Abstract
Podisus nigrispinus Dallas (Heteroptera: Pentatomidae), released in biological control programs, is a predator of Lepidopteran and Coleopteran species. Lemongrass essential oil and its constituents can be toxic to this natural enemy. The major constituents of lemongrass essential oil are neral (31.5%), citral (26.1%), and geranyl acetate (2.27%). Six concentrations of lemongrass essential oil and of its citral and geranyl acetate constituents were applied to the thorax of P. nigrispinus nymphs and adults. The walking and respiratory behavior of the P. nigrispinus third-instar nymphs, treated with citral and geranyl acetate at the LD50 and LD90 doses, were analyzed with video and respirometer. The lemongrass essential oil toxicity increased from first- to fifth-instar P. nigrispinus nymphs. The P. nigrispinus respiration rates (μL de CO2 h−1/insect) with citral and geranyl acetate in the LD50 and LD90 differed. Nymphs exposed to the lemongrass essential oil and its constituents on treated surfaces presented irritability or were repelled. Podisus nigrispinus adults were tolerant to the lemongrass essential oil and its constituents, geranyl acetate and citral. The altered respiratory activity with geranyl acetate and the fact that they were irritated and repelled by citral suggest caution with regard to the use of the lemongrass essential oil and its constituents in integrated pest management incorporating this predator, in order to avoid diminishing its efficiency against the pests.
Similar content being viewed by others
Introduction
Predatory insects play an important role in insect communities, and are used in biological control to reduce herbivorous arthropod populations1,2. The predatory bug, Podisus nigrispinus Dallas (Heteroptera: Pentatomidae) can control Lepidopteran and Coleopteran pest species which prey on agricultural crops and forest plantations in the Americas3,4,5. The biology and ecology of P. nigrispinus, including its development, morphology6, predator-prey interaction4, and feeding strategies such as extraoral digestion1 have been studied. This insect is reared in the laboratory and released in biological control programs in cotton7, soybean4, and tomato8 crops.
Synthetic insecticides may induce resistance in insects9, cause toxic reactions in mammals10 and other non-target organisms such as parasitoids, pollinators, and predators11,12,13, and may also leave residues14 and cause environmental pollution15. Exposure to insecticide causes adverse effects on the development, longevity and fecundity, and may alter behavior related to mobility and feeding16,17,18. The search for safer insecticides for human health and the environment has resulted in the development of specific compounds for pests which are selective for non-target organisms19,20. In this sense, effective use of P. nigrispinus in integrated pest management (IPM) programs depends on the compatibility of the predator with the other control methods being employed21.
Plant essential oils represent an alternative for pest control with low pollution and quick degradation in the environment, making them suitable for managing insects even in organic farming22,23,24. Plant essential oils are volatile substances, mainly composite mixtures of terpenoids which are used for their aromatic qualities. In plants, terpenoids are products of secondary metabolism and are found in glandular hairs or secretory cavities of the plant cell wall in bark, flowers, fruits, leaves, roots and stems25. Essential oils and their constituents cause lethal and sublethal effects on insects, such as biocide activity, infertility, irritability, phagoinhibition and repellency23,26,27. Essential plant oils can control pests22,23,26,27.
Lemongrass, Cymbopogon citratus (DC. Stapf.), a plant native to India and Sri Lanka28, has antifungal29, anti-inflammatory30 and anti-protozoa31 properties. Predatory insects have a tolerance in relation to essential oils, which emphasizes the importance of the potential success of these natural enemies in IPM programs24. Essential oils have been shown to possess toxic effects against lepidopteran pests such as Euprosterna elaeasa Dyar (Limacodidae)32, Spodoptera exigua Hübner33 and Trichoplusia ni Hübner (Noctuidae)34, and these insects are natural prey of the P. nigrispinus in Brazilian agricultural crops. However, the lethal and sublethal effects caused by essential oils have also been demostrated on this predatory bug35. Podisus nigrispinus is a predator of defoliating pests in different crop systems, but the action of essential oils as insecticide on this natural enemy of those pests needs further studies in order to avoid harming this natural ally.
The objective of this study was to evaluate the lethal and sublethal effects of lemongrass essential oil and its terpenoid constituents (geranyl acetate and citral) on P. nigrispinus.
Results
Lemongrass essential oil toxicity test
Lethal doses of the lemongrass oil increased from first to fifth instars with LD50 of 1.08 to 139.30 μg/insect−1 and LD90 of 2.02 to 192.05 μg/insect−1. The LD50 and LD90 of the lemongrass for third instar P. nigrispinus nymphs was 21.58 and 28.35 μg/insect−1, respectively. Mortality was always <1% in the control (Table 1).
Composition of lemongrass essential oil
A total of 13 compounds from the lemongrass essential oil were identified, which accounted for 95.98% of its total composition (Table 2). The primary compounds of the lemongrass oil were neral (31.5%), citral (26.1%), nonan-4-ol (6.54%), camphene (5.19%), 6-metil-hept-5-en-2-one (4.36%), citronelal (3.83%), β-caryophyllene (3.26%), citronelol (2.95%), caryophyllene oxide (2.63%), γ-muurolene (2.46%), limonene (2.32%), geranyl acetate (2.27%), and geranial (2.15%).
Toxicity of lemongrass commercial constituents
The dose response with geranyl acetate showed that this compound has lower toxicity than citral in third-instar P. nigrispinus nymphs, with LD50 = 33.44 (30.99–37.23) μg/insect−1 and LD90 = 48.34 (42.78–59.99) μg/insect−1, compared to LD50 = 25.56 (23.98–27.60) μg/insect−1 and LD90 = 35.39 (32.15–41.61) μg/insect−1 for the citral (Fig. 1).
Mortality curve, estimated by the dose-response (Probit), of Podisus nigrispinus (Heteroptera: Pentatomidae) nymphs for geranyl acetate and citral at two lethal doses (LD50 and LD90) (X2; P < 0.001). Dotted lines denote 95% confidence intervals. Black dot represents LD50 (citral) and blue LD50 (geranyl acetate) selected to assess the toxic effects.
Effects on the respiratory rate
The respiration rate (μL de CO2 h−1/insect) of third-instar P. nigrispinus nymphs differed between the LD50 and LD90 of geranyl acetate (F2,48 = 4.81; P < 0.001) (Fig. 2a), and of citral (F2,48 = 22.19; P < 0.001) (Fig. 2b). The respiratory rate for third-instar P. nigrispinus nymphs differed between 1 and 3 h of exposure to geranyl acetate (F2,48 = 5.12, P < 0.001) or citral (F2,48 = 8.32; P < 0.001).
Effects on locomotor behavior
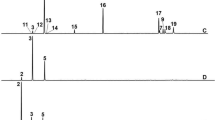
The representative bands of the P. nigrispinus trail in the treated arena part indicated that the geranyl acetate repelled and by citral irritated this predator(Fig. 3). The walking distance with geranyl acetate was shorter (F2,11 = 8.21, P < 0.002) (Fig. 4a) and the immobile period longer (F2,11 = 0.41, P = 0.669), (Fig. 4c). The walking distance of P. nigrispinus exposed to citral did not differ from the control (F2,11 = 0.83, P = 0.449) (Fig. 4b), but the immobile period of this predator (F2,11 = 4.99, P < 0.016) was longer with this compound (Fig. 4d).
Representative locomotor activity tracks of Podisus nigrispinus (Heteroptera: Pentatomidae) nymphs for 10 minutes in filter paper arrays (9 cm in diameter) impregnated in the upper half of each arena with geranyl acetate (a: Control, b: LD50 and c: LD90) or citral (d: Control, e: LD50 and f: LD90). Red tracks indicate high-speed walking and green indicates low speed (initial).
Discussion
The increase in the lethal doses (LD50 and LD90) of the lemongrass essential oil from first to fifth instars of P. nigrispinus suggests that this predator progressively developed a tolerance as it matured. The toxicity of this essential oil is similar to that reported in other studies for this insect36 and for other predators37,38, showing a relatively favorable safety profile for P. nigrispinus, whether through direct use of the oil or by the individual components of the oil serving as precursors for the synthesis of active ingredients of new selective insecticides. Insects may present selectivity mechanisms such as reduction of insecticide penetration through the cuticle, or site insensitivity and/or detoxification or metabolization of the insecticide by enzymes39 to reduce the effect on acetylcholinesterase40 or inhibition of octopamine receptors41. Comparing the contact toxicity of lemongrass essential oil on developmental of P. nigrispinus nymphs showed that the first and second instar were more susceptible followed by the third, fourth and fifth instars; this indicates that high quantities of the lemongrass essential oil are toxic in the early stages of this insect, and that they become more tolerant with age.
The chemical composition of lemongrass essential oil revealed 13 constituents, identified and quantified. Neral, citral, nonan-4-ol, camphene, 6-metil-hept-5-en-2-one, and citronelal were the main compounds that were detected, according to previous reports on terpenoids obtained from lemongrass essential oil42,43,44. However, variations in the abundance of the constituents was observed, including geranial as its main compound42,43,44, depending on the extracted organ, plant age, geographical area of the collection and extraction method45,46. Terpenoids are frequently found in plants, where they play numerous vital roles in plant physiology as well as important functions in all cellular membranes47. Also, the defensive role in plants containing simple terpenoids has been demonstrated, as well as more complex compounds48. In this study, terpenoids are the most abundant constituents of lemongrass essential oil, but the relative proportions of the constituents with insecticide potential45 can vary.
The low toxicity of citral or geranyl acetate for P. nigrispinus may be related to the cuticle of this insect, which acts as a barrier, as reported for Bombyx mori Linnaeus (Lepidoptera: Bombycidae) exposed to deltamethrin49. Cuticular lipids prevent the desiccation and penetration of xenobiotics into insects50, as well as promoting thickening and cuticle composition that can delay the penetration of insecticidal molecules into the body of the insect51, thus reducing the essential oil effect post-application due to rapid degradation or evaporation in the environment52. The lack of detoxifying enzymatic activity (inhibitors of cytochrome P450s, esterases or glutathione S-transferases)53 was observed in Trichoplusia ni (Hübner) (Lepidoptera: Noctuidae) treated with citral, which penetrated through the cuticular layer54. Toxic constituents can affect multiple regions of the insect body, causing necrotic areas which increase progressively throughout the entire insect body26,55. One possible explanation for the low toxicity caused in P. nigrispinus is that there may be differences in the penetration rate of the lemongrass constituents into the body, coupled with the ability of this insect to rapidly detoxify.
The terpenoid constituents of lemongrass essential oil had a negative effect on the P. nigrispinus respiration rate. The reduction of the respiratory rate of third-instar P. nigrispinus nymphs after exposure to citral and geranyl acetate may be due to muscle paralysis, disruption of oxidative phosphorylation processes and dysregulation of the breathing activities18,22,26,56. In this study, P. nigrispinus nymphs exposed to the terpenoid constituents of the essential oil developed low respiration rates, which further unbalanced the organism physiology, as described for Sitophilus granarius Linnaeus (Coleoptera: Curculionidae)57 and Tenebrio molitor Linnaeus (Coleoptera: Tenebrionidae)55.
The short distance traveled in the arenas towards the opposite side from the geranyl acetate by P. nigrispinus nymphs suggest repellent activity. Various insect pests show altered behavioral responses when exposed to lemongrass essential oil constituents, as reported for Bemisia tabaci Gennadius (Hemiptera: Aleyrodidae)58, Culex quinquefasciatus Say (Diptera: Culicidae)59, and Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae)60, influencing the olfactory orientation and insect walking behavior. Insects can identify the presence of compounds, as reported for Acyrthosiphon pisum (Harris) (Hemiptera: Aphididae), after the activation of olfactory receptors in the presence of geranyl acetate61. The results indicate that P. nigrispinus exhibits behavioral avoidance by means of repellence to geranyl acetate, minimizing contact with insecticide-contaminated surfaces. In contrast, nymphs of P. nigrispinus, exposed in arenas with citral, presented irritability and decreased resting periods, which may be related to intoxication in the octapaminergic system, causing hyperactivity or hyperextension in the legs and abdomen62. Essential oils have caused sublethal effects such as increased heart rate, changes in the cAMP level in the nervous system and decreased binding to octopamine receptors, as decribed for Periplaneta americana Linnaeus (Blattodea: Blattidae)62.
Podisus nigrispinus tolerates lemongrass essential oil and its constituents, but geranyl acetate repelled this predator and citral caused irritability. This suggests caution in the use of lemongrass essential oil and these constituents in integrated pest management involving P. nigrispinus. This study may support future research with Cymbopogon citratus and its constituents in the search for bioinsecticides, based on nanoscience, against pests but without effect on this predator.
Methods
Insect mass rearing
Nymphs and adults of P. nigrispinus were obtained from the mass rearing of the ‘Laboratorio de Controle Biológico’ (LCBI) of the ‘Universidade Federal de Viçosa’ (UFV) in Viçosa, Minas Gerais state, Brazil. This predatory bug is reared at room temperature at 25 ± 1 °C, 70 ± 10% RH, and 12 h photophase. Podisus nigrispinus eggs were placed in Petri dishes (12 × 1.5 cm) with cotton soaked with water. Nymphs and adults of this insect were monitored in cubic wooden cages (30 × 30 × 30 cm) covered with nylon. These nymphs and adults were fed ad libitum with Tenebrio molitor L. (Coleoptera: Tenebrionidae) pupae and received Eucalyptus sp. (Myrtaceae) leaves and water2.
Essential oil toxicity test
The lemongrass essential oil was acquired from the ‘Destilaria Bauru Ltda.’ company (Catanduva, São Paulo, Brazil), extracted by hydrodistillation on an industrial scale63. Lemongrass essential oil was diluted in 1 mL of acetone to obtain a stock solution. Six different doses of lemongrass were prepared and used to assess the insecticide toxicity and determine relevant toxicological endpoints; a dilution series of doses (8.1, 16.2, 31.2, 62.5, 125, and 250 µg/insect−1) was used to determine dose-mortality relationship and lethal dose (LD50 and LD90). Acetone was used as a control. Each solution (1 μL) was applied to the thorax of first-, second-, third-, fourth- and fifth-instar nymphs using a micropipette. For each nymph instar, fiften nymphs were tested, placed individually in Petri dishes with one T. molitor pupa per day and cotton soaked with water. The number of dead nymphs in each Petri dish was counted after 36 h.
Identification of the lemongrass essential oil constituents
Quantitative analyses of lemongrass essential oil were performed in triplicate using a gas chromatograph (GC-17A, Shimadzu, Kyoto, Japan) equipped with flame ionization detector (FID). Chromatographic conditions were: a fused silica capillary column (30 m × 0.22 mm) with a DB-5 bonded phase (0.25 μm film thickness); carrier gas N2 at a flow rate of 1.8 mL min−1; injector temperature of 220 °C; detector temperature of 240 °C; column temperature programmed to begin at 40 °C (remaining isothermal for 2 min) and increase at 3 °C min−1 to 240 °C (remaining isothermal at 240 °C for 15 min); 1 μL injection volume (1% w/v in dichloromethane); 1:10 split ratio and 115 kPa column pressure.
Constituents were identified using a gas chromatograph coupled with a mass detector GC/MS (CGMS-QP 5050 A; Shimadzu, Kyoto, Japan). The injector and detector temperatures were 220 °C and 300 °C, respectively. The initial column temperature was 40 °C for 3 min, with a programmed temperature increasing of 3 °C/min to 300 °C, where it was maintained for 25 min. The split mode ratio was 1:10. One microliter of lemongrass essential oil containing 1% (w/v in dichloromethane) was injected and helium used as carrier gas with a flow rate constant of 1.8 mL−1 on the Rtx®-5MS capillary column (30 m, 0.25 mm × 0.25 μm, Bellefonte, USA) using Crossbond® stationary phase (35% diphenyl, 65% dimethyl polysiloxane). The Mass Spectrometer was programmed to detect masses in the range of 29–450 DA with 70 eV ionization energy. Constituents were identified by comparisions of the mass spectra with those available from the National Institute of Standards and Technology (NIST08, NIST11) libraries, the Wiley Spectroteca database (7th edition), and by the retention indices.
Toxicity of lemongrass constituents
Geranyl acetate (97.0% purity) and citral (95.0% purity), identified as constituents of the lemongrass essential oil, were obtained from Sigma Aldrich (Darmstadt, Germany). The efficacy of these constituents was determined by their lethal doses (LD50 and LD90) in the laboratory. Six different doses of each constituent were prepared and used to assess the insecticide toxicity and determine relevant toxicological endpoints; a dilution series of doses (8.1, 16.2, 31.2, 62.5, 125, and 250 µg/insect−1) was used to determine dose-mortality relationship and lethal dose. Acetone was used as a control. Each solution (1 μL) was applied to the thorax of third-instar nymphs using a micropipette. Fiften nymphs were tested, placed individually in Petri dishes with one T. molitor pupa per day, and cotton soaked with water. The number of dead nymphs in each Petri dish was counted after 36 h.
Testing the respiratory rate
Respiration rate bioassays were conducted for 3 h after P. nigrispinus nymphs were exposed to geranyl acetate or and citral (LD50 and LD90 levels). Insects treated with distilled water were used as control. Carbon dioxide (CO2) production (μL of CO2 h−1/insect) was measured with a TR3C CO2 Analyzer (Sable System International, Las Vegas, USA) according to methods adapted from previous studies18,22,55. A third-instar nymph of P. nigrispinus was placed in each respirometry chamber (25 mL) connected to a closed system. After insect acclimation, CO2 production was measured for 12 h at 27 ± 2 °C. Subsequently, compressed oxygen gas (99.99% pure) was introduced into the chamber at 100 mL min−1 for 2 min. The gas flow forces the CO2 through an infrared reader, which continuously measures the CO2 contained inside the chamber. Before and after the experiment, P. nigrispinus nymphs were weighed on an analytical balance (Sartorius BP 210D, Göttingen, Germany). Ten replicates were used for each insecticide treatment and control following a completely randomized design.
Testing locomotion behavior
Nymphs of P. nigrispinus were placed in a Petri dish (90 mm diameter × 15 mm high) lined with filter paper (Whatman no. 1). Then the inner walls of the Petri dish were covered with polytetrafluoroethylene (Dupont®, Barueri, SP, Brazil) to prevent insect escape. Behavioral locomotor response bioassays were conducted in arenas half-treated with 250 µL of geranyl acetate or citral; dishes treated with acetone only were used as control. One P. nigirispinus nymph was released at the center of the arena treated with geranyl acetate or citral (on filter paper) and kept in the Petri dish for 10 min. Forty-eight third-instar P. nigrispinus nymphs were used for each lethal dose (16 per each treatment: control, geranyl acetate or citral), following a completely randomized design. For each insect, walking activity within the arena was recorded using a digital camcorder (XL1 3CCD NTSC, Canon, Lake Success, NY, USA) equipped with a 16 × video lens (Zoom XL 5.5–88 mm, Canon, Lake Success, NY, USA). A video tracking system (ViewPoint LifeSciences, Montreal, Quebec, Canada) was used to analyze the videos and measure the distances that the insects walked and the time spent resting on each half of the arena. Insects that spent less than 1 s on the half of the arena treated with the essential oil or constituent were considered repelled, whereas those that remained less than 50% of the time on the insecticide-treated surface were considered to have been irritated26,55,57.
Statistical analysis
Dose-mortality data were subjected to Probit analysis, generating a dose-mortality curve64. Respiration rates were subjected to two-way ANOVA and Tukey’s HSD test (P < 0.05). Locomotor behavior response data were analyzed by one-way ANOVA, and a Tukey Honestly Significant Difference (HSD) test was also used for comparison of means at the 5% significance level. Toxicity, respiration rate, and locomotor behavior response data were analyzed using SAS for Windows v. 9.065.
References
Martínez, L. C. et al. Stink bug predator kills prey with salivary non-proteinaceous compounds. Insect Biochem. Mol. Biol. 68, 71–78 (2016).
Neves, R. C. D. S., Torres, J. B. & Zanuncio, J. C. Production and storage of mealworm beetle as prey for predatory stinkbug. Biocontrol Sci. Techn. 20, 1013–1025 (2010).
De Oliveira, H. N., Espindula, M. C., Duarte, M. M., Pereira, F. F. & Zanuncio, J. C. Development and reproduction of Podisus nigrispinus (Hemiptera: Pentatomidae) fed with Thyrinteina arnobia (Lepidoptera: Geometridae) reared on guava leaves. Braz. Arch. Biol. Techn. 54, 429–434 (2011).
Ferreira, J. A., Zanuncio, J. C., Torres, J. B. & Molina-Rugama, A. J. Predatory behaviour of Podisus nigrispinus (Heteroptera: Pentatomidae) on different densities of Anticarsia gemmatalis (Lepidoptera: Noctuidae) larvae. Biocontrol Sci. Techn. 18, 711–719 (2008).
Zanuncio, J. C. et al. Predation rate of Spodoptera frugiperda (Lepidoptera: Noctuidae) larvae with and without defense by Podisus nigrispinus (Heteroptera: Pentatomidae). Braz. Arch. Biol. Techn. 51, 121–125 (2008).
Martínez, L. C., Fialho, M. D. C. Q., Zanuncio, J. C. & Serrão, J. E. Ultrastructure and cytochemistry of salivary glands of the predator Podisus nigrispinus (Hemiptera: Pentatomidae). Protoplasma 251, 535–543 (2014).
De Jesus, F. G., Junior, A. L. B., Alves, G. C. & Zanuncio, J. C. Behavior, development, and predation of Podisus nigrispinus (Hemiptera: Pentatomidae) on Spodoptera frugiperda (Lepidoptera: Noctuidae) fed transgenic and conventional cotton cultivars. Ann. Entomol. Soc. Am. 107, 601–606 (2014).
Torres, J. B., Evangelista, W. S., Barras, R. & Guedes, R. N. C. Dispersal of Podisus nigrispinus (Het., Pentatomidae) nymphs preying on tomato leaf miner: effect of predator release time, density and satiation level. J. Appl. Entomol. 126, 326–332 (2002).
Qayyum, M. A. et al. Multiple resistances against formulated organophosphates, pyrethroids, and newer-chemistry insecticides in populations of Helicoverpa armigera (Lepidoptera: Noctuidae) from Pakistan. J. Econ. Entomol. 108, 286–293 (2015).
Arslan, M. et al. Sex-related effects of imidacloprid modulated by piperonyl butoxide and menadione in rats. Part II: genotoxic and cytotoxic potential. Drug Chem. Toxicol. 39, 81–86 (2016).
Penagos, D. I., Cisneros, J., Hernández, O. & Williams, T. Lethal and sublethal effects of the naturally derived insecticide spinosad on parasitoids of Spodoptera frugiperda (Lepidoptera: Noctuidae). Biocontrol Sci. Techn. 15, 81–95 (2005).
Catae, A. F. et al. MALDI-imaging analyses of honeybee brains exposed to a neonicotinoid insecticide. Pest Manag. Sci. 75, 607–615 (2019).
Martínez, L. C. et al. Permethrin induces histological and cytological changes in the midgut of the predatory bug, Podisus nigrispinus. Chemosphere 212, 629–637 (2018).
Liu, X. et al. Residue analysis of four diacylhydrazine insecticides in fruits and vegetables by quick, easy, cheap, effective, rugged, and safe (QuEChERS) method using ultra-performance liquid chromatography coupled to tandem mass spectrometry. Anal. Bioanal. Chem. 401, 1051–1058 (2011).
Wan, N. F. et al. An ecological indicator to evaluate the effect of chemical insecticide pollution management on complex ecosystems. Ecol. Indic. 53, 11–17 (2015).
Martínez, L. C., Plata-Rueda, A., Zanuncio, J. C. & Serrão, J. E. Comparative toxicity of six insecticides on the rhinoceros beetle (Coleoptera: Scarabaeidae). Fla. Entomol. 97, 1056–1063 (2014).
Fiaz, M. et al. Toxicological and morphological effects of tebufenozide on Anticarsia gemmatalis (Lepidoptera: Noctuidae) larvae. Chemosphere 212, 337–345 (2018).
Plata-Rueda, A. et al. Chlorantraniliprole–mediated effects on survival, walking abilities, and respiration in the coffee berry borer. Hypothenemus hampei. Ecotox. Environ. Safe. 172, 53–58 (2019).
Biondi, A. et al. The non-target impact of spinosyns on beneficial arthropods. Pest Manag. Sci. 68, 1523–1536 (2012).
Martínez, L. C. et al. Toxicity and cytotoxicity of the insecticide imidacloprid in the midgut of the predatory bug. Podisus nigrispinus. Ecotox. Environ. Safe. 167, 69–75 (2019).
De Castro, A. A. et al. Demographic parameters of the insecticide-exposed predator Podisus nigrispinus: implications for IPM. BioControl 60, 231–239 (2015).
Fiaz, M. et al. Squamocin induce histological and ultrastructural changes in the midgut cells of Anticarsia gemmatalis (Lepidoptera: Noctuidae). Ecotox. Environ. Safe. 156, 1–8 (2018).
Martínez, L. C., Plata-Rueda, A., Zanuncio, J. C. & Serrão, J. E. Bioactivity of six plant extracts on adults of Demotispa neivai (Coleoptera: Chrysomelidae). J. Insect Sci. 15, 34, https://doi.org/10.1093/jisesa/iev021 (2015).
Pavela, R. & Benelli, G. Essential oils as eco-friendly biopesticides? Challenges and constraints. Trends Plant Sci. 21, 1000–1007 (2016).
Dev, S. T. In: Rowe, J. W. (Ed), Natural products of woody plants. Springer-Verlag, Berlin, pp. 691–807 (1989).
Plata-Rueda, A. et al. Insecticidal activity of garlic essential oil and their constituents against the mealworm beetle, Tenebrio molitor Linnaeus (Coleoptera: Tenebrionidae). Sci. Rep. 7, 46406 (2017).
Amaral, K. D., Martínez, L. C., Lima, M. A. P., Serrão, J. E. & Della Lucia, T. M. C. Azadirachtin impairs egg production in Atta sexdens leaf-cutting ant queens. Environ. Pollut. 243, 809–814 (2018).
Zheng, G., Kenney, P. M. & Lam, L. K. T. Potential anticarcinogenic natural products isolated from lemongrass oil and galanga root oil. J. Agr. Food Chem. 41, 153–156 (1993).
Khan, M. S. A. & Ahmad, I. In vitro antifungal, anti-elastase and anti-keratinase activity of essential oils of Cinnamomum-, Syzygium- and Cymbopogon-species against Aspergillus fumigatus and Trichophyton rubrum. Phytomedicine 19, 48–55 (2011).
Gbenou, J. D. et al. Phytochemical composition of Cymbopogon citratus and Eucalyptus citriodora essential oils and their anti-inflammatory and analgesic properties on Wistar rats. Mol. Biol. Rep. 40, 1127–1134 (2013).
Santin, M. R. et al. In vitro activity of the essential oil of Cymbopogon citratus and its major component (citral) on Leishmania amazonensis. Parasitol. Res. 105, 1489–1496 (2009).
Hernández-Lambraño, R., Caballero-Gallardo, K. & Olivero-Verbel, J. Toxicity and antifeedant activity of essential oils from three aromatic plants grown in Colombia against Euprosterna elaeasa and Acharia fusca (Lepidoptera: Limacodidae). Asian Pac. J. Trop. Biomed. 4, 695–700 (2014).
Murcia-Meseguer, A., Alves, T. J., Budia, F., Ortiz, A. & Medina, P. Insecticidal toxicity of thirteen commercial plant essential oils against Spodoptera exigua (Lepidoptera: Noctuidae). Phytoparasitica 46, 233–245 (2018).
Jiang, Z. L., Akhtar, Y., Zhang, X., Bradbury, R. & Isman, M. B. Insecticidal and feeding deterrent activities of essential oils in the cabbage looper, Trichoplusia ni (Lepidoptera: Noctuidae). J. Appl. Entomol. 136, 191–202 (2012).
Zanuncio, J. C. et al. Toxic effects of the neem oil (Azadirachta indica) formulation on the stink bug predator, Podisus nigrispinus (Heteroptera: Pentatomidae). Sci. Rep. 6, 30261 (2016).
Poderoso, J. C. M., Correia-Oliveira, M. E., Chagas, T. X., Zanuncio, J. C. & Ribeiro, G. T. Effects of plant extracts on developmental stages of the predator Podisus nigrispinus (Hemiptera: Pentatomidae). Fla. Entomol. 99, 113–117 (2016).
Castilhos, R. V., Grützmacher, A. D. & Coats, J. R. Acute toxicity and sublethal effects of terpenoids and essential oils on the predator Chrysoperla externa (Neuroptera: Chrysopidae). Neotrop. Entomol. 47, 311–317 (2018).
Chellappandian, M. et al. Toxicological effects of Sphaeranthus indicus Linn. (Asteraceae) leaf essential oil against human disease vectors, Culex quinquefasciatus Say and Aedes aegypti Linn., and impacts on a beneficial mosquito predator. Environ. Sci. Pollut. Res. 25, 10294–10306 (2018).
Hemingway, J. The molecular basis of two contrasting metabolic mechanisms of insecticide resistance. Insect. Biochem. Mol. Biol. 30, 1009–1015 (2000).
López, M. D. & Pascual-Villalobos, M. J. Mode of inhibition of acetylcholinesterase by monoterpenoids and implications for pest control. Ind. Crop. Prod. 31, 284–288 (2010).
Kostyukovsky, M., Rafaeli, A., Gileadi, C., Demchenko, N. & Shaaya, E. Activation of octopaminergic receptors by essential oil constituents isolated from aromatic plants: possible mode of action against insect pests. Pest Manag. Sci. 58, 1101–1106 (2002).
Abegaz, B., Yohannes, P. G. & Dieter, R. K. Constituents of the essential oil of Ethiopian Cymbopogon citratus Stapf. J. Nat. Prod. 46, 424–426 (1983).
Bassolé, I. H. N. et al. Chemical composition and antimicrobial activity of Cymbopogon citratus and Cymbopogon giganteus essential oils alone and in combination. Phytomedicine 18, 1070–1074 (2011).
Chisowa, E. H., Hall, D. R. & Farman, D. I. Volatile constituents of the essential oil of Cymbopogon citratus Stapf grown in Zambia. Flavour Fragr. J. 13, 29–30 (1998).
Schaneberg, B. T. & Khan, I. A. Comparison of extraction methods for marker compounds in the essential oil of lemon grass by GC. J. Agric. Food Chem. 50, 1345–1349 (2002).
Olivero-Verbel, J., Nerio, L. S. & Stashenko, E. E. Bioactivity against Tribolium castaneum Herbst (Coleoptera: Tenebrionidae) of Cymbopogon citratus and Eucalyptus citriodora essential oils grown in Colombia. Pest Manag. Sci. 66, 664–668 (2010).
Loza-Tavera, H. Monoterpenes in essential oils. In: Chemicals via higher plant bioengineering. Springer US, 49–62 (1999).
Lerdau, M., Litvak, M. & Monson, R. Plant chemical defense: monoterpenes and the growth-differentiation balance hypothesis. Trends Ecol. Evol. 9, 58–61 (1994).
Xiong, G. et al. Cuticular protein defective Bamboo mutant of Bombyx mori is sensitive to environmental stresses. Pestic. Biochem. Physiol. 148, 111–115 (2018).
Yu, Z. et al. The ABC transporter ABCH-9C is needed for cuticle barrier construction in Locusta migratoria. Insect Biochem. Mol. Biol. 87, 90–99 (2017).
Balabanidou, V., Grigoraki, L. & Vontas, J. Insect cuticle: a critical determinant of insecticide resistance. Curr. Opin. Insect Sci. 27, 68–74 (2018).
Pavela, R. & Sedlák, P. Post-application temperature as a factor influencing the insecticidal activity of essential oil from Thymus vulgaris. Ind. Crop. Prod. 113, 46–49 (2018).
Tak, J. H. & Isman, M. B. Metabolism of citral, the major constituent of lemongrass oil, in the cabbage looper, Trichoplusia ni, and effects of enzyme inhibitors on toxicity and metabolism. Pestic. Biochem. Physiol. 133, 20–25 (2016).
Tak, J. H., Jovel, E. & Isman, M. B. Effects of rosemary, thyme and lemongrass oils and their major constituents on detoxifying enzyme activity and insecticidal activity in Trichoplusia ni. Pestic. Biochem. Physiol. 140, 9–16 (2017).
Martínez, L. C. et al. Toxic effects of two essential oils and their constituents on the mealworm beetle, Tenebrio molitor. Bull. Entomol. Res. 108, 716–725 (2018).
Corrêa, A. S. et al. Are mitochondrial lineages, mitochondrial lysis and respiration rate associated with phosphine susceptibility in the maize weevil Sitophilus zeamais? Ann. Appl. Biol. 165, 137–146 (2014).
Plata-Rueda, A. et al. Terpenoid constituents of cinnamon and clove essential oils cause toxic effects and behavior repellency response on granary weevil, Sitophilus granarius. Ecotoxicol Environ Safe. 156, 263–270 (2018).
Deletre, E., Chandre, F., Barkman, B., Menut, C. & Martin, T. Naturally occurring bioactive compounds from four repellent essential oils against Bemisia tabaci whiteflies. Pest Manag. Sci. 72, 179–189 (2016).
Leal, W. S. & Uchida, K. Application of GC-EAD to the determination of mosquito repellents derived from a plant, Cymbopogon citratus. J. Asia-Pac. Entomol. 1, 217–221 (1998).
Bossou, A. D. et al. Characterization of volatile compounds from three Cymbopogon species and Eucalyptus citriodora from Benin and their insecticidal activities against Tribolium castaneum. Ind. Crop. Prod. 76, 306–317 (2015).
Zhang, R. et al. Molecular basis of alarm pheromone detection in aphids. Curr. Biol. 27, 55–61 (2017).
Enan, E. Insecticidal activity of essential oils: octopaminergic sites of action. Comp. Biochem. Physiol. C. 130, 325–337 (2001).
Dapkevicius, A., Venskutonis, R., Van Beek, T. A. & Linssen, J. P. H. Antioxidant activity of extracts obtained by different isolation procedures from some aromatic herbs grown in Lithuania. J. Sci. Food. Agr. 77, 140–146, https://doi.org/10.1002/(SICI)1097-0010(199805)77:1<140::AID-JSFA18>3.0.CO;2-K (1998).
Finney, D. J. Probit Analysis. Cambridge University Press, Cambridge, UK (1964).
SAS Institute. The Statistical Analysis System. SAS Institute, Cary, NC, USA, http://www.sas.com (2002).
Acknowledgements
To “Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (grant number 151566/2018-6), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)- Finance Code 001, Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), and “Programa Cooperativo sobre Proteção Florestal’ (PROTEF) do Instituto de Pesquisas e Estudos Florestais (IPEF)” for finantial support. David Michael Miller, a professional editor and proofreader and a native English speaking, has reviewed and edited this article for structure, grammar, punctuation, spelling, word choice, and readability.
Author information
Authors and Affiliations
Contributions
B.P.B., A.P.R., L.C.M., J.E.S. and J.C.Z. designed the research; B.P.B., A.P.R., L.C.M., A.G.C., M.A.S., B.M.C.C. and C.F.W. performed the experiments; B.P.B., L.C.M., A.G.C., B.M.C.C., M.A.S., J.E.S. and C.F.W. analyzed the data; All authors wrote and approved the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Brügger, B.P., Martínez, L.C., Plata-Rueda, A. et al. Bioactivity of the Cymbopogon citratus (Poaceae) essential oil and its terpenoid constituents on the predatory bug, Podisus nigrispinus (Heteroptera: Pentatomidae). Sci Rep 9, 8358 (2019). https://doi.org/10.1038/s41598-019-44709-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-44709-y
This article is cited by
-
The antioxidant and antimicrobial activity of ethanolic extract in roots, stems, and leaves of three commercial Cymbopogon species
BMC Complementary Medicine and Therapies (2024)
-
A sustainable green-approach for biofabrication of chitosan nanoparticles, optimization, characterization, its antifungal activity against phytopathogenic Fusarium culmorum and antitumor activity
Scientific Reports (2024)
-
Insecticidal activity of Thymus pallescens de Noë and Cymbogon citratus essential oils against Sitophilus zeamais and Tribolium castaneum
Scientific Reports (2024)
-
Enhancement of lemongrass essential oil physicochemical properties and antibacterial activity by encapsulation in zein-caseinate nanocomposite
Scientific Reports (2024)
-
Development and Characterization of Cymbopogon winterianus (Jowitt) Essential Oil-Based Nano-Emulsion for Larvicidal and Antifeedant Activity Against Spodoptera litura (Fab.) (Lepidoptera: Noctuidae)
BioNanoScience (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.