Abstract
Species interactions are known to be key in driving patterns of biodiversity across the globe. Plant-plant interactions through heterospecific pollen (HP) transfer by their shared pollinators is common and has consequences for plant reproductive success and floral evolution, and thus has the potential to influence global patterns of biodiversity and plant community assembly. The literature on HP transfer is growing and it is therefore timely to review patterns and causes of among-species variation in HP receipt at a global scale, thus uncovering its potential contribution to global patterns of biodiversity. Here we analyzed published data on 245 species distributed across five continents to evaluate latitudinal and altitudinal patterns of HP receipt. We further analyzed the role of floral symmetry and evolutionary history in mediating patterns of HP receipt. Latitude and elevation affected the likelihood and intensity of HP receipt indicating that HP transfer increases in species-rich communities and in areas with high abundance of vertebrate pollinators. Floral symmetry and evolutionary history determined HP load size across plant communities worldwide. Overall, our results suggest that HP receipt may have the potential to contribute to global geographic patterns of plant diversity by imposing strong selective pressures in species-rich areas across the globe.
Similar content being viewed by others
Introduction
Understanding the factors that generate and organize plant diversity in nature has been a long-standing goal in ecology. The importance of indirect plant-plant interactions (i.e. pollinator competition and facilitation) in these two processes has been widely studied, and these have been shown to play a major role1,2,3,4,5. In contrast, the ecological and evolutionary consequences of direct plant-plant interactions via heterospecific pollen (hereafter HP) transfer have received considerably less attention. In co-flowering communities high levels of pollinator sharing6,7,8,9 and heterospecific pollen (hereafter HP) transfer are common (e.g. up to 70% of total pollen load10,11,12). Further evidence shows that HP receipt can decrease plant reproductive success (~20% decrease in seed production) by physically or chemically interfering with conspecific ovule fertilization10,13. These negative effects are widespread and have been shown from animal13- and wind-dispersed HP donors14, even if HP deposition occurs in small amounts (e.g. <5 pollen grains15). As a result, HP transfer can be a strong, but perhaps underestimated force driving floral evolution13,16,17,18 and co-flowering community assembly10,19. Knowledge on the full extent as well as the causes and consequences of HP receipt is thus a key step towards a more complete understanding of the processes that generate and organize plant diversity in nature.
Increasing evidence suggests that HP transfer is common in natural communities10,11,20,21,22,23. However, the frequency and intensity of HP receipt varies greatly among plant species (2–100% of flowers, 0.1–74% of total pollen load10), and the underlying causes of this variation are largely unknown. To date, this variation has been evaluated among-species within a single plant community or community type20,21,23,24. However, patterns and factors mediating HP receipt might also vary across large geographical scales24. Knowledge of large-scale geographic patterns of HP transfer dynamics is central for uncovering its potential for contributing to global trends in floral diversification and in mediating patterns of community assembly across plant communities worldwide.
A global pattern of latitudinal and altitudinal variation in plant species diversity has been widely demonstrated, with increasing species diversity with decreasing latitude25,26,27,28,29,30,31, and at mid to low elevations26,32,33,34. Interestingly, it has also been shown that HP receipt can increase with increasing plant species richness35. Thus, it is plausible that plant species growing in communities near the equator and at low elevations, where plant diversity tends to be the highest, will be at greater risk of receiving HP. Evidence of such geographic pattern in HP receipt could suggest a potential role of HP in contributing to global patterns of floral diversification and plant diversity distribution. High levels of HP receipt can select for HP tolerance and avoidance strategies10,13, thus imposing strong selective pressures on a wide array of morphological1,16,17,36,37,38 and reproductive traits18,39,40. When HP transfer is low, and/or inconsistent, these selective pressures can be predicted to be minimal, while the contrary would be expected when HP transfer is high10,13. Higher amounts of HP transfer in regions near the equator could also help explain the global decrease in plant reproductive success observed in these areas41. Thus, selection on traits that maximize reproductive success by avoiding or tolerating HP effects can be expected to be stronger in species-rich areas, leading to further diversification and contributing to observed latitudinal patterns of plant biodiversity. Biotic interactions have long been predicted to play a key role in generating latitudinal patterns of biodiversity42,43, and plant-plant interactions via HP transfer may not be the exception.
In addition to a plant’s geographical location (latitude and elevation), differences in floral symmetry (radial vs. bilateral), a broad indicator of the level of pollinator generalization, may also contribute to among-species variation in HP receipt20,21,24. Plants with radial flowers are expected to be visited by a higher number of pollinator species and to receive larger and more diverse HP loads compared to those with bilateral flowers21,24. This prediction has been tested within single communities with inconclusive results20,21, and thus whether floral symmetry (pollinator generalization) is a mediator of HP transfer dynamics acting across large geographical scales is not yet tested. It is also possible that other species-shared floral characteristics may influence HP receipt (e.g. stigma size, style exertion11,23), and thus closely related species can be expected to receive similar amounts of HP as a result of their shared evolutionary history. However, to our knowledge, the strength of the phylogenetic signal underlying patterns of HP receipt has not been evaluated in any system.
Uncovering the factors that mediate among-species variation in HP receipt at large geographical and evolutionary scales is key if we want to predict its potential ecological and evolutionary consequences, particularly in light of large community-wide changes in pollen transfer dynamics11 that result from human disturbances22,23. In this study we analyze published data on 245 species to evaluate the effects of latitude, elevation, pollinator generalization and evolutionary history in mediating patterns of HP receipt at a global scale. Specifically we ask the following questions: 1) Does the likelihood and intensity of HP receipt increase with decreasing latitude and/or elevation? 2) Is the likelihood and intensity of HP receipt greater in flowers with radial (generalized) versus bilateral (specialized) symmetry? 3) Does the effect of floral specialization in mediating patterns of HP receipt depend on a plant species’ geographic location (latitude or elevation)? And finally, 4) is there a phylogenetic signal on the likelihood and/or intensity of HP receipt?
Results
Our dataset included species located in five continents, and their distribution ranged from 63°N to 41°S in latitude and from 0 to 3336 meters above sea level (Fig. 1; Supplementary Data). Average HP load size ranged from 0 to 368.5 pollen grains (mean ± SE; 11.83 ± 2.15).
We found a significant phylogenetic signal in HP load size itself (λ = 0.99, K = 0.81, P < 0.05 for both; Fig. 2) and in the residuals of the model (λ = 0.7, P < 0.01). We also found a significant effect of latitude (t211 = 2.7, P < 0.01) and elevation (t211 = 3.5, P = 0.001) on average HP load size on stigmas. More importantly, however, we found a significant latitude by elevation interaction (t211 = −3.8, P = 0.001; Fig. 3) indicating that both act in combination to influence patterns of HP receipt (in flowers that receive ≥1 HP grain; Fig. 3). Our results also showed that HP load size (≥1 HP grain received) is significantly larger in radial (14.64 ± 3.6) compared to bilateral (11.6 ± 2.8) flowers (t211 = 3.06, P < 0.01), however this effect varied with elevation (symmetry by elevation interaction: t211 = 2.5, P = 0.01). While HP receipt increased for both type of flowers (radial and bilateral) with decreasing elevation, the increase was significantly more pronounced for bilateral flowers (Fig. 4). Radial flowers on the other hand, receive more HP than bilateral flowers at high elevations and the increase in HP receipt with decreasing elevation was less steep (Fig. 4). It is important to note that even though the range of elevations was larger for radial compared to bilateral flowers (Fig. 4) this same result was observed when we only considered the altitudinal range for which we have data for both, radial and bilateral flowers (up to 2000 m.a.s.l, N = 153; symmetry by elevation interaction, P = 0.03). The interaction between latitude and symmetry was not significant (P > 0.05) and its exclusion improved the overall fit of the model.
Phylogenetic relationships among the 245 species evaluated in this study. Heterospecific pollen load size (log transformed) for each species is mapped onto the phylogeny and represented by the color of each branch. Phylogenetic relationships were generated from the maximally resolved tree of seed plants within Phylomatic.
Variation in heterospecific pollen (HP) load size (log transformed) across 217 species according to their altitudinal (meters above sea level) and latitudinal location. Different colors reflect variation in the intensity of HP receipt and the predicted surface indicates geographic areas of high and low intensity of HP receipt.
Variation in heterespoecific pollen (HP) load size (log transformed) across 217 species according to their floral symmetry and altitudinal location (meters above sea level). Plant species have been divided based on their floral symmetry into radial (black circles) and bilateral (red triangles) flowers. Both slopes are significant at P < 0.05 (see results).
There was no phylogenetic signal on the likelihood (presence/absence) of receiving HP itself (D = 0.9, P > 0.05) or in the residuals of the model (S2 = 0.25, P > 0.05). Elevation (Z241 = −2.1, P = 0.02) but not latitude (Z241 = −1.01, P = 0.3) significantly affected the likelihood of receiving HP. As before, we found a significant latitude by elevation interaction (z241 = 2.06, P = 0.03; Fig. 5). Neither floral symmetry nor its interactions with latitude and elevation were significant (P > 0.05 for all) and their exclusion improved the overall fit of the model.
Discussion
Our study revealed a high incidence of HP receipt at a global scale. Of the 245 species evaluated, 88% (217) showed some degree of HP receipt, thus emphasizing the ubiquity of these direct plant-plant interactions in nature11,22,24. We also found strong evidence suggesting that species’ geographic location (latitude and elevation) and degree of pollinator generalization (as indicated by flower symmetry) are strong predictors of the likelihood and intensity of HP receipt across plant communities worldwide.
Our results revealed that elevation and latitude interactively influence the intensity of HP receipt. In high latitude sites, HP receipt tends to be higher at low elevations (Fig. 3). This pattern is consistent with the higher diversity of floral resources at these lower elevations28,30,32,34, which would lead to higher incidence of pollinator movements, and pollen transfer, between plant species. Indeed, some of our studies that observed high levels of HP transfer18,35 come from plant biodiversity hotspots that occur at relatively low elevations and high latitudes, such as the California floristic province44,45 and the Mediterranean basin in Europe46,47 (Fig. 1). However, at low latitudes (e.g. tropical regions), HP receipt increased with increasing elevation (Fig. 3). Although this pattern seems inconsistent with our initial prediction it may indicate that not only the diversity of the co-flowering community but the composition of the pollinator community plays an important role in mediating patterns of HP receipt24. For instance, marked differences in pollinator species composition across altitudinal gradients in the tropics can be expected. Pollinator community composition in tropical rainforests (ca. 1000–5000 m.a.s.l.) can consist of a high diversity and abundance of vertebrate species such as bats, hummingbirds, and even monkeys17,48,49,50,51. Vertebrate pollinators are typically large in size and are known to carry and deposit large HP loads compared to invertebrate pollinators (e.g. beetles, bees, flies, butterflies) that are more common at low elevations (0–100 m.a.s.l.) in tropical and sub-tropical regions52,53,54. For instance, in a species-rich cloud forest in Ecuador (1300–2300 m.a.s.l.) bat species have been shown to deliver large and diverse HP loads to stigmas17,55. Hummingbirds at high elevations (1200 m.a.s.l.) in Costa Rica have also been observed carrying large HP loads of up to six different plant species51. Large vertebrate pollinators are less diverse and abundant outside of the tropics48,56, and thus the diversity of the co-flowering community may play a larger role in mediating patterns of HP receipt at these higher latitudes. Overall, these results suggest that differences in pollinator body size and foraging behavior may mediate the frequency and amount of HP transfer24,57.
Interestingly, even though HP load size increased with elevation in the tropics (Fig. 3), the likelihood of receiving HP was the lowest in this region (Fig. 5). In a similar manner, the likelihood of receiving HP was the highest in high-latitude and low-elevation areas (Fig. 5), where the intensity of HP receipt (HP load size) was the lowest (Fig. 3). These results suggest a potential decoupling of these two processes such that the likelihood of receiving HP and the intensity of HP receipt (HP load size) may be driven by different forces (e.g. random events vs, pollinator size). It is important to point out that in our dataset the number of cases where no HP was received is limited and thus more studies are needed (see below). Nonetheless, our results suggest that distinguishing between these two ecological processes (i.e. likelihood and intensity of HP receipt) is key in order to develop a more predictive understanding of the factors that mediate patterns of HP transfer in nature and how these may vary as a result of human-mediated disturbances22,23.
Our findings of higher levels of HP receipt in geographic regions that are predicted to possess high levels of plant diversity, such as in cloud forests and Mediterranean communities, suggest that HP transfer could act as a strong selective force contributing to higher floral diversification in these regions. It has been proposed that HP receipt can lead to the evolution of several HP tolerance and avoidance strategies10,13. In fact, HP receipt has been shown to exert a wide variety of selective pressures on plants including morphological traits (e.g. flower size, shape, color, style length and stigma size)1,10,16,17,36,38, physiological processes (e. g. pollen tube growth and germination)10,24, mating systems37,39,40, and flowering phenology19,58,59. High levels of HP receipt cannot only impose selection via female fitness but also through male fitness costs13,17,60. For instance, it has been shown that HP transfer can be a strong driver of specialization in pollination systems due to high costs of conspecific pollen loss to heterospecific flowers60. Thus, it is not unreasonable to expect that higher levels of HP transfer can impose strong and wide-ranging selective pressures that contribute differentially to floral diversification across the globe. Global patterns in HP receipt may also contribute to the high levels of pollen limitation observed in species-rich areas41, further strengthening its role in floral evolution and plant community assembly10,13 in these regions. HP pollen receipt is thus an untested mechanism that might contribute to overall patterns of pollen limitation. Even though the importance of biotic interactions in contributing to global patterns of diversity has been well documented for a large number of antagonistic and mutualistic interactions42,43, the potential for HP transfer interactions in contributing to these patterns has so far been overlooked.
Our results revealed that radial flowers, which are considered more generalized in their pollination system than bilateral flowers20,21,61, receive slightly higher amounts of HP, and that HP load size increases with decreasing elevation in both types of flowers (Fig. 4). However, the increase in HP receipt with decreasing elevation was more pronounced for bilateral compared to radial flowers, which tend to receive higher amounts of HP at high elevations (Fig. 4). These results support our prediction of higher HP receipt in generalized, open flowers (radial symmetry), compared to specialized ones (bilateral symmetry). Interestingly, however, our results also suggest that differences in HP receipt between the two flower types (radial vs. bilateral) diminish with decreasing elevation (Fig. 4), where plant diversity tends to be the highest. Overall, these results suggest that floral symmetry (pollinator generalization) may only be a good predictor of HP receipt in plant communities with low species richness such as those at high elevations. These results also suggest that, in low-elevation areas that tend to be species-rich, HP transfer is high across all species regardless of floral symmetry. We also detected a significant phylogenetic signal in the intensity of HP receipt even after accounting for floral symmetry, suggesting that other shared plant traits are still important in mediating the amount of HP received (e.g. stigma area and style exertion11,23). On the other hand, we did not detect a phylogenetic signal on the probability of receiving HP. This suggests that whether plants receive HP or not may be strongly influenced by random ‘incidental’ pollination events (e.g. indiscriminate visits to flowers by young bees, misperception of floral cues by inexperienced floral visitors)24,62, or by wind-dispersed pollen transfer63, thus diminishing the importance of shared floral characteristics.
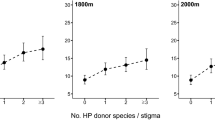
It is important to acknowledge that even though our findings are consistent with the prediction of higher intensity of HP receipt in areas with high plant diversity and with high abundance of large vertebrate pollinators across the globe, these patterns do not necessarily reflect causation. Experimental assessment of patterns, and the ecological and evolutionary consequences of HP receipt, across gradients of plant35 and pollinator diversity would be valuable in confirming the predictions outlined in this study. It is also important to note that even though we observed strong global geographic trends the number of studies documenting patterns of HP receipt is still limited, and strongly biased towards temperate systems (largely concentrated in the United States and Europe; Fig. 1). Studies on HP transfer in diverse regions in Africa and South America are largely underrepresented. Furthermore, in species-rich areas, HP loads may not only be large but also diverse (e.g. >7 species11), leading to stronger and synergistic negative effects on plant fitness64 with so far unknown consequences. However, we were unable to test for global geographic patterns in the diversity of the HP load given the small number of studies that have reported average or total number of HP donor species per stigma (8 studies). Bias in studies of HP receipt to date is not only geographical but also phylogenetic. For instance, large groups of plants such as monocotyledons have been poorly represented in these studies (Fig. 2). Thus, we stress the need to evaluate patterns of variation in the diversity as well as in the intensity of HP receipt at larger phylogenetic scales, particularly in tropical regions where its ecological and evolutionary consequences might be stronger. Such studies are critical in order to develop a more predictive understanding of the ecological and evolutionary consequences of plant-plant interactions via HP transfer in natural communities across the globe.
Methods
Data set
To evaluate patterns of HP receipt at a global scale we collected data from published studies that have reported an average amount of HP on stigmas for one or multiple species in nature. We avoided studies where the diversity and/or composition of the co-flowering community had been experimentally manipulated and only considered studies that reported naturally deposited HP loads. We started by gathering data reported in Appendix S1 in Ashman and Arceo-Gómez10. This dataset contained 77 species from 17 studies from 1986 to 201210. We complemented this data by conducting a literature search for studies published between 2012 and 2017 using ISI Web of Science and Google Scholar (key words: heterospecific pollen*, pollen transfer*, pollen load*, pollen*, pollinator sharing*, pollination*). We also included two unpublished datasets, one from the sand dune ecosystem in Yucatan, Mexico (6 species; Parra-Tabla V. unpublished data) and one from a grassland community in Hampton Creek Cove Park Natural Park in Tennessee, USA (26 species; Arceo-Gómez G. unpublished data). In total we compiled information for 279 study cases from 28 different studies distributed across five different continents (Fig. 1). In some cases, data on HP deposition was reported for the same species at the same location multiple times (e.g. different years) and in these cases an average per species at that location was estimated. If the same species was sampled in different geographic locations (i.e. elevation or latitude) one study (species/location combination) was randomly selected for data analyses since phylogenetic models (see below) do not allow for replication of species in the dataset. As a result, 34 observations from 14 species were excluded from this study but the selection of species did not influence the results (Arceo-Gómez G. unpublished data). In total, we analyzed data for 245 species from 26 different studies distributed across five different continents (Fig. 1) and across 52 plant families (Fig. 2; Supplementary Data). For each species, we recorded information on average HP load size (average number of HP grains on stigmas). When data was not available in the text we extracted it from figures using DataThief65. When studies only reported the total amount of HP found on stigmas we used sample size data reported to estimate an average. For each species, we also documented its latitudinal (i.e. GPS coordinates) and altitudinal location (meters above sea level). Latitudinal coordinates were converted to decimal degrees and the absolute values were used in analyses. Data on species altitudinal and latitudinal location was gathered from the original study. When information regarding elevation was not provided in the original study, it was estimated using the GPS coordinates reported and topographic data from Google Earth. We also recorded information on floral symmetry and categorized each species as actinomorphic (radial flowers) or zygomorphic (bilateral flowers). Floral symmetry has been commonly used as a broad indicator of pollinator generalization (radial flowers) and specialization (bilateral flowers20,21,61,66). When information on floral symmetry for HP recipient species was not available in the original study it was gathered from additional published sources.
Data analyses
We evaluated the effects of elevation, latitude, floral symmetry and their interaction on the likelihood and intensity (HP load size) of HP receipt using phylogenetic least square models (PGLS) to account for species’ shared evolutionary history67,68. For this, we constructed a phylogeny using the most recent megatree in ‘Phylomatic’ (R20160415.new) as our base tree69. The final phylogenetic tree was adjusted with branch lengths scaled to time using the BLADJ function in ‘Phylocom’70. With this information we estimated phylogenetic signal on the response variables themselves (likelihood and intensity of HP receipt) and on the residuals of each model68. For this, we calculated Pagel’s λ71 and K-statistic indexes72,73 using the function ‘phylosig’ in R74. λ is a scaling parameter for the covariance matrix of species traits, relative to the covariance expected under Brownian evolution73. K is a scaled ratio of the trait similarity variance among species over the contrasts phylogenetic variance72,73. These two indexes vary between zero (no phylogenetic signal) and 1 (complete phylogenetic signal under a Brownian model of trait evolution) and are considered the most robust indexes of phylogenetic signal even in the presence of polytomies73,75. We evaluated if phylogenetic signal was significantly different from zero using a likelihood ratio test and null model analysis (1000 randomizations) for ‘λ’ and ‘K’ respectively using Phytools76 and the caper packages77 in R74. If observed phylogenetic signal was not different from zero then a non-phylogenetic model was used in the analysis.
When evaluating effects on the intensity of HP receipt we were interested in evaluating how our predictor variables influenced HP load size and thus we only used the subset of species that receive ≥1 pollen grains for this analysis (N = 217). Heterospecific pollen load size was log transformed. The analysis was conducted using the package APE78 in R74. For evaluating effects on the likelihood of receiving HP we used the entire data set (N = 245). For this, we converted data for each species into a binary trait, 0 (no HP received) or 1 (HP received) and used logistic regression79 to analyze its relationship with latitude, elevation and floral symmetry. For this particular analysis we used the ‘D-statistic’ and ‘S2’ indexes for estimating phylogenetic signal on the response variable itself and on the residuals of the model, as these are more appropriate for binary data79,80. Since no phylogenetic signal was found (see results), we used a non-phylogenetic model to evaluate effects on the likelihood of receiving HP. Estimation of phylogenetic signal was conducted using the package Phylom in R81.
We conducted backwards stepwise regression in all the analyses and used Akaike information criterion (AIC) to avoid overparametrization of the models and identify the models with the best fit. We predicted that the likelihood and intensity of HP receipt would decrease at high latitudes and in high elevations and it would be greater for radial compared to bilateral flowers.
Data Availability
All data generated and analyzed during this study are included in this published article (and its Supplementary Information Files).
References
Caruso, C. M. Competition for pollination influences selection on floral traits of Ipomopsis aggregata. Evolution. 54, 1546–1557 (2000).
Moeller, D. A. Facilitative interactions among plants via shared pollinators. Ecology 85, 3289–3301 (2004).
Ghazoul, J. Floral diversity and the facilitation of pollination. J. Ecol. 94, 295–304 (2006).
Sargent, R. D. & Ackerly, D. D. Plant-pollinator interactions and the assembly of plant communities. Trends Ecol. Evol. 23, 123–130 (2008).
Mitchell, R. J., Flanagan, R. J., Brown, B. J., Waser, N. M. & Karron, J. D. New frontiers in competition for pollination. Ann. Bot. 103, 1403–1413 (2009).
Olesen, J. M. & Jordano, P. Geographic Patterns in Plant-Pollinator Mutualistic Networks. Ecology 83, 2416–2424 (2002).
Bascompte, J., Jordano, P., Melián, C. J. & Olesen, J. M. The nested assembly of plant-animal mutualistic networks. Proc. Natl. Acad. Sci. 100, 9383–7 (2003).
Bascompte, J. & Jordano, P. Plant-Animal Mutualistic Networks: The Architecture of Biodiversity. Annu. Rev. Ecol. Evol. Syst. 38, 567–593 (2007).
Martín González, A. M., Dalsgaard, B. & Olesen, J. M. Centrality measures and the importance of generalist species in pollination networks. Ecol. Complex. 7, 36–43 (2010).
Ashman, T. L. & Arceo-Gómez, G. Toward a predictive understanding of the fitness costs of heterospecific pollen receipt and its importance in co-flowering communities. Am. J. Bot. 100, 1061–1070 (2013).
Fang, Q. & Huang, S. Q. A directed network analysis of heterospecific pollen transfer in a biodiverse community. Ecology 94, 1176–1185 (2013).
Tur, C., Sáez, A., Traveset, A. & Aizen, M. A. Evaluating the effects of pollinator-mediated interactions using pollen transfer networks: Evidence of widespread facilitation in south Andean plant communities. Ecology Letters 19 (2016).
Morales, C. L. & Traveset, A. Interspecific Pollen Transfer: Magnitude, Prevalence and Consequences for Plant Fitness. CRC. Crit. Rev. Plant Sci. 27, 221–238 (2008).
Arceo-Gómez, G., Jameel, M. I. & Ashman, T.-L. Effects of heterospecific pollen from a wind-pollinated and pesticide-treated plant on reproductive success of an insect-pollinated species. Am. J. Bot. 105, 836–841 (2018).
Thomson, J. D., Andrews, B. J. & Plowright, R. C. The effect of a foreign pollen on ovule development in Diervilla lonicera (Caprifoliaceae). New Phytol. 90, 777–783 (1982).
Armbruster, W. S., Edwards, M. E. & Debevec, E. M. Floral character displacement generates assemblage structure of Western Australian triggerplants (Stylidium). Ecology 75, 315–329 (1994).
Muchhala, N. & Thomson, J. D. Interspecific competition in pollination systems: Costs to male fitness via pollen misplacement. Funct. Ecol. 26, 476–482 (2012).
Arceo-Gómez, G., Raguso, R. A. & Geber, M. A. Can plants evolve tolerance mechanisms to heterospecific pollen effects? An experimental test of the adaptive potential in Clarkia species. Oikos 125, 718–725 (2016).
Waser, N. M. Interspecific pollen transfer and competition between co-occurring plant species. Oecologia 36, 223–236 (1978).
McLernon, S. M., Murphy, S. D. & Aarssen, L. W. Heterospecific pollen transfer between sympatric species in a midsuccessional old‐field community. Am. J. Bot. 83, 1168–1174 (1996).
Montgomery, B. R. & Rathcke, B. J. Effects of floral restrictiveness and stigma size on heterospecific pollen receipt in a prairie community. Oecologia 168, 449–458 (2012).
Emer, C., Vaughan, I. P., Hiscock, S. & Memmott, J. The impact of the invasive alien plant, impatiens glandulifera, on pollen transfer networks. PLoS One 10, 1–16 (2015).
Johnson, A. L. & Ashman, T. L. Consequences of invasion for pollen transfer and pollination revealed in a tropical island ecosystem. New Phytol. 221, 142–154 (2018).
Arceo-Gómez, G. et al. Patterns of among- and within-species variation in heterospecific pollen receipt: The importance of ecological generalization. Am. J. Bot. 103, 396–407 (2016).
Pianka, E. R. Latitudinal gradients in species diversity: A review of concepts. Am. Nat. 100, 33–46 (1966).
Gentry, A. H. Changes in plant community diversity and floristic composition on environmental and geographical gradients. Ann. Missouri Bot. Gard. 1–34 (1988).
Kevin, J. Gaston. Global patterns in biodiversity. Nature 405, 220 (2000).
Willig, M. R., Kaufman, D. M. & Stevens, R. D. Latitudinal gradients of biodiversity: pattern, process, scale, and synthesis. Annu. Rev. Ecol. Evol. Syst. 34, 273–309 (2003).
Hillebrand, H. On the generality of the latitudinal diversity gradient. Am. Nat. 163, 192–211 (2004).
Kreft, H. & Jetz, W. Global patterns and determinants of vascular plant diversity. Proc. Natl. Acad. Sci. 104, 5925–5930 (2007).
Mittelbach, G. G. et al. Evolution and the latitudinal diversity gradient: speciation, extinction and biogeography. Ecol. Lett. 10, 315–331 (2007).
Givnish, T. J. Altitudinal gradients in tropical forest composition, structure, and diversity in the Sierra de Manantlán. J. Ecol. 86, 999–1020 (1998).
Odland, A. & Birks, H. J. B. The altitudinal gradient of vascular plant richness in Aurland, western Norway. Ecography. 22, 548–566 (1999).
Ohlemüller, R. & Wilson, J. B. Vascular plant species richness along latitudinal and altitudinal gradients: a contribution from New Zealand temperate rainforests. Ecol. Lett. 3, 262–266 (2000).
Arceo-Gómez, G. & Ashman, T.-L. Coflowering community context influences female fitness and alters the adaptive value of flower longevity in Mimulus guttatus. Am. Nat. 183, E50–E63 (2014).
Muchhala, N. & Potts, M. D. Character displacement among bat-pollinated flowers of the genus Burmeistera: analysis of mechanism, process and pattern. Proc. R. Soc. B Biol. Sci. 274, 2731–2737 (2007).
Smith, R. A. & Rausher, M. D. Selection for character displacement is constrained by the genetic architecture of floral traits in the ivyleaf morning glory. Evol. Int. J. Org. Evol. 62, 2829–2841 (2008).
Hopkins, R. & Rausher, M. D. Pollinator-mediated selection on flower color allele drives reinforcement. Science. 335, 1090–1092 (2012).
Fishman, L. & Wyatt, R. Pollinator‐mediated competition, reproductive character displacement, and the evolution of selfing in Arenaria uniflora (Caryophyllaceae). Evolution. 53, 1723–1733 (1999).
Randle, A. M., Spigler, R. B. & Kalisz, S. Shifts to earlier selfing in sympatry may reduce costs of pollinator sharing. Evolution. 72, 1587–1599 (2018).
Vamosi, J. C. et al. Pollination decays in biodiversity hotspots. Proc. Natl. Acad. Sci. 103, 956–961 (2006).
Schemske, D. W., Mittelbach, G. G., Cornell, H. V., Sobel, J. M. & Roy, K. Is there a latitudinal gradient in the importance of biotic interactions? Annu. Rev. Ecol. Evol. Syst. 40, 245–269 (2009).
Henriques‐Silva, R., Kubisch, A. & Peres‐Neto, P. R. Latitudinal‐diversity gradients can be shaped by biotic processes: new insights from an eco‐evolutionary model. Ecography. 42, 259–271 (2018).
Stebbins, G. L. & Major, J. Endemism and speciation in the California flora. Ecol. Monogr. 35, 1–35 (1965).
Safford, H. D., Viers, J. H. & Harrison, S. P. Serpentine endemism in the California flora: a database of serpentine affinity. Madrono 222–257 (2005).
Cowling, R. M., Rundel, P. W., Lamont, B. B., Arroyo, M. K. & Arianoutsou, M. Plant diversity in Mediterranean-climate regions. Trends Ecol. Evol. 11, 362–366 (1996).
Médail, F. & Quézel, P. Biodiversity hotspots in the Mediterranean Basin: setting global conservation priorities. Conserv. Biol. 13, 1510–1513 (1999).
Bawa, K. S. Plant-pollinator interactions in tropical rain forests. Annu. Rev. Ecol. Syst. 21, 399–422 (1990).
Gautier-Hion, A. & Maisels, F. Mutualism between a leguminous tree and large African monkeys as pollinators. Behav. Ecol. Sociobiol. 34, 203–210 (1994).
Graham, G. L. Bats versus birds: comparisons among Peruvian volant vertebrate faunas along an elevational gradient. J. Biogeogr. 657–668 (1990).
Borgella, R. Jr., Snow, A. A. & Gavin, T. A. Species Richness and Pollen Loads of Hummingbirds Using Forest Fragments in Southern Costa Rica 1. Biotropica 33, 90–109 (2001).
Gottsberger, G., Camargo, J. M. F. & Silberbauer-Gottsberger, I. A bee-pollinated tropical community: the beach dune vegetation of Ihla de Sao Luis, Maranhao, Brazil. Bot. Jahrb∞cher f∞r Syst. Pflanzengeschichte und Pflanzengeographie 109(4), 469–500 (1988).
Machado, I. C. & Lopes, A. V. Floral traits and pollination systems in the Caatinga, a Brazilian tropical dry forest. Ann. Bot. 94, 365–376 (2004).
Campos-Navarrete, M. J., Parra-Tabla, V., Ramos-Zapata, J., Díaz-Castelazo, C. & Reyes-Novelo, E. Structure of plant-Hymenoptera networks in two coastal shrub sites in Mexico. Arthropod. Plant. Interact. 7, 607–617 (2013).
Muchhala, N. & Jarrin, V. P. Flower Visitation by Bats in Cloud Forests of Western Ecuador1. Biotropica 34, 387–395 (2002).
Waser, N. M. Specialization and generalization in plant-pollinator interactions: A historical perspecitve. In Plant-pollinator interactions from specialization to generalization 3–17 (2006).
Herrera, C. M. Components of pollinator “quality”: comparative analysis of a diverse insect assemblage. Oikos 79–90 (1987).
Campbell, D. R. & Motten, A. F. The Mechanism of Competition for Pollination between Two Forest Herbs. Ecology 66, 554–563 (1985).
Aizen, M. A. & Vazquez, D. P. Flower performance in human-altered habitats. In Ecology and Evolution of Flowers 184, 159–180 (2006).
Muchhala, N., Brown, Z., Armbruster, W. S. & Potts, M. D. Competition drives specialization in pollination systems through costs to male fitness. Am. Nat. 176, 732–743 (2010).
Gong, Y.-B. & Huang, S.-Q. Floral symmetry: pollinator-mediated stabilizing selection on flower size in bilateral species. Proc. R. Soc. B Biol. Sci. 276, 4013–4020 (2009).
Wang, G., Compton, S. G. & Chen, J. The mechanism of pollinator specificity between two sympatric fig varieties: a combination of olfactory signals and contact cues. Ann. Bot. 111, 173–181 (2013).
Pleasants, J. M. et al. Corn pollen deposition on milkweeds in and near cornfields. Proc. Natl. Acad. Sci. 98, 11919–11924 (2001).
Arceo-Gómez, G. & Ashman, T. L. Heterospecific pollen deposition: Does diversity alter the consequences? New Phytol. 192, 738–746 (2011).
Flower, A., McKenna, J. W. & Upreti, G. Validity and reliability of GraphClick and DataThief III for data extraction. Behav. Modif. 40, 396–413 (2016).
Sargent, R. D. Floral symmetry affects speciation rates in angiosperms. Proc. R. Soc. London. Ser. B Biol. Sci. 271, 603–608 (2004).
Grafen, A. The uniqueness of the phylogenetic regression. J. Theor. Biol. 156, 405–423 (1992).
Revell, L. J. Phylogenetic signal and linear regression on species data. Methods Ecol. Evol. 1, 319–329 (2010).
Webb, C. O. & Donoghue, M. J. Phylomatic: tree assembly for applied phylogenetics. Mol. Ecol. Notes 5, 181–183 (2005).
Webb, C. O., Ackerly, D. D. & Kembel, S. W. Phylocom: software for the analysis of phylogenetic community structure and trait evolution. Bioinformatics 24 (2008).
Pagel, M. Inferring the historical patterns of biological evolution. Nature 401, 877 (1999).
Blomberg, S. P., Garland, T. & Ives, A. R. Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution. 57, 717–745 (2003).
Münkemüller, T. et al. How to measure and test phylogenetic signal. Methods Ecol. Evol. 3, 743–756 (2012).
R Development Core TEAM. A language and environment for statistical computing. R Foundation for Statistical Computing (2012).
Molina-Venegas, R. & Rodríguez, M. Á. Revisiting phylogenetic signal; strong or negligible impacts of polytomies and branch length information? BMC Evol. Biol. 17, 53 (2017).
Revell, L. J. phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217–223 (2012).
Orme, D. The caper package: comparative analysis of phylogenetics and evolution in R. R Packag. version 5, 1–36 (2013).
Paradis, E., Claude, J. & Strimmer, K. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290 (2004).
Ives, A. R. & Garland, T. Phylogenetic regression for binary dependent variables. In Modern phylogenetic comparative methods and their application in evolutionary biology 231–261 (2014).
Fritz, S. A. & Purvis, A. Selectivity in mammalian extinction risk and threat types: A new measure of phylogenetic signal strength in binary traits. Conserv. Biol. 24, 1042–1051 (2010).
Ho, L. S. T. & Ané, C. Package “Phylolm”-Phylogenetic Linear Regression (2014).
Acknowledgements
The authors thank J. Daniels for helping with data collection and R. Price for input regarding data analyses. This work was funded by an RDC (17-005) grant to GAG and by Consejo Nacional de Ciencia y Tecnología (248406) grant to VPT.
Author information
Authors and Affiliations
Contributions
G.A.G. developed the concept and wrote the manuscript, A.S. and C.A. analyzed the data and prepared figures, T.L.A contributed to concept development and data collection, T.K. and J.M.B. contributed to manuscript writing and editing, B.S. collected data and V.P.T. contributed to concept development and data collection. All authors contributing to manuscript editing.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arceo-Gómez, G., Schroeder, A., Albor, C. et al. Global geographic patterns of heterospecific pollen receipt help uncover potential ecological and evolutionary impacts across plant communities worldwide. Sci Rep 9, 8086 (2019). https://doi.org/10.1038/s41598-019-44626-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-44626-0
This article is cited by
-
Pollination success increases with plant diversity in high-Andean communities
Scientific Reports (2021)
-
Pollinator sharing among co-flowering plants mediates patterns of pollen transfer
Alpine Botany (2021)
-
Floral traits are associated with the quality but not quantity of heterospecific stigmatic pollen loads
BMC Ecology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.