Abstract
Microbial community diversity and composition are important for the maintenance of mangrove ecosystem. Bacterial and archaeal community composition of the Bamenwan Mangrove Wetland soil in Hainan, China, was determined using pyrosequencing technique. Bacterial community composition presented differences among the five soil samples. Rhizobiales with higher abundance were observed in inner mangrove forest samples, while Desulfobacterales were in the seaward edge samples, and Frankiales, Gaiellales and Rhodospirillales in the landedge sample. For archaea, Crenarchaeota and Euryarchaeota dominated in five samples, but the proportion in each samples were different. Dominant archaeal community composition at the order level was similar in the seaward edge samples. The dominant archaeal clusters in the two inner mangrove forest samples were different, with Soil Crenarchaeotic Group (SCG) and Halobacteriales in sample inside of Bruguiera sexangula forest and SCG, Methanosarcinales and Marine Benthic Group B (MBGB) in sample inside of Xylocarpus mekongensis forest. The dominant archaeal clusters in land sample were unique, with Terrestrial Group and South African Gold Mine Group 1. The metabolic pathways including metabolism, genetic information processing, environmental information processing, cellular processes, organismal systems and human diseases were all detected for bacterial and archaeal functional profiles, but metabolic potentials among five samples were different.
Similar content being viewed by others
Introduction
Mangroves are intertidal estuarine wetlands ecosystem along the tropical and subtropical coasts, covering approximately 60 to 75% of the global coastline1. Mangrove ecosystems serve a variety of important ecological and economic functions, including protecting coastlines from storm damage and erosion, degrading environmental contaminants, and providing nursery habitats for numerous aquatic organisms2,3,4,5,6. Despite the known ecological importance of mangrove forests, human activities place the forests under the rising threat of extinction3.
Mangrove ecosystems are one of the most productive ecosystems in the world. They are characterized by high levels of salinity, high redox potential and organic matter contents, and high rates of nutrient recycling1,7,8. Under such unique environmental conditions, the mangrove habitat contains abundant and characteristic microbial resources1,7,8, which make mangrove as the hotspots for microbial diversity. The mangrove microbiota is composed of a combination of terrestrial soil, marine and freshwater microorganisms1,7,8. These microorganisms play critical roles in mangrove ecosystem maintenance and function1,7,8. Microbes contribute to biogeochemical cycles and serve as a primary nutrition source to plants and animals8. Microbial diversity and activity are essential for the productivity, conservation, and recovery of mangroves9. Microbial diversity in mangrove soil is influenced by biogeographical, ecological, and anthropogenic factors1,8. Additionally, mangrove plants and microorganisms have a close relationship. For example, many microorganisms are beneficial to the growth of mangrove plants. Nitrogen-fixing bacteria, phosphate-solubilizing bacteria, and sulfate-reducing bacteria have been isolated and cultured from mangrove soils10,11. Mangrove plants provide microbial colonization sites and nutrients for microbial growth12,13. Despite these known connections, the details of rhizosphere effect of mangrove plants on the microbial communities remain unclear due to limited studies on these microenvironments.
Many microbiological studies in mangrove ecosystems have been reported in the last few years. Some of these studies have isolated and identified microbial strains with the potential for biotechnical use14. Some studies have characterized the microbial groups present in the mangrove ecosystems, such as archaea14,15,16, fungi17 and cyanobacterial18, as well as characterized the diversity of special microbial populations involving nutrient biogeochemical cycling, such as nitrogen19, sulfur20 and carbon21. Some studies have characterized bacterial community structures in rhizosphere and bulk mangrove soils22,23. Previous studies have shown that diverse and variable microbial communities harbored in mangrove ecosystem24. The anaerobic and high salinity environment of the mangrove wetlands provide conditions for archaea to thrive, so the domain archaea is particularly important for mangrove ecosystem25,26. However, previous studies have mainly focus on bacterial communities, so there was a lack of information regarding archaeal communities in mangrove soils. The archaeal communities in mangrove soils have been examined using clone libraries19,20,27,28,29,30,31,32, but details of archaeal community diversity in mangrove wetlands could not be revealed using this method. This is the first characterization of the archaeal community within mangrove ecosystem soil that has been reported using data from high-throughput next generation sequencing. Next generation sequencing, like pyrosequencing and Illumina sequencing provides a relatively detailed picture of microbial communities compared to other methods. In this study, we describe details of bacterial and archaeal communities that exist within different sites of the mangrove wetland using pyrosequencing. These results may be helpful for guidance to isolate bacteria of interest within these distinct sites.
Results
Sequencing and quality control
A total of 94013 and 100585 valid reads, for bacteria and archaea, respectively, were obtained for five soil samples in the Bamenwan Mangrove Wetland (Table 1). The average read lengths for bacterial and archaeal samples were 597 bp and 499 bp, respectively. Table 1 shows that 77–82% of the raw reads met quality and length standards for bacteria. After initial quality check mentioned above, the chimera, Achaea and singleton reads were also checked and filtered out. Finally, 7951, 8072, 7757, 8992 and 8039 effective bacterial sequences were extracted from each of the five samples, respectively, for use in downstream bioinformatic analyses (Table 1). For archaea, 86–89% of the raw reads met quality and length standards. 13640, 3056, 15636, 16203 and 12819 effective archaeal sequences were obtained from each of five samples, respectively, for use in downstream bioinformatic analyses after the initial quality check (Table 1).
Bacterial diversity indices and community structure
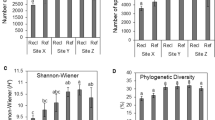
The effective sequences were clustered into operational taxonomic units (OTUs) using 97% similarity cutoff with UPARSE, which is a method with a focus on reducing OTU inflation. The OTU number range for all five samples was 427 to 870 OTUs using a distance cutoff level of 3% (Table 1). The BM5 sample contained the lowest OUT number. Diversity was highest in the BM4 sample and the lowest in BM5 sample (Table 1, Fig. 1). The Shannon diversity index also revealed that the BM5 sample had the lowest bacterial diversity among the five samples (Table 1). The rarefaction curves of the five samples did not reach a plateau, indicating that the data did not contain enough sequence depth to ascertain the full bacterial diversity (Fig. 1).
Thirty-four phyla were detected within the five samples. The top 10 phyla present in the samples were Proteobacteria, Actinobacteria, Chloroflexi, Acidobacteria, Firmicutes, Bacteroidetes, Gemmatimonadetes, Cyanobacteria, Nitrospirae, and Chlorobi (Fig. 2). Proteobacteria was the predominant phylum in all five samples. The highest percentages of Proteobacteria were detected in the samples BM3 and BM4 (51.40% and 53.53% of the total sequences in each sample, respectively). The lowest percentage of Proteobacteria was detected in sample BM5 (31.53%) (Table S1). Actinobacteria was the second most abundant phylum, with the highest percentage of 48.40% in the sample BM5 and lower percentages of 10.10% and 8.86% in the samples BM1 and BM2, respectively (Table S1). The percentages of Chloroflexi were higher in the samples BM1 and BM2, (16.30% and 15.28%, respectively), than those of other three samples, (which ranged from 2.99–6.54%) (Table S1). Acidobacteria were detected in greater abundance in the sample BM5 (10.37%) than in the other four samples (4.25–8.43%) (Table S1). The percentages of Cyanobacteria and Deferribacteres in the samples BM1 (3. 55% and 1.31%, respectively) and BM2 (4.78% and 3.39%, respectively) were higher than those of the other three samples (0.53% and 0.23% on average, respectively) (Table S1). Gemmatimonadetes were detected in greater numbers in the samples BM3 and BM4 (2.98% and 4.07%, respectively) than those of the other three samples (which ranged from 0.27–1.50%) (Table S1). In summary, the percentage abundance of different bacterial phyla differed between the five samples. Compared with the other four samples, the composition of the bacterial community in sample BM5 was distinct, and dominated by Actinobacteria (representing 48.70% of the total sequences), Proteobacteria (31.53%), and Acidobacteria (10.37%). However, some predominant bacterial clusters were similar between some samples. For example, the predominant phyla in samples BM1 and BM2 were similar. These samples were composed of Proteobacteria (with 44.20% and 43.27% of the total sequences in each sample, respectively)), Chloroflexi (with 16.30% and 15.28%, respectively) and Actinobacteria (with 10.10% and 8.86%, respectively). The bacterial community composition in samples BM3 and BM4 were both mainly dominated by Proteobacteria (with 51.40% and 53.53% of the total sequences in each sample, respectively), Actinobacteria (with 24.82% and 16.70%, respectively), Acidobacteria (with 7.30% and 8.43%, respectively) and Chloroflexi (with 6.54% and 6.57%, respectively) (Table S1).
In the Proteobacteira phylum, Alphaproteobacteria (40.51% of the Proteobacteira phylum on average), Deltaproteobacteria (32.89%) and Gammaproteobacteria (23.01%) were the main three classes detected in the five samples (Table S2). However, the proportion of these three classes differed among the five samples. Alphaproteobacteria was present in the highest abundance (77.99% in the Proteobacteira phylum) in sample BM5, and in the lowest abundance (11.34%) in sample BM2 (Table S2). Deltaproteobacteria was detected in the highest abundance (62.67%) in sample BM2, and in the lowest abundance (3.27%) in sample BM5 (Table S2). Gammaproteobacteria was detected in the highest abundance (36.25%) in sample BM1, and in the lowest abundance (16.61%) in sample BM5 (Table S2).
Within Alphaproteobacteria, the dominant orders were Rhizobiales and Rhodospirillales, with the highest abundance detected in samples BM3 (21.62% of total sequences) and BM5 (14.96% of total sequences), respectively (Table S3). The majority of Deltaproteobacteria sequences belonged to Desulfobacterales and Syntrophobacterales, which were both present in high abundance in samples BM1 and BM3 (accounting for 13.80% and 3.83%, respectively) compared to the other three samples (accounting for 3.15% and 0.73%, respectively) (Table S3). Within Gammaproteobacteria, the dominant orders were Xanthomonadales and Chromatiales. Xanthomonadales were detected in all five samples with percent abundances ranging from 1.92–7.23%. Chromatiales were not detected in sample BM5 but presented in the other four samples at similar percentages ranging from 2.66% to 3.46% (Table S3). Acidimicrobiales, Frankiales, Gaiellales, Solirubrobacterales, as the major orders of Actinobacteria, were present in high proportions in sample BM5 (especially for Frankiales, with a percent abundance of 22.20%) (Table S3). In summary, In summary, the bacterial community composition differed between the five samples the level of order. The main orders detected in sample BM1 were Desulfobacterales (10.51%), Xanthomonadales (7.23%), Anaerolineales (5.94%), Rhizobiales (5.82%) and Acidimicrobiales (4.50%). The main orders detected in sample BM2 were Desulfobacterales (17.08%), Anaerolineales (7.04%), Nitrospira (4.35%), Rhizobiales (3.90%) and Syntrophobacterales (3.79%). The main orders detected in sample BM3 were Rhizobiales (21.62%), Acidimicrobiales (7.77%), Solirubrobacterales (7.14%) and Gaiellales (6.41%), Xanthomonadales (4.55%). The main orders detected in sample BM4 were Rhizobiales (19.54%), Desulfobacterales (6.29%), Gaiellales (5.94%), Acidimicrobiales (4.10%) and Solirubrobacterales (4.06%). The main orders detected in sample BM5 were Frankiales (22.20%), Gaiellales (15.31%), Rhizobiales (8.66%), Rhodospirillales (14.96%) and Solirubrobacterales (5.25%) (Table S3).
The results of principal component analysis (PCA) at OTUs levels indicated that the five samples could be separated into three groups (Fig. S1a). The BM1 and BM2 samples were grouped together, and the BM3 and BM4 samples clustered together. Sample BM5 was separated from the other four samples. The results of PCoA analysis using the weighted and unweighted UniFrac metrics gave results that were similar to the PCA results (Fig. S1b,c).
Archaeal diversity indices and community structure
For archaea, the OTU number, the taxon richness level reflected by Chao1 and ACE estimators, and the Shannon diversity index were relatively higher in samples BM1 and BM2, and lowest in sample BM5 (Table 1). The archaeal richness and diversity were higher in the soil inside Xylocarpus mekongensis mangrove forest (sample BM4) than inside the Bruguiera sexangula mangrove forest (sample BM5) (Fig. 3). Like the bacterial rarefaction curves, the archaeal rarefaction curves of the five samples did not reach a plateau, indicating the sequencing depth of these samples was not sufficient to fully assess archaeal diversity (Fig. 3).
Three different archaeal phyla, Crenarchaeota, Euryarchaeota and Thaumarchaeota, were detected in this study (Fig. 4). Crenarchaeota and Euryarchaeota were dominat in the five samples, but the proportion of each phylum differed between samples. The relatively higher proportion of Euryarchaeota were in samples BM2 (43.25%) and BM1 (30.85%), and then in samples BM4 (24.06%) and BM3 (15.66%), and the lowest proportion in BM5 (11.11%) (Fig. 4). The observed trend in Crenarchaeota abundance was the reverse of the trend observed in Euryarchaeota. The highest abundance was detected in sample BM5 (88.86%), then in samples BM3 (84.34%) and BM4 (75.76%), and relatively lower proportion in the samples BM1 (68.06%) and BM2 (55.97%) (Fig. 4). Crenarchaeota were only observed at low abundance in sample BM1 (0.02%). The Halobacteriales, Methanosarcinales and Thermoplasmatales orders dominated within Euryarchaeota, with the highest abundances observed in BM2 (20.8%), BM4 (13.9%) and BM2 (18.4%), respectively (Table S4). The observed Crenarchaeota mainly belonged to the Marine Benthic Group B (MBGB), Miscellaneous Crenarchaeotic Group (MCG), Soil Crenarchaeotic Group (SCG), South African Gold Mine Group 1(SAGMCG-1), Group C3, and the Terrestrial Group (Table S4). MCG was found to be relatively abundant in BM1 (36.20%) and BM2 (16.80%), and followed in BM3 (2.40%) and BM4 (8.40%). SCG was detected at high abundance in BM3 (75.60%) and BM4 (43.00%), and at low abundance in the other three samples (ranging from 0.90% to 2.40%) (Table S4). MBGB and Group C3 accounted for a large proportion of samples BM1, BM2 and BM4, but were absent in the sample BM5. Terrestrial Group and SAGMCG-1, as the predominant orders with 68.70% and 14.80%, respectively, were only detected in the sample BM5 (Table S4).
In summary, similar patterns in abundance in archaeal community composition at the order level were detected in samples BM1 and BM2. Both samples contained MCG, MBGB, Halobacteriales, Thermoplasmatales and Group C3. In these two samples, however, the percentages of MCG and Halobacteriales showed the reverse trend (Table S3). The most abundant archaeal clusters differed between the two mangrove forest samples, with SCG (75.60%) and Halobacteriales (8.10%) present in BM3 and SCG (43.00%), Methanosarcinales (13.90%) and MBGB (12.60%) present in BM4. Compared to the former four samples, the dominant archaeal clusters in BM5 were unique, containing Terrestrial Group (68.70%) and SAGMCG-1 (14.80%) (Table S4).
The results of PCA at OTUs levels indicated that the five samples could be separated into four groups (Fig. S1d). BM5 was separated from the other four samples. The BM1 and BM2 samples were grouped together, and the BM3 and BM4 samples clustered with one another. Similar results were also found in PCoA analysis using the weighted and unweighted UniFrac metrics (Fig. S1e,f). The PCA and PCoA results were consistent with the community composition results mentioned above.
Functional properties predicted by PICRUSt
PICRUSt was used to explore the different metabolic potentials among the samples from different sites in the mangrove wetland. The metabolic pathways including metabolism, genetic information processing, environmental information processing, cellular processes, organismal systems and human diseases were all detected for bacterial and archaeal functional profiles (Figs 5 and 6). For bacteria, PICRUSt analysis revealed that metabolic pathways such as energy metabolism, glycan biosynthesis and metabolism, replication and repair and translation were more abundant in the seaward edge samples, and membrane transport pathway was more abundant in inner samples, and pathways related to amino acid metabolism, carbohydrate metabolism, xenobiotics biodegradation and metabolism, lipid metabolism and transcription were more abundant in the landedge sample (Fig. 5). For archaea, the pathways such as nucleotide metabolism, metabolism of cofactors and vitamins, lipid metabolism, glycan biosynthesis and metabolism, translation and replication and repair were more abundant in seaward edge samples, and amino acid metabolism, membrane transport, and xenobiotics biodegradation and metabolism were more abundant in the other three samples (Fig. 6).
Discussion
Soil microbial communities are active and vital components of mangrove ecosystems and play essential roles in nutrient biogeochemical cycling, organic matter remineralization and contaminant degradation33. The microbial communities in the mangrove soil are significantly affected by bio-geographical, anthropological, and ecological factors. These factors include the food web within the mangrove ecosystem, nutrient cycling, and the presence of inorganic or organic compounds in the soil34. Recently, microbial diversity and community structures have been studied in several mangrove ecosystems, such as those located in Brazil35 and Hong Kong36. In this study, a barcoded pyrosequencing analysis of 16S rRNA gene was performed to characterize the bacterial and archaeal communities present within five different sites in Bamenwan Mangrove Wetland in Hainan, China.
Metagenomic data collected from mangrove soil samples in Sao Paulo State, Brazil revealed an abundance of Proteobacteria (47.1–56.3%), Firmicutes (10.5–13.8%), Actinobacteria (5.4–12.2%), and Bacteroidetes (3.8–11.8%)30. In this study, Proteobacteria (31.53–53.53%), Actinobacteria (8.86–48.70%), Acidobacteria (4.25–10.37%), and Chloroflexi (2.99–16.30%) were dominant bacterial groups detected in the two samples taken from inside of the mangrove forest. In the previous two studies, Proteobacteria and Actinobacteria were both the predominant bacterial groups, which might suggest these bacterial groups are cosmopolitan inside of mangrove environments. However, differences between the abundant bacterial groups detected in these studies were also obvious. For example, Firmicutes and Bacteroidetes were detected at 0.76–5.43% and 0.40–6.28%, respectively, in the present study. These differences might be due to biogeographical, ecological (such as different mangrove plant community composition), and anthropogenic factors (such as nearby aquiculture and urbanization).
In this study, bacterial community structure among soil samples from the seaward edge, the inner and the landedge of the Bamenwan mangrove wetland presented differences. The two previous studies26,31 reported that spatial differences had a significant effect on bacterial community composition. For example, Actinobacteria, Acidobacteria, Nitrospirae, and Verrucomicrobia were enriched in the nutrient-rich inner mangrove soils, but Proteobacteria and Deferribacterias were enriched in the outer mangrove soils. Jiang et al.35 compared the bacterial communities in the mangrove wetland with that of freshwater reservoirs and marine sediments, and found that diverse groups of bacteria with functions related to the primary production were enriched in the mangrove wetland. These studies suggest that the intertidal mangrove wetland has a unique bacterial community. The mangrove wetland may be unique at the level of bacterial composition because it is the only forest ecosystem in marine environments, making it a unique intertidal ecosystem. The mangrove plant root exudates secreted from the roots of plants into the rhizosphere and surrounding sediment may act as nutrient sources or inhibitors for microbial populations31.
Rhizobiales was observed in the rhizospheres of mangrove trees26 and bulk soils around the mangrove tree roots. This suggests Rhizobiales may be common in mangrove soil bacterial communities and the presence of Rhizobiales is not restricted to one mangrove tree species. Rhizobiales are comprised of nitrogen-fixing bacteria and plant symbionts. Recently, a genomic analysis indicated that Rhizobiales are very metabolically versatile and are capable of degrading aromatic compounds26. Our results indicate the proportion of Rhizobiales in the two inner mangrove wetland samples is much higher than the proportion in the seaward and landedge samples. This suggests that Rhizobiales contribute to nitrogen-fixation in mangrove soils and promote mangrove plant growth. In addition, some specific orders are present within the two samples, such as Anaerolineales, Gemmatimonadales, Nitrospira, Syntrophobacterales detected in the soil sample from Xylocarpus mekongensis forest. This suggests mangrove plants have some impact on bacterial community composition not only in the rhizosphere, but also in the bulk soils around plant roots. Previous studies have illustrated that soil physiochemical properties, such as salinity, soil pH, nutrient concentration and composition, and root exudates, are the major factors influencing the activity and microbial community of mangrove soils31,37.
Mangrove soils are anoxic environments26, with high levels of salinity, high redox potential, and high amounts of organic matter and sulphates38. Accordingly, sulphur transformation is one of most active chemical cycles in the mangrove ecosystem39,40,41,42. Sulphur transformation, in its various forms, is mediated by microorganisms1,43. Microbial sulfate reduction is important for anaerobic degradation of organic matter in marine soils7,44 and is often mediated by bacterial clusters belonging to Deltaproteobacteria, specifically, Desulfobacterales44,45. In this study, Desulfobacterales were the dominant cluster in the four soil samples (including the seaward edge and the inner) except the landedge sample (BM5). This result was consistent with previous results as described abundant in mangrove soils46. These results suggested that Desulfobacterales as dominant clusters contributed greatly to sulfur transformation in the mangrove ecosystem. The results additionally suggest that sulfate reduction may be a primary pathway for anaerobic degradation of organic matter in mangrove soils. Desulfobacterales was also found to be abundant in polluted sites47, where they are associated with anaerobic degradation of hydrocarbons48,49. Taketani et al.50,51 report that Deltaproteobacteria are stimulated by oil pollution. These results suggest that mangrove and adjacent coastal ecosystems not only contain high levels of organic matter, but also high levels of pollutants. Indeed, it has been reported that the offshore areas (including mangrove ecosystems) of China are heavily polluted with organic contaminants and heavy metals due to the rapid economic development of coastal regions52. In addition, some other known sulfate-reducing bacterial clusters were also detected in this study, such as Syntrophobacterales presented in the seaward edge and the inner of mangrove forest soils, which indicated the diversity of sulphidogenic prokaryotes in mangrove ecosystem53.
In this study, bacterial clusters involved in other types of biogeochemical cycling, like phosphate and nitrogen cycling, were also observed. Nitrospira detedcted in all samples in this study, was described as nitrite-oxidizing bacteria in nitrogen cycle51. Rhizobiales as nitrogen-fixing bacteria were also detected in all samples. Deferribacteraleswere detected in the mudflat, marine edge and mangrove forest soils, and were considered as potential heterotrophic nitrate reducers50,51.
Frankiales, belonging to Actinobacteria, was the most dominant order detected, with up to 22.20% in the landedge sample (BM5). Acidothermus was the major genus of Frankiales (Table S3). This genus contains a single species, namely A. cellulolyticus, which is thermophilic, acidophilic and could produce many thermostable cellulose-degrading enzymes54. Gaiellales, a member of order Actinobacteria, was also abundant in the landedge sample (BM5). However, little is known about the physiology of Gaiellales. It is a novel order within the class Actinobacteria55. Rhodospirillales, the third most abundant order detected in the landedge sample, is comprised of many acetic acid-producing bacteria. These bacteria may be responsible for the low pH of the land sample soil.
Many bacterial members from Bacillales are considered beneficial to plant growth and also have protective effects against diseases56. Bacillales account for 1.35% of the bacteria detected in the present study, on average. Some isolates from the root and rhizosphere soil in the mangrove environment were phosphorus-solubilizing bacteria57,58. Bacillales may play a special role in mangrove ecosystems by engaging in long term promotion of plant growth. It is possible the Bacillales promote growth by producing endospores under stressful environmental conditions and by secreting large quantities of enzymes, such as phytase, a critical component of the phosphorous cycle59.
As showun from the previous studies, MBGB and MCG are ubiquitous in marine environments60,61,62. In the present study, a higher relative abundance of the two archaeal clusters was observed in seaward edge samples. No sequences were observed in the landedge sample. This distribution pattern was consistent with the previous reports. It is hypothesized that MCG and MBGB are to be anaerobic heterotrophs that consume buried carbon63,64,65. Lloyd et al.66 reports that MCG plays an important role in degradation of detrital proteins in anoxic marine soils. Based on these studies, the prevalence of MBGB and MCG in coastal sediments could contribute to the degradation of organic matter.
Methods
Site characterization and sample collection
Surface soil (top 30 cm) samples were collected from the Bamenwan mangrove wetland in Hainan, China (19°30′N, 110°15′E). Samples were taken from the mangrove forest dominated by Avicennia marina (BM1), Aegiceras corniculatum (BM2), Bruguiera sexangula (BM3), Xylocarpus mekongensis (BM4) and Pongamia pinnata (BM5), respectively. BM1 and BM2 sited in the seaward edge of the magrove wetland, BM3 and BM4 sited in the inner and BM5 sited in the landedge. Bulk soils, each in triplicate in each location, were collected in December 2011. The three replicates in one location were taken approximately 5 m apart, and then pooled together.
DNA extraction and 454 pyrosequencing
Microbial DNA was extracted from five soil samples using the FastPrep® SPIN Kit for Soil (MP Biomedicals, U.S.) according to manufacturer’s protocols. The bacterial 16S ribosomal RNA gene were amplified by polymerase chain reaction (95 °C for 2 min, followed by 25 cycles at 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s and a final extension at 72 °C for 5 min) using primers 341F (5′-CCTACGGGAGGCAGCAG-3′)-1073R (5′-ACGAGCTGACGACARCCATG-3′), and the archaeal 16S ribosomal RNA gene were amplified by polymerase chain reaction (95 °C for 2 min, followed by 30 cycles at 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s and a final extension at 72 °C for 5 min) using primers 344F (5′-ACGGGGYGCAGCAGGCGCGA-3′)-915R (5′-GTGCTCCCCCAATTCCT-3′). PCR reactions were performed in a 20 μL mixture containing 4 μL of 5 × FastPfu Buffer, 2 μL of 2.5 mM dNTPs, 0.8 μL of each primer (5 μM), 0.4 μL of FastPfu Polymerase, and 10 ng of template DNA. After purification using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, U.S.) and quantification using QuantiFluor™ -ST (Promega, U.S.), a mixture of amplicons was used for pyrosequencing on a Roche 454 GS FLX+ Titanium platform (Roche 454 Life Sciences, Branford, CT, U.S.) according to standard protocols. The raw reads were deposited into the NCBI Sequence Read Archive (SRA) database (Accession Number: SRP040784 for bacteria and SRP041275 for archaea).
Processing of pyrosequencing data
The resulting sequences were processed using QIIME (version 1.17)67. After removing sequences with average quality score <25 over a 50 bp sliding window and sequences shorter than 200 bp, with homopolymers longer than six nucleotides, and containing ambiguous base calls or incorrect primer sequences, high-quality sequences were produced. Operational Taxonomic Units (OTUs) were clustered with 97% similarity cutoff using UPARSE (version 7.1 http://drive5.com/uparse/)19,68 and chimeric sequences were identified and removed using UCHIME. The phylogenetic affiliation of each 16S rRNA gene sequence was analyzed by RDP Classifier (http://rdp.cme.msu.edu/) against the silva (SSU115)16S rRNA database using confidence threshold of 70%19,32,68. The beta diversity analysis was carried out using UniFrac to compare the results of PCA at the OTU level with the community ecology package, R-forge (vegan 2.0 package was used to generate a PCA figure)35,69.
Phylogenetic investigation of communities by reconstruction of unobserved states (PICRUSt) analysis
PICRUSt analysis was performed to predict metagenomic functional content based on the software package (PICRUSt v1. 0. 0)70. This approach exploits the relationship between phylogeny and function by combining 16 s data with a database of reference genomes (Greengenes) to predict the presence of gene families. The 16S rDNA sequences were clustered into a collection of OTUs using a closed-reference OUT picking protocol (QIIME1.8.0)71. The obtained OUT table was normalized by 16S rRNA gene copy number, and then used to predict metagenomic functional content based on the PICRUSt software package70. Functional predictions were exported as KEGG orthologs.
Data Availability
The datasets generated during the current study are available in the NCBI Sequence Read Archive (SRA) database (Accession Number: SRP040784 for bacteria and SRP041275 for archaea).
References
Holguin, G., Vazquez, P. & Bashan, Y. The role of sediment microorganisms in the productivity, conservation, and rehabilitation of mangrove ecosystems: an overview. Biol. Fert. Soils. 33, 265–278 (2001).
Alongi, D. M. Present state and future of the world’s mangrove forests. Environ. Conserv. 29, 331–349 (2002).
Duke, N. C. et al. A world without mangroves? Science. 317, 41–42 (2007).
Wang, P. & Chen, G. Q. Contaminant transport in wetland flows with bulk degradation and bed absorption. J Hydrol. 552, 674–683 (2017).
Wang, P. & Chen, G. Q. Environmental dispersion in a tidal wetland with sorption by vegetation. Communications in Nonlinear Science and Numerical Simulation. 22, 348–366 (2015).
Wang, P., Zeng, L. & Huai, W. Transient dispersion of an initial point pollutant concentration in wetland flows. Environmental Science and Pollution Research., https://doi.org/10.1007/s11356-11018-13376-11351 (2018).
Clark, M. W., McConchie, D., Lewis, D. & Saenger, P. Redox stratification and heavy metal partitioning in Avicennia-dominated mangrove sediments: a geochemical model. Chem. Geol. 149, 147–171 (1998).
Alongi, D. M. The dynamics of benthic nutrient pools and fluxes in tropical mangrove forests. J. Mar. Res. 54, 123–148 (1996).
Ananda, K. & Sridhar, K. Diversity of endophytic fungi in the roots of mangrove species on the west coast of India. Can. J. Microbiol. 48, 871–878 (2002).
Alongi, D. M. Bacterial productivity and microbial biomass in tropical mangrove sediments. Microb. Ecol. 15, 59–79 (1988).
Kathiresan, K. & Bingham, B. L. Biology of mangroves and mangrove ecosystems. Adv. Mar. Biol. 40, 81–251 (2001).
Dos Santos, H. F. et al. Mangrove bacterial diversity and the impact of oil contamination revealed by pyrosequencing: bacterial proxies for oil pollution. PLoS One. 6, e16943 (2011).
Gomes, N. et al. Mangrove microniches determine the structural and functional diversity of enriched petroleum hydrocarbon-degrading consortia. FEMS Microbiol. Ecol. 74, 276–290 (2010).
Zhou, H. W. et al. Polycyclic aromatic hydrocarbon-induced structural shift of bacterial communities in mangrove sediment. Microb. Ecol. 58, 153–160 (2009).
Sahoo, K. & Dhal, N. Potential microbial diversity in mangrove ecosystems: a review. Indian J. Mar. Sci. 38, 249–256 (2009).
Lopez-Fuentes, E. et al. Bacterial community in the roots and rhizosphere of Hypericum silenoides Juss. 1804. Afr. J. Microbiol. Res. 6, 2704–2711 (2012).
Doornbos, R. F., van Loon, L. C. & Bakker, P. A. Impact of root exudates and plant defense signaling on bacterial communities in the rhizosphere. A review. Agron. Sustain. Dev. 32, 227–243 (2012).
Holguin, G., Guzman, M. A. & Bashan, Y. Two new nitrogen-fixing bacteria from the rhizosphere of mangrove trees: Their isolation, identification and in vitro interaction with rhizosphere Staphylococcus sp. FEMS Microbiol. Lett. 101, 207–216 (1992).
Dias, A. C. F. et al. Archaeal communities in the sediments of three contrasting mangroves. J. Soil Sediment. 11, 1466–1476 (2011).
Fasanella, C. C. et al. The selection exerted by oil contamination on mangrove fungal communities. Water Air Soil Poll. 223, 4233–4243 (2012).
Rigonato, J. et al. Drivers of cyanobacterial diversity and community composition in mangrove soils in south-east Brazil. Environ. Microbiol. 15, 1103–1114 (2013).
Wang, Y., Li, X. & Gu, J. Differential responses of ammonia/ammonium-oxidizing microorganisms in mangrove sediment to amendment of acetate and leaf litter. Appl. Environ. Microb. 98, 3165–3180 (2014).
Wang, H., Su, J., Zheng, T. & Yang, X. Impacts of vegetation, tidal process, and depth on the activities, abundances, and community compositions of denitrifiers in mangrove sediment. Appl. Environ. Microb. 98, 9375–9387 (2014).
Varon-Lopez, M. et al. Sulphur-oxidizing and sulphate-reducing communities in Brazilian mangrove sediments. Environ. Microbiol. 16, 845–855 (2014).
Cleary, D. F., Smalla, K., Mendonça-Hagler, L. C. & Gomes, N. C. Assessment of variation in bacterial composition among microhabitats in a mangrove environment using DGGE fingerprints and barcoded pyrosequencing. PLoS One. 7, e29380 (2012).
Gomes, N. C. et al. Assessing variation in bacterial composition between the rhizospheres of two mangrove tree species. Estuar. Coast Shelf S. 139, 40–45 (2014).
Lyimo, T. J., Pol, A., Harhangi, H. R., Jetten, M. S. & Op den Camp, H. J. Anaerobic oxidation of dimethylsulfide and methanethiol in mangrove sediments is dominated by sulfate-reducing bacteria. FEMS Microbiol. Ecol. 70, 483–492 (2009).
Liang, J. et al. Recovery of novel bacterial diversity from mangrove sediment. Mar. Biol. 150, 739–747 (2007).
Gomes, N. C. M. et al. Exploring the diversity of bacterial communities in sediments of urban mangrove forests. FEMS Microbiol. Ecol. 66, 96–109 (2008).
Ghosh, A. et al. Culture independent molecular analysis of bacterial communities in the mangrove sediment of Sundarban, India. Saline Systems. 6, 1–11 (2010).
Andreote, F. D. et al. The microbiome of Brazilian mangrove sediments as revealed by metagenomics. PLoS One. 7, e38600 (2012).
Mendes, L. W., Taketani, R. G., Navarrete, A. A. & Tsai, S. M. Shifts in phylogenetic diversity of archaeal communities in mangrove sediments at different sites and depths in southeastern Brazil. Res. Microbiol. 163, 366–377 (2012).
Edgar, R. C. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 10, 996–998 (2013).
Amato, K. R. et al. Habitat degradation impacts black howler monkey (Alouatta pigra) gastrointestinal microbiomes. ISME J. 7, 1344–1353 (2013).
Jiang, X.-T. et al. Illumina sequencing of 16S rRNA tag revealed spatial variations of bacterial communities in a mangrove wetland. Microb. Ecol. 66, 96–104 (2013).
Gruber, N. & Galloway, J. N. An Earth-system perspective of the global nitrogen cycle. Nature. 451, 293–296 (2008).
Wang, Y. et al. Comparison of the levels of bacterial diversity in freshwater, intertidal wetland, and marine sediments by using millions of illumina tags. Appl. Environ. Microb. 78, 8264–8271 (2012).
Baek, S.-H. et al. Isolation and characterization of bacteria capable of degrading phenol and reducing nitrate under low-oxygen conditions. Curr. Microbiol. 47, 462–466 (2003).
Philippot, L., Raaijmakers, J., Lemanceau, P. & van der Putten, W. Going back to the roots: the microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 11, 789–799 (2013).
Berg, G. & Smalla, K. Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol. Ecol. 68, 1–13 (2009).
Perryman, S. E., Rees, G. N., Walsh, C. J. & Grace, M. R. Urban stormwater runoff drives denitrifying community composition through changes in sediment texture and carbon content. Microb. Ecol. 61, 932–940 (2011).
Bañeras, L., Ruiz-Rueda, O., López-Flores, R., Quintana, X. & Hallin, S. The role of plant type and salinity in the selection for the denitrifying community structure in the rhizosphere of wetland vegetation. Int. Microbiol. 15, 89–99 (2012).
Attri, K., Kerkar, S. & LokaBharathi, P. Ambient iron concentration regulates the sulfate reducing activity in the mangrove swamps of Diwar, Goa, India. Estuar. Coast Shelf S. 95, 156–164 (2011).
Lyimo, T. J., Pol, A. & Op den Camp, H. J. Sulfate reduction and methanogenesis in sediments of Mtoni mangrove forest, Tanzania. Ambio. 31, 614–616 (2002).
Meyer, B. & Kuever, J. Molecular analysis of the diversity of sulfate-reducing and sulfur-oxidizing prokaryotes in the environment, using aprA as functional marker gene. Appl. Environ. Microb. 73, 7664–7679 (2007).
Fan, L.-F., Tang, S.-L., Chen, C.-P. & Hsieh, H.-L. Diversity and composition of sulfate-and sulfite-reducing prokaryotes as affected by marine-freshwater gradient and sulfate availability. Microb. Ecol. 63, 224–237 (2012).
Canfield, D. E. et al. Pathways of organic carbon oxidation in three continental margin sediments. Mar. Geol. 113, 27–40 (1993).
Orphan, V. et al. Comparative analysis of methane-oxidizing archaea and sulfate-reducing bacteria in anoxic marine sediments. Appl. Environ. Microb. 67, 1922–1934 (2001).
Leloup, J. et al. Sulfate-reducing bacteria in marine sediment (Aarhus Bay, Denmark): abundance and diversity related to geochemical zonation. Environ. Microbiol. 11, 1278–1291 (2009).
Taketani, R. G., Dos Santos, H. F., van Elsas, J. D. & Rosado, A. S. Characterisation of the effect of a simulated hydrocarbon spill on diazotrophs in mangrove sediment mesocosm. Antonie van Leeuwenhoek. 96, 343–354 (2009).
Taketani, R. G., Yoshiura, C. A., Dias, A. C. F., Andreote, F. D. & Tsai, S. M. Diversity and identification of methanogenic archaea and sulphate-reducing bacteria in sediments from a pristine tropical mangrove. Antonie van Leeuwenhoek. 97, 401–411 (2010).
NBO. National Bureau of Oceanography of China. Bull Mar Environ Qual. 2008–2009 (2009).
Zhang, W. et al. Microbial diversity in polluted harbor sediments II: Sulfate-reducing bacterial community assessment using terminal restriction fragment length polymorphism and clone library of dsrAB gene. Estuar. Coast Shelf S. 76, 682–691 (2008).
Guan, J. et al. Functional genes (dsr) approach reveals similar sulphidogenic prokaryotes diversity but different structure in saline waters from corroding high temperature petroleum reservoirs. Appl. Environ. Microb. 98, 1871–1882 (2014).
Castro, H. F., Williams, N. H. & Ogram, A. Phylogeny of sulfate-reducing bacteria. FEMS Microbiol. Ecol. 31, 1–9 (2000).
Gittel, A., Sørensen, K. B., Skovhus, T. L., Ingvorsen, K. & Schramm, A. Prokaryotic community structure and sulfate reducer activity in water from high-temperature oil reservoirs with and without nitrate treatment. Appl. Environ. Microb. 75, 7086–7096 (2009).
Mohagheghi, A., Grohmann, K., Himmel, M., Leighton, L. & Updegraff, D. Isolation and characterization of Acidothermus cellulolyticus gen. nov., sp. nov., a new genus of thermophilic, acidophilic, cellulolytic bacteria. Int. J. Syst. Bacteriol. 36, 435–443 (1986).
Tucker, M. P., Mohagheghi, A., Grohmann, K. & Himmel, M. E. Ultra-thermostable cellulases from Acidothermus cellulolyticus: comparison of temperature optima with previously reported cellulases. Nat. Biotechnol. 7, 817–820 (1989).
Albuquerque, L. et al. Gaiella occulta gen. nov., sp. nov., a novel representative of a deep branching phylogenetic lineage within the class Actinobacteria and proposal of Gaiellaceae fam. nov. and Gaiellales ord. nov. Syst. Appl. Microbiol. 34, 595–599 (2011).
Richardson, A. E. & Simpson, R. J. Soil microorganisms mediating phosphorus availability update on microbial phosphorus. Plant Physiol. 156, 989–996 (2011).
Guerrero-Olazarán, M., Rodríguez-Blanco, L., Carreon-Treviño, J. G., Gallegos-López, J. A. & Viader-Salvadó, J. M. Expression of a Bacillus phytase C gene in Pichia pastoris and properties of the recombinant enzyme. Appl. Environ. Microb. 76, 5601–5608 (2010).
Kerovuo, J., Lauraeus, M., Nurminen, P., Kalkkinen, N. & Apajalahti, J. Isolation, characterization, molecular gene cloning, and sequencing of a novel phytase from Bacillus subtilis. Appl. Environ. Microb. 64, 2079–2085 (1998).
Francis, C. A., Roberts, K. J., Beman, J. M., Santoro, A. E. & Oakley, B. B. Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. P. Natl. Acad. Sci. USA 102, 14683–14688 (2005).
Leininger, S. et al. Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature. 442, 806–809 (2006).
Wuchter, C. et al. Archaeal nitrification in the ocean. P. Natl. Acad. Sci. USA 103, 12317–12322 (2006).
Stahl, D. A. & de la Torre, J. R. Physiology and diversity of ammonia-oxidizing archaea. Annu. Rev. Microbiol. 66, 83–101 (2012).
Caporaso, J. G. et al. QIIME allows analysis of high-throughput community sequencing data. Nature Methods. 7, 335–336 (2010).
Yan, B., Hong, K. & Yu, Z.-N. Archaeal communities in mangrove soil characterized by 16S rRNA gene clones. J. Microbio. 44, 566–571 (2006).
Lozupone, C., Lladser, M. E., Knights, D., Stombaugh, J. & Knight, R. UniFrac: an effective distance metric for microbial community comparison. ISME J. 5, 169–172 (2011).
Langille, M. et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 31, 814–821 (2013).
Odamaki, T. et al. Age-related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study. BMC Microbiol. 16, 1–12 (2016).
Acknowledgements
This research was supported by National nature science foundation of China project (31400010). We thank Dr. Tian Xiao for technical assistance.
Author information
Authors and Affiliations
Contributions
M.L., Y.T., S.B. and H.H. conceived and designed the experiments; M.L. performed the experiments: M.L. and Y.T. analyzed the data; M.L. wrote the paper.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, M., Huang, H., Bao, S. et al. Microbial community structure of soils in Bamenwan mangrove wetland. Sci Rep 9, 8406 (2019). https://doi.org/10.1038/s41598-019-44788-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-44788-x
This article is cited by
-
Community structures of mangrove endophytic and rhizosphere bacteria in Zhangjiangkou National Mangrove Nature Reserve
Scientific Reports (2023)
-
Untapped rich microbiota of mangroves of Pakistan: diversity and community compositions
Folia Microbiologica (2023)
-
Microbial Metabolites Beneficial to Plant Hosts Across Ecosystems
Microbial Ecology (2023)
-
Draft genome sequence of Hahella sp. CR1 and its ability in producing cellulases for saccharifying agricultural biomass
Archives of Microbiology (2023)
-
Microbial diversity and ecological interactions of microorganisms in the mangrove ecosystem: Threats, vulnerability, and adaptations
Environmental Science and Pollution Research (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.