richness of primary parasitoid, richness of hyperparasitoid and (hyper)parasitism rates using linear mixed-effect models with county and year as random effects (package “lme4”43). Arcsine-square-root transformations were used for parasitism data to meet assumptions of the approach. Explanatory landscape variables in the full models were percentages of cropland, vegetable, orchard, grassland and woodland. To avoid the multicolinearity, we selected the landscape variables with a restriction that models with one landscape variable did not include the other correlated landscape variables. The analyses were conducted at each spatial scale separately. We performed an automated model selection (dredge function) based on Akaike’s information criterion for small sample sizes (AICc), and ranked alternative models using model probabilities known as Akaike weights (wi) (package “MuMIn”44). If one of the models was strongly supported (i.e., wi > 0.90), we based inferences on that model. However, in cases where no single model was superior, coefficients for each predictor variable were derived from the competing models using a model averaging procedure45. To test for spatial autocorrelation, we computed Moran’s I test for the residuals of the best-fitting models for each response variable (package “spdep”46,47). The analysis revealed no spatial autocorrelation. We used the packages ‘bipartite’48 for plotting food web structures.
Results
Effect of year and county
In total, 3911 parasitoids were recorded across all of the sites, of which 84.7% were primary parasitoids, and 15.3% hyperparasitoids. The food web metrics: generality, vulnerability and interaction evenness varied noticeably between the two years, but were similar between counties. In contrast, link density was significantly affected by county but not by year (Table 1). Primary parasitoid richness and hyperparasitism rate varied significantly between years, while the aphid mortality, active primary parasitism and hyperparasitoid richness were not affected by year or county (Table 1).
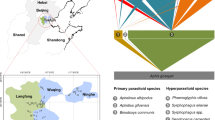
The quantified food webs of each year were pooled separately and are depicted in Fig. 1 (see supplementary material Table S1 for species names and abundances). The dominant primary parasitoid was Binodoxys communis (Gahan) (Fig. 1; code 3). The dominant hyperparasitoid species were Pachyneuron aphidis (Bouche) and Syrphophagus aphidivorus (Mayr) (Fig. 1; code 12 and code 13). The quantitative food webs (Fig. 1) showed that the remarkable changes occurred between years. Food webs in 2015 were dominated by two trophic interactions (two hyper parasitoids P. aphidis and S. aphidivorus parasitizing B. communis; code 12, 13 and 3 in Fig. 1). Food webs in 2016 were dominated by a single trophic interaction (a hyper parasitoid S. aphidivorus parasitizing B. communis; code 13 and 3 in Fig. 1).
Quantitative host–parasitoid food webs for the year 2015 (a) and year 2016 (b). Lower bars represent host abundance (primary parasitoids); upper bars represent hyperparasitoid abundance (drawn at different scales). The width at the basis of the wedges reflects the pooled frequency of each host-parasitoid interaction. Species codes and abundances are given in Supplementary Table S1.
Landscape effects
Generality increased with percentage cropland in both years (Fig. 2a, Table 2). Multiple comparisons of models for generality evaluated at different radii (0.5, 1, 2, 3 km) demonstrated a similar result (see supplementary materials Tables S3 and S4). Across all of the spatial scales, the AICc of the model at 2 km was lowest, indicating that this was the best fitting models for generality. Vulnerability decreased as percentage woodland increased, although this effect was only significant at 0.5 km (Fig. 2c, Table 2, see also supplementary materials Tables S3 and S4). Link density and interaction evenness were not affected by landscape composition because no landscape variables entered the best fitting models (Table 2).
Effect of cropland and woodland percentages on food web metrics: generality (a), vulnerability (c), hyperparasitism rate (b) and hyperparasitoid richness (d). Hyperparasitism rate was arcsine-square-root transformed for analysis. Red circles and lines show results from the year 2015, while blue triangle and lines represent the year 2016. Regression lines are shown for descriptive purposes (see text for statistical results).
Aphid mortality was not affected by landscape factors. Active primary parasitism increased with percentage woodland at 0.5 km, while hyperparasitism decreased with increasing percentage woodland at same scale (Fig. 2b, Table 2, see also supplementary materials Tables S5 and S6). Primary parasitoid richness decreased as percentage vegetable land increased at 1 km, while hyperparasitoid richness decreased as percentage woodland increased at 0.5 km (Fig. 2d, Table 2, see also supplementary materials Tables S5 and S6).
Aphid mortality was positively correlated with vulnerability (Pearson correlation r = 0.571, p = 0.013) but negatively correlated with generality (r = −0.556, p = 0.017). Active primary parasitism was negatively correlated with vulnerability (r = −0.701, p = 0.001), link density (r = −0.510, p = 0.031) and hyperparasitism (r = −0.751, p < 0.001). Hyperparasitism was positively correlated with vulnerability (r = 0.974, p < 0.001) but negatively correlated with both generality (r = −0.704, p = 0.001) and primary parasitoid richness (r = −0.536, p = 0.022).
Discussion
Here, we aimed in part to determine how the food web structures of parasitoid-hyperparasitoid in greenhouses responded to landscape composition. We found that cropland increased generality (i.e., the mean number of primary parasitoid per hyperparasitoid species). The positive effect of cropland on primary parasitoids has two possible explanations. First, parasitoids might benefit from the increased availability of host resources. Parasitoids are quite closely linked with aphids based on host-parasitoid relation and aphid honeydew as food source18. Crops (e.g. soybean) usually harbor many aphids, which act as host for primary parasitoids. Most of the primary parasitoids identified here, such as B. communis and Lysiphlebus fabarum, are generalist; the possible hosts of these parasitoids include soybean aphid, Aphis glycines Matsumura49 and A. gossypii. Second, additional cropland areas might increase the chances of parasitoids migration from fields to greenhouses. Increased dispersal among habitat patches facilitates food web complexity, dispersal may thus play a determinant role in food web structure50. Therefore, the surrounding cropland increased the complexity of parasitoid-hyperparasitoid food web. The higher generality in crop-dominant landscapes may therefore actually be a result of bottom-up effect from aphid-parasitoid interactions outside the greenhouses. Such pattern of trophic cascade is very common in host-parasitoid systems51.
Aphid control was not enhanced by increased cropland percentages. In fact, aphid mortality owing to parasitism was not affected by landscape factors, probably because hyperparasitoid activity offset the function of primary parasitoid assemblage. As the hyperparasitoids tended toward polyphagy with little host specificity, they also benefited by the increase in primary parasitoids. This effect was also indicated by the hyperparasitism rate, which was positively related to aphid mortality. Biocontrol potential (i.e., active primary parasitism), was negatively correlated with hyperparasitism and positively correlated with woodland percentages at 0.5 km scale. This suggested that hyperparasitoid-focused landscape management might be a promising strategy for the improvement of biological control measures.
Woodland areas were negatively correlated with vulnerability (i.e., the mean number of hyperparasitoid per primary parasitoid species), hyperparasitoid richness and hyperparasitism. Parasitoid species were on average attacked by a fewer hyperparasitoid species in landscapes with higher percentages of woodland, perhaps because hyperparasitoid species richness was also lower in these types of landscapes. These results suggested that woodland habitats provided little for hyperparasitoids in terms of alternative hosts and other resources. The negative relationship between hyperparasitoid and woodland was significant at a small scale (0.5 km radius), supporting the view that species losses are usually greatest in the smallest fragments52. As spatial scale increased, more potentially suitable habitats could be available, allowing hyperparasitoids populations to persist. High parasitoid diversity can promote high rates of parasitism53. Therefore, high rate of hyperparasitism was related to complex consumer communities (high vulnerability). In addition, hyperparasitism was negatively correlated with generality, indicating that bottom-up control is stronger than top-down control when complexity of host community increases. Lack of relationships between landscape features and parasitoid-hyperparasitoid interactions evenness (or link density) might be explained by countervailing effects of generality and vulnerability. Overall hyperparasitism rates were lowest in landscapes with the greatest percentages of woodland habitats at 0.5 km. This finding raises the question of species-area relationship for hyperparasitoids54. It suggests that the scale of 0.5 km radius may be an effective scale for habitat management aimed at reducing the hyperparasitoid diversity and hyperparasitism. The food web metrics respond to landscape composition at different spatial scales. Thus, studies at multiple scales are required for the analysis of food web metrics.
In greenhouses, methods that introduce non-pest hosts on non-crop plants to maintain parasitoid populations have been widely adopted55. Thus, alternative prey or food sources can promote natural enemy population growth prior to pest outbreaks and increase biocontrol services. Our study is the first to analyze the effect of surrounding landscape on the food web of parasitoids in greenhouses. Food web structure in greenhouses located in different types of landscapes can be expected to differ. Management of the surrounding habitats may be a promising strategy to enhance biological control in greenhouses. Recently, methods involving the establishment of native plants around greenhouses have been studied for natural enemy conservation in a greenhouse-dominated landscape56,57. More importantly, these plants should increase the number of natural enemies in the surroundings and facilitate the migration of natural enemies to the greenhouses when necessary.
The difference in primary parasitoid richness between 2015 and 2016 was one of the factors driving the changes in food-web structure. The variation in richness between years may primarily result from differences in sampling duration. In 2015, sampling was conducted at the watermelon fruit stage, corresponding to the peak and decline in aphid population. However, samples were collected earlier in 2016, during the watermelon flower stage. More primary parasitoid species were identified in 2016 than in 2015, indicating that primary parasitoid primarily occurred early in the growing season. Hyperparasitoids mainly act later in the season, namely in the period when aphid population densities break down owing to decreasing resource quality21. This discrepancy between the appearance points of primary and hyperparasitoids should be considered in future studies. More intensive sampling, with the same number of sampling events each year, is required to cover the entire aphid infestation period; this type of sampling program might yield more details about parasitoid dynamics. Although our approach suffered greatly from the limited number of samples we were able to obtain, our results provide a valuable framework and basis for future studies.
A complex host-parasitoid food web structure does not necessarily lead to improved biocontrol. On the contrary, top-down control in host-parasitoid systems appears to be strong in simplified food webs with only one or a few key stone species51,58. Here, we focused on the third and fourth levels of trophic interactions (i.e., primary parasitoid and hyperparasitoid) to characterize the link between food web structure and pest control service. Our result showed that hyperparasitism could be higher when host community was less complex (lower generality) and consumer community was more complex (higher vulnerability). This may be related to simple interaction structures between hyperparasitoids and the dominant primary parasitoid B. communis. In the landscapes with high cropland proportion, parasitoid communities are possibly under bottom-up control, and thus exert little or no influence on pest control. In the landscapes with high proportion of woodland, active primary parasitism (biocontrol potential) tended to increase, and both hyperparasitoid richness and hyperparasitism decreased. This suggested that the top-down force of primary parasitoid was increased when hyperparasitoid was limited. Thus, landscape management programs that aim to increase primary parasitoid should consider the side effects of increasing the hyperparasitism, as these effects may dampen biocontrol potential. It may be useful to use landscape management techniques to improve biocontrol by targeting, and thus limiting the impact of, hyperparasitoid.
References
Veres, A., Petit, S., Conord, C. & Lavigne, C. Does landscape composition affect pest abundance and their control by natural enemies? A review. Agric. Ecosyst. Environ. 166, 110–117, https://doi.org/10.1016/j.agee.2011.05.027 (2013).
Tscharntke, T. & Brandl, R. Plant-insect interactions in fragmented landscapes. Annu. Rev. Entomol. 49, 405–430, https://doi.org/10.1146/annurev.ento.49.061802.123339 (2004).
Thies, C. & Tscharntke, T. Landscape structure and biological control in agroecosystems. Science 285, 893, https://doi.org/10.1126/science.285.5429.893 (1999).
Roland, J. & Taylor, P. D. Insect parasitoid species respond to forest structure at different spatial scales. Nature 386, 710–713, https://doi.org/10.1038/386710a0 (1997).
Bukovinszky, T., van Veen, F. J. F., Jongema, Y. & Dicke, M. Direct and indirect effects of resource quality on food web structure. Science 319, 804–807, https://doi.org/10.1126/science.1148310 (2008).
Morris, R. J., Lewis, O. T. & Godfray, H. C. J. Experimental evidence for apparent competition in a tropical forest food web. Nature 428, 310, https://doi.org/10.1038/nature02394 (2004).
Bianchi, F. J., Booij, C. J. & Tscharntke, T. Sustainable pest regulation in agricultural landscapes: a review on landscape composition, biodiversity and natural pest control. Proc. R. Soc. B-Biol. Sci. 273, 1715–1727, https://doi.org/10.1098/rspb.2006.3530 (2006).
Benton, T. G., Vickery, J. A. & Wilson, J. D. Farmland biodiversity: is habitat heterogeneity the key? Trends Ecol. Evol. 18, 182–188, https://doi.org/10.1016/s0169-5347(03)00011-9 (2003).
Tscharntke, T., Klein, A. M., Kruess, A., Steffan-Dewenter, I. & Thies, C. Landscape perspectives on agricultural intensification and biodiversity-ecosystem service management. Ecol. Lett. 8, 857–874, https://doi.org/10.1111/j.1461-0248.2005.00782.x (2005).
Gagic, V. et al. Food web structure and biocontrol in a four-trophic level system across a landscape complexity gradient. Proc. R. Soc. B-Biol. Sci. 278, 2946–2953, https://doi.org/10.1098/rspb.2010.2645 (2011).
Kaartinen, R., Stone, G. N., Hearn, J., Lohse, K. & Roslin, T. Revealing secret liaisons: DNA barcoding changes our understanding of food webs. Ecol. Entomol. 35, 623–638, https://doi.org/10.1111/j.1365-2311.2010.01224.x (2010).
Tylianakis, J. M., Tscharntke, T. & Lewis, O. T. Habitat modification alters the structure of tropical host-parasitoid food webs. Nature 445, 202–205, https://doi.org/10.1038/Nature05429 (2007).
Thies, C., Steffan-Dewenter, I. & Tscharntke, T. Effects of landscape context on herbivory and parasitism at different spatial scales. Oikos 101, 18–25, https://doi.org/10.1034/j.1600-0706.2003.12567.x (2003).
Kruess, A. Effects of landscape structure and habitat type on a plant-herbivore-parasitoid community. Ecography 26, 283–290, https://doi.org/10.1034/j.1600-0587.2003.03402.x (2003).
Rand, T. A., van Veen, F. J. F. & Tscharntke, T. Landscape complexity differentially benefits generalized fourth, over specialized third, trophic level natural enemies. Ecography 35, 97–104, https://doi.org/10.1111/j.1600-0587.2011.07016.x (2012).
Zhao, Z.-H. et al. Responses of cereal aphids and their parasitic wasps to landscape complexity. J. Econ. Entomol. 107, 630–637, https://doi.org/10.1603/EC13054 (2014).
Menalled, F. D., Marino, P. C., Gage, S. H. & Landis, D. A. Does agricultural landscape structure affect parasitism and parasitoid diversity? Ecol. Appl. 9, 634–641, https://doi.org/10.2307/2641150 (1999).
Vollhardt, I. M. G., Tscharntke, T., Wackers, F. L., Bianchi, F. J. J. A. & Thies, C. Diversity of cereal aphid parasitoids in simple and complex landscapes. Agric. Ecosyst. Environ. 126, 289–292, https://doi.org/10.1016/j.agee.2008.01.024 (2008).
Zhao, Z.-H. et al. Effects of inter-annual landscape change on interactions between cereal aphids and their natural enemies. Basic Appl. Ecol. 14, 472–479, https://doi.org/10.1016/j.baae.2013.06.002 (2013).
Landis, D. A., Wratten, S. D. & Gurr, G. M. Habitat management to conserve natural enemies of arthropod pests in agriculture. Annu. Rev. Entomol. 45, 175–201, https://doi.org/10.1146/annurev.ento.45.1.175 (2000).
Thies, C., Roschewitz, I. & Tscharntke, T. The landscape context of cereal aphid-parasitoid interactions. Proc. R. Soc. B-Biol. Sci. 272, 203–210, https://doi.org/10.1098/rspb.2004.2902 (2005).
Tougeron, K. & Tena, A. Hyperparasitoids as new targets in biological control in a global change context. Biol. Control https://doi.org/10.1016/j.biocontrol.2018.09.003 (2018).
Gomez-Marco, F. et al. Untangling the aphid-parasitoid food web in citrus: Can hyperparasitoids disrupt biological control? Biol. Control 81, 111–121, https://doi.org/10.1016/j.biocontrol.2014.11.015 (2015).
Rosenheim, J. A. Higher-order predators and the regulation of insect herbivore populations. Annu. Rev. Entomol. 43, 421–447, https://doi.org/10.1146/annurev.ento.43.1.421 (1998).
Ortiz-Martinez, S. A. & Lavandero, B. The effect of landscape context on the biological control of Sitobion avenae: temporal partitioning response of natural enemy guilds. J. Pest Sci. 91, 41–53, https://doi.org/10.1007/s10340-017-0855-y (2018).
Plećaš, M. et al. Landscape composition and configuration influence cereal aphid-parasitoid-hyperparasitoid interactions and biological control differentially across years. Agric. Ecosyst. Environ. 183, 1–10, https://doi.org/10.1016/j.agee.2013.10.016 (2014).
Lohaus, K., Vidal, S. & Thies, C. Farming practices change food web structures in cereal aphid–parasitoid–hyperparasitoid communities. Oecologia 171, 249–259, https://doi.org/10.1007/s00442-012-2387-8 (2013).
Gullino, M. L., Albajes, R. & van Lenteren, J. C. In Integrated Pest and Disease Management in Greenhouse Crops (eds Albajes, R., Gullino, M. L., van Lenteren, J. C. & Elad, Y.) 1–15 (Springer Netherlands 1999).
Messelink, G. J., Sabelis, M. W. & Janssen, A. In Integrated Pest Management and Pest Control-Current and Future Tactics (eds Larramendy, M. L. & Soloneski, S.) 191 (InTech 2012).
Enkegaard, A. & Brødsgaard, H. F. In An Ecological and Societal Approach to Biological Control (eds Eilenberg, J. & Hokkanen, H. M. T.) 91–112 (Springer Netherlands 2006).
Blackman, R. L. & Eastop, V. F. In Aphids as crop pests (eds Van Emden, H. & Harringtons, R. V.) 1–29 (CAB International 2007).
Dong, Z., Li, Y., Liu, S. & Zhang, Z. Genetic diversity of the melon aphid Aphis gossypii (Hemiptera: Aphididae) in a diversified vegetable growing area. Appl. Entomol. Zool. 53, 259–266, https://doi.org/10.1007/s13355-018-0552-1 (2018).
Dong, Z., Liu, S. & Zhang, Z. Efficacy of using DNA barcoding to identify parasitoid wasps of the melon-cotton aphid (Aphis gossypii) in watermelon cropping system. BioControl 63, 677–685, https://doi.org/10.1007/s10526-018-9894-4 (2018).
Dong, Z., Li, Y. & Zhang, Z. Genetic diversity of melon aphids Aphis gossypii associated with landscape features. Ecol. Evol. 8, 6308–6316, https://doi.org/10.1002/ece3.4181 (2018).
Powell, W. The identification of hymenopterous parasitoids attacking cereal aphids in Britain. Syst. Entomol. 7, 465–473, https://doi.org/10.1111/j.1365-3113.1982.tb00457.x (1982).
Andrews, F. G. In Occasional Papers. Entomology (USA) (1978).
Fueloep, D., Miko, I., Seltmann, K., Penzes, Z. & Melika, G. The description of Alloxysta chinensis, a new Charipinae species from China (Hymenoptera, Figitidae). Zootaxa 3637, 394–400, https://doi.org/10.11646/zootaxa.3637.3.11 (2013).
Shaw, M. & Huddleston, T. In Handbooks for the identification of British insects Vol. 7 (eds Dolling, W. R. & Askew, R. R.) 126 (British Museum 1991).
Starý, P. & Schlinger, E. I. Revision of the Far East Asian Aphidiidae (Hymenoptera). Vol. 3 (Springer Science & Business Media 1967).
Bersier, L.-F., Banašek-Richter, C. & Cattin, M.-F. Quantitative descriptors of food-web matrices. Ecology 83, 2394–2407, https://doi.org/10.1890/0012-9658(2002)083[2394:QDOFWM]2.0.CO;2 (2002).
Gagic, V. et al. Agricultural intensification and cereal aphid–parasitoid–hyperparasitoid food webs: network complexity, temporal variability and parasitism rates. Oecologia 170, 1099–1109, https://doi.org/10.1007/s00442-012-2366-0 (2012).
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/ (2018).
Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using. J. Stat. Softw. 67, lme4, https://doi.org/10.18637/jss.v067.i01 (2015).
Bartoń, K. MuMIn: Multi-Model Inference. R package version 1.40.4 https://CRAN.R-project.org/package=MuMIn (2018).
Burnham, K. P. & Anderson, D. R. Model selection and multimodel inference: a practical information-theoretic approach. (Springer 2002).
Bivand, R., Hauke, J. & Kossowski, T. Computing the Jacobian in Gaussian spatial autoregressive models: An illustrated comparison of available methods. Geogr. Anal. 45, 150–179, https://doi.org/10.1111/gean.12008 (2013).
Bivand, R. & Piras, G. Comparing implementations of estimation methods for spatial econometrics. J. Stat. Softw. 63, 1–36, https://doi.org/10.18637/jss.v063.i18 (2015).
Dormann, C. F., Gruber, B. & Fruend, J. Introducing the bipartite package: analysing ecological networks. R News 8/2, 8–11 (2008).
Zhou, K. et al. Effects of land use and insecticides on natural enemies of aphids in cotton: First evidence from smallholder agriculture in the North China Plain. Agric. Ecosyst. Environ. 183, 176–184, https://doi.org/10.1016/j.agee.2013.11.008 (2014).
Murakami, M., Hirao, T. & Kasei, A. Effects of habitat configuration on host–parasitoid food web structure. Ecol. Res. 23, 1039–1049, https://doi.org/10.1007/s11284-008-0478-0 (2008).
Rodríguez, M. Á. & Hawkins, B. A. Diversity, function and stability in parasitoid communities. Ecol. Lett. 3, 35–40, https://doi.org/10.1046/j.1461-0248.2000.00115.x (2000).
Pardini, R., Bueno, A. D. A., Gardner, T. A., Prado, P. I. & Metzger, J. P. Beyond the fragmentation threshold hypothesis: Regime shifts in biodiversity across fragmented landscapes. Plos One 5, e13666, https://doi.org/10.1371/journal.pone.0013666 (2010).
Tylianakis, J. M., Tscharntke, T. & Klein, A. M. Diversity, ecosystem function, and stability of parasitoid host interactions across a tropical habitat gradient. Ecology 87, 3047–3057, https://doi.org/10.1890/0012-9658(2006)87[3047:DEFASO]2.0.CO;2 (2006).
Holt, R. D., Lawton, J. H., Polis, G. A. & Martinez, N. D. Trophic rank and the species-area relationship. Ecology 80, 1495–1504, https://doi.org/10.1890/0012-9658(1999)080[1495:TRATSA]2.0.CO;2 (1999).
Frank, S. D. Biological control of arthropod pests using banker plant systems: Past progress and future directions. Biol. Control 52, 8–16, https://doi.org/10.1016/j.biocontrol.2009.09.011 (2010).
Rodriguez, E., Gonzalez, M., Paredes, D., Campos, M. & Benitez, E. Selecting native perennial plants for ecological intensification in Mediterranean greenhouse horticulture. Bull. Entomol. Res. 108, 694–704, https://doi.org/10.1017/s0007485317001237 (2018).
Cotes, B. et al. Spider communities and biological control in native habitats surrounding greenhouses. Insects 9, https://doi.org/10.3390/insects9010033 (2018).
Hawkins, B. A., Mills, N. J., Jervis, M. A. & Price, P. W. Is the biological control of insects a natural phenomenon? Oikos 86, 493–506, https://doi.org/10.2307/3546654 (1999).
Acknowledgements
We thank Yifan Li, Yuansheng Li and Baofeng Mu for providing assistance with sampling and the farmers for allowing us to work on their properties. We thank Dr. Xu Zhang (Institute of Zoology, Chinese Academy of Sciences) for assistance in parasitoids identification. This study was supported by National Key R&D Program of China (2017YFD0200300), National Natural Science Foundation of China (No. 31501646), and Scientific Research Project of Beijing Municipal Education Commission (KM201710020006).
Author information
Authors and Affiliations
Contributions
Z.D. and Z.Z. conceived and designed the research. Z.D. and L.S. conducted experiments. X.M. contributed to the research design. Z.D. wrote the manuscript. All of the authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dong, Z., Men, X., Liu, S. et al. Food web structure of parasitoids in greenhouses is affected by surrounding landscape at different spatial scales. Sci Rep 9, 8442 (2019). https://doi.org/10.1038/s41598-019-44857-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-44857-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.