Abstract
Gallstone disease (GD) is one of the most common presentations to surgical units worldwide and shares several risk factors with cardiovascular disease (CVD). CVD remains the most common cause of death worldwide and results in considerable economic burden. Recent observational studies have demonstrated an association between GD and CVD with some studies demonstrating a stronger association with cholecystectomy. We present the findings of a meta-analysis assessing the relationship between GD and CVD. A total of fourteen cohort studies with over 1.2 million participants were included. The pooled hazard ratio (HR, 95% confidence interval [CI]) for association with GD from a random-effects model is 1.23 (95%CI: 1.16–1.30) for fatal and non-fatal CVD events. The association was present in females and males. Three studies report the relationship between cholecystectomy and CVD with a pooled HR of 1.41 (95%CI: 1.21–1.64) which compares to a HR of 1.30 (95%CI: 1.07–1.58) when cholecystectomy is excluded although confounding may influence this result. Our meta-analysis demonstrates a significant relationship between GD and CVD events which is present in both sexes. Further research is needed to assess the influence of cholecystectomy on this association.
Similar content being viewed by others
Introduction
Cardiovascular disease (CVD) remains the most common cause of death worldwide1,2,3. Despite significant advances in the prevention and treatment of CVD, the societal and economic burden is considerable and continues to rise4. CVD is preventable and it is cost-effective to promote risk factor modification in those at risk of CVD5. Early identification of at-risk populations may allow these changes to be implemented at a time when they are most effective.
Gallstone disease (GD) forms one of the most common presentations to surgical units worldwide6,7,8 and patients with gallstones have a higher prevalence of risk factors for cardiovascular disease including obesity and elements of the metabolic syndrome9,10. The presence of gallstones is associated with atherosclerosis11 and patients with gallstones may be at high risk of progression to symptomatic CVD.
Several observational studies have been published reporting a link between GD and CVD although some studies have found no difference12,13,14,15,16,17,18,19,20,21,22. While recent meta-analyses have demonstrated an association between GD and CVD, there is significant variation in the effect size of contributing studies. No meta-analysis to date has adequately accounted for confounding and the heterogeneity remains unexplained21,23,24. Further uncertainty is present over the role of cholecystectomy in modifying the risk of CVD with some authors concluding that cholecystectomy may directly cause CVD24. Analyses of cholecystectomy and CVD have not sufficiently accounted for the presence of confounding variables such as gallstone severity and inflammation. It is likely that these factors account for any apparent association between cholecystectomy and CVD. An additional cohort study examining the relationship between screen-detected GD and CVD has also been published with assessment of the impact of cholecystectomy on CVD22.
We therefore undertook a systematic review with meta-analysis of longitudinal studies assessing the impact of GD on CVD. We performed additional analyses assessing the impact of cholecystectomy on this association.
Results
Search Results
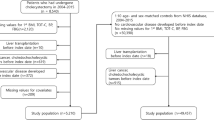
The search identified 5497 references of which 984 were duplicates. Searching of bibliographies identified six other potentially relevant references. Following screening of titles and abstracts, 118 articles remained for full text review. After exclusion of 93 articles (not longitudinal studies, not including GD or not including CVD), a total of 25 citations referring to fourteen cohort studies were included in this systematic review (Fig. 1). One publication reported two cohort studies14 and one publication reported three separate cohort studies as well as a meta-analysis21. Two cohort studies included participants recruited from the same National Health Insurance Research Database (Taiwan) and recruited controls from overlapping inclusion periods17,18. To prevent bias in our meta-analysis we conducted all analyses twice: first using the larger of the two studies18 and second using the smaller study17. We present results from the first set of analyses and describe any differences from the second set of analyses.
Study Characteristics
There were 14 prospective and retrospective cohort studies reported in eleven primary publications and reported in a further fourteen duplicate publications including conference abstracts. The characteristics of the studies are reported in Table 1
. Two studies reported CVD mortality, seven studies reported coronary heart disease (CHD), one study reported cerebrovascular disease and four studies reported multiple CVD outcomes. Four studies relied on baseline ultrasound for diagnosis of GD, one study used cholecystography, two studies used International Classification of Disease (ICD) database records of GD verified in hospital records, five studies used self-reported GD at baseline with four of these verifying reports through hospital records and two studies do not report on ascertainment of exposure to GD and define exposure as “gallbladder disease” rather than GD. Both of the studies which consider “gallbladder disease” do not provide adequate numeric data to be incorporated into a meta-analysis and are therefore considered only in the qualitative analysis. It was felt that “gallbladder disease” was most likely to represent gallstones in these studies given the relative rarity of other gallbladder pathologies although caution is needed when considering the findings of these two studies. All of the remaining studies define exposure as “gallstone disease”. Three studies report separate outcomes for participants with GD who do not undergo cholecystectomy compared to controls and participants who undergo cholecystectomy compared to controls. Five studies report the rate of cholecystectomy which varied from approximately 31% to 73%. Four studies included only females and three studies included only males. Length of follow-up varied from 4.5 to 32 years with a median of 20 years.
Nine studies report adjusted HR of which eight are included in the main analysis (one includes duplication of controls from the same database). Two studies reported in the same publication report odds ratio (OR) and are considered in the qualitative analysis. Two studies report relative risk (RR) of which one does not report a sample size and the studies were therefore considered qualitatively. One study does not report numeric data. Overall this review includes over 1,269,137 participants (number of participants not provided in two studies). The quantitative meta-analysis included 1,229,171 participants (186,589 cases and 1,042,582 controls).
Gallstone Disease and the risk of Cardiovascular Disease
Ten of the fourteen studies reported a statistically significant increase in CVD due to GD. Three studies report non-statistically significant findings of which two demonstrate a trend towards an increase in CVD with GD and one reports a trend towards reduced CHD with GD in a female-only cohort. The study reporting no numeric data examined CHD and states: “There was no significant relationship with a history of gallbladder disease”.
All Cardiovascular Disease
After controlling for confounding variables participants with GD had a 23% increased risk of CVD compared to controls (95% CI = 1.16–1.30, Fig. 2). We identified considerable heterogeneity (I2 = 76.3%, p = 0.0001).
Forest plot demonstrating relationship between GD and CVD. Squares and horizontal lines represent individual studies with 95% confidence intervals. Area of squares is proportional to study weighting in random effects meta-analysis. Diamond (blue) represents pooled HR from meta-analysis with 95% CI. Reference line (black) represents hazard ratio of 1.00 (no association between GD and CVD). Summary line (red) represents summary estimate and demonstrates increased risk of CVD with GD (23%).
Coronary Heart Disease
Seven studies reported CHD as a separate outcome including 843,312 participants (56,040 cases and 787,272 controls). Participants with GD had a 24% increased risk of CHD compared to controls (95% CI = 1.16–1.32). We identified considerable heterogeneity when CHD was considered (I2 = 70.7%, p = 0.0023).
Cerebrovascular Disease
Four studies reported cerebrovascular disease as a separate outcome although two studies used controls from the same database. Three studies were pooled by meta-analysis including 458,518 participants (140,844 cases and 317,674 controls). Participants with GD had a 29% increased risk of cerebrovascular disease compared to controls (95% CI = 1.26–1.32). We did not identify heterogeneity when cerebrovascular disease was considered (I2 = 0.00%, p = 0.76).
Subgroup Analyses and Sensitivity Analyses
We performed subgroup analyses for male-only participants, female-only participants, non-diabetic participants, diabetic participants, screen-detected GD and symptomatic GD (Table 2). We identified a statistically significant association between GD and CVD in all subgroups. One study which reported RR reported a summary estimate <1.00 but was not statistically significant and included only 322 cases compared to 103,562 cases included in our meta-analysis. We therefore demonstrate that the relationship between GD and CVD is present in both men and women (Fig. 3). The difference between diabetic and non-diabetic participants is not statistically significant although a trend towards a stronger effect suggests that GD may have a greater relative importance as a predictor of CVD in non-diabetic participants. CVD was significantly more common in both screen-detected and symptomatic GD. Heterogeneity remained moderate to substantial in all subgroup analyses other than cerebrovascular disease, screen-detected GD and cholecystectomy-only.
Two studies from the same database report similar increases in CVD with GD for normotenstive and hypertensive participants. One study reports similar increases in CVD with GD for hyperlipidemic and non-hyperlipidemic participants. Three studies from the same publication were pooled in a meta-analysis for non-obese participants and for non-smokers revealing similar results in both analyses to the overall cohorts. Two studies from the same database report HRs for comorbidity-free participants as well as age-stratified subgroups for which results are similar to the overall cohorts except in the study assessing effect of GD on cerebrovascular where younger and comorbidity-free participants appear to have relatively greater burden of CVD risk. One study stratified participants according to GD duration at baseline and found no difference between groups.
We performed sensitivity analyses by iteratively excluding one study from the analysis to detect its impact on heterogeneity. Heterogeneity remained substantial for all sensitivity analyses with one study removed. Heterogeneity was noted to fall (I2 = 49%) when both Taiwanese studies using data from the same database were excluded.
We performed a meta-regression which did not detect any significant effect of follow-up, year of publication, quality assessment score, degree of adjustment for confounding variables or variance (p > 0.4 in all cases). In addition, none of the explanatory variables accounted for heterogeneity (pseudo-R2 = 0.00%, I2 = 70.4%).
Effect of Cholecystectomy on risk of Cardiovascular Disease
Three studies report separate data for cholecystectomy compared to controls and for GD without cholecystectomy compared to controls. One study reports a separate analysis of cholecystectomy versus GD without cholecystectomy. Pooled adjusted HR for cholecystectomy compared to controls was 1.41 (95%CI: 1.21–1.64) with insignificant heterogeneity (p = 0.250, I2 = 27.8%). Pooled adjusted HR for GD without cholecystectomy compared to controls was 1.30 (95%CI:1.07–1.58) with moderate heterogeneity (p = 0.161, I2 = 45.3%). The study compared cholecystectomy to GD without cholecystectomy directly found no statistically significant difference (HR 1.24, 95%CI: 0.85–1.81). One study also reported mild GD (cholelithiasis or choledocholithiasis excluding definition of severe GD) and severe GD (acute calculous cholecystitis, calculous pancreatitis, cholangitis, surgical procedure for GD or endoscopic procedure for GD) and reported a stronger association in mild gallstone disease (HR 1.34, 95%CI: 1.24–1.46 for non-severe GD versus HR 1.20, 95%CI: 1.02–1.40 for severe GD).
One report including three cohort studies limited exposure to cholecystectomy only and stated: “CHD risk was similar to when our definition of gallstone disease included both history of gallstones and cholecystectomy” although does not provide numeric data for this subgroup21. One report including two cohort studies states: “Cholecystectomy status proved to be superior to gallstone disease diagnosis in discriminating between those individuals who went on to develop coronary disease and those who did not”. This report did not provide data for GD with and without cholecystectomy14.
Overall four studies favour a stronger relationship between GD and CVD when cholecystectomy alone is considered and six studies favour a weaker relationship or no difference. No studies report a statistically significant difference between the two groups. Only one study16 adjusted for inflammatory markers (C-reactive protein) and found a non-significant trend towards lower risk of CVD in patients undergoing cholecystectomy compared to those with gallstones who did not undergo cholecystectomy.
Publication Bias
We did not detect publication bias through visual inspection of the funnel plot or by Egger’s regression test (p = 0.51, Fig. 4).
Discussion
This systematic review with meta-analysis includes over 1.2 million participants and demonstrates a significant association between GD and CVD with an increase of 23% between cases and controls following adjustment for confounding variables. The association with GD is present for CHD, cerebrovascular disease and PVD as well as for men and women. There is trend towards a relatively stronger association in young and otherwise healthy individuals although no subgroup differences were statistically significant. The review highlights a scarcity of high quality evidence for the association between cholecystectomy and CVD.
Three separate meta-analyses investigating the relationship between GD and CVD have been published and report similar findings to our review with a statistically significant increase in CVD with GD which is present in several subgroups21,23,24.
Possible mechanisms linking GD and CVD include dyslipidemia, chronic inflammation and abnormalities in the ABCG8 cholesterol transporter gene25,26,27. Patients with the D19H and T400k polymorphisms of the ABCG8 gene have been demonstrated to have elevated risk of both CVD and GD suggesting a shared aetiology through aberrant cholesterol metabolism28,29. The presence of gallstones is known to be associated with inflammation of the gallbladder mucosa7. The presence of inflammation is already known to promote formation of atherosclerotic plaques even at distant sites and it is possible that low-grade inflammation associated with gallstones triggers a similar response30,31. GD is also associated with obesity, hyperlipidemia, diabetes mellitus and hypertension10,32 which is responsible in part for an increase in CVD. Adjustment of the reported HR for these confounding variables results in a reduction of CVD risk although, in all but two studies the association remains statistically significant. Other aetiological mechanisms such as altered gut microbiota and reduced levels of serum insulin-like growth factor are implicated in gallstone development and development of atherosclerotic plaques that have not been accounted for in any existing studies33,34,35,36.
In another meta-analysis, Fan et al. conclude that cholecystectomy is likely to be responsible for increased risk of CVD compared to GD without cholecystectomy. They cite evidence of altered serum lipid levels post-cholecystectomy in animal models, protective effects of biliary hormones on lipid and glucose metabolism and post-cholecystectomy alteration in gut microbiota. We do not feel that the evidence is sufficient to support this hypothesis. Only one cohort study makes direct comparison between cholecystectomy and GD without cholecystectomy and finds no significant difference. Changing the definition of exposure from GD to cholecystectomy is reported to have no significant impact in three studies although numeric data is not provided. Two studies report a trend towards higher rates of CVD following cholecystectomy and one publication reporting two studies only published data for cholecystectomy as this was found to be a stronger predictor of CVD than GD as a whole. Three studies found a trend towards a weaker association between GD and CVD when cholecystectomy only was considered. Of the studies which find cholecystectomy to be a weaker predictor of CVD than GD as a whole, one of these studies16 adjusts for C-reactive protein suggesting that inflammation may be responsible for any apparent increase in CVD in the cholecystectomy group. None of the studies which demonstrate a trend towards higher rates of CVD in the cholecystectomy group, compared to those with gallstones who do not require cholecystectomy, adjust for inflammatory markers. Symptoms arising from inflammation of the gallbladder are a key determinant of the need for cholecystectomy, meaning that patients who require cholecystectomy may have suffered from more extensive systemic inflammation. For these reasons we do not feel that cholecystectomy contributes directly to increased risk of CVD and is, instead, more likely to represent those patients who have a greater burden from other CVD risk factors.
Our meta-analysis identified substantial heterogeneity for which we did not identify the likely cause. It is possible that additional confounding variables at participant- or study-level have not been recorded or reported. A total of five studies reported having additional data available, which we were unable to obtain, relating to differences between rate of CVD following cholecystectomy compared to GD without cholecystectomy. This suggests that the review may be subject to selective reporting bias with only those articles finding a difference between the two comparisons publishing the results. Our review identified a persistent direction of effect in all but one study and included data from three studies not previously considered in any other review on the same topic. The findings are consistent in several populations and subgroups but do not demonstrate a causal link between GD or cholecystectomy and CVD.
Our review has confirmed the significant association between GD and CVD and highlights the importance of recognizing GD as a risk factor for CVD. We have demonstrated that risk of CVD is elevated in GD for both males and females. We have demonstrated that cholecystectomy may not increase the risk of CVD as previously thought. Our review identifies the need for further research into the impact of chronic inflammation on the risk of CVD in patients with GD and in particular whether this accounts for any apparent increase in CVD with cholecystectomy.
Methods
A systematic review with meta-analysis was undertaken in accordance with the Meta-analysis of Observation Studies in Epidemiology (MOOSE) statement37 and the PRISMA statement38.
Search Strategy
In October 2017 we ran searches in MEDLINE, EMBASE, Science Citation Index and Social Sciences Citation Index Expanded and Literatura Latino-americana e do Caribe em Ciências da Saúde (LILACS) from earliest date possible. The search strategies are shown in Supplementary Table S1. We combined terms into three categories (GD; CVD; and observational studies) using the OR operator before combining the three categories using the AND operator.
Study Selection
We included studies meeting the following criteria:
-
Longitudinal cohort study
-
Assessing the relationship between GD and CVD
We included cholecystectomy as well as GD without cholecystectomy, we also included screen-detected as well as symptomatic GD. Studies which reported “gallbladder disease” and were thought to represent gallstones rather than rarer gallbladder pathology were considered only in the qualitative analysis. We included fatal and non-fatal CVD events and considered CHD, cerebrovascular disease and peripheral vascular disease (PVD). Each subgroup of CVD was considered separately in subanalyses where possible as well as combined into one single composite outcome of CVD. No relevant non-English language articles were identified. When identified studies were reported in multiple publications we extracted unique data from each publication.
Data Extraction
We extracted the following data from each study: authors, publication year, study population, baseline characteristics, sample size, study design, country, ascertainment of exposure, detection of CVD events, definition of CVD, follow-up duration, confounding variable adjustment, rate of cholecystectomy and adjusted effect measures (hazard ratio [HR], relative risk [RR] or odds ratio [OR]) with 95% confidence interval. We assessed each study according to the Newcastle-Ottawa Quality Assessment Scale (NOS)39. Prior to data extraction we defined an appropriate follow-up period of at least 10 years to allow for CVD events to occur and a follow-up rate of >90%. We contacted authors to request additional data when required.
Data Analysis
We assimilated data using DerSimonian-Laird random effects meta-analysis40. We used fully adjusted (multivariable) effect measures from each study when available. We did not transform each unique effect measures into one single effect measure and as such studies reporting HR, RR or OR are considered in three distinct groups. All effect measures were log-transformed for purposes of data analysis prior to back-transformation for presentation of results.
Subgroup analyses were performed for the following groups:
-
Male-only participants
-
Female-only participants
-
Diabetic participants
-
Non-diabetic participants
-
Screen-detected GD
-
Symptomatic GD
-
Gallstones (not including cholecystectomy) versus controls
-
Cholecystectomy only versus controls
Discrete subgroups were compared using a Wald-type test with p value significance set at <0.01. We assessed influences of moderator variables by meta-regression using a mixed effects model and the Knapp and Hartung adjustment41. For the mixed effects model and meta-regression we included length of follow-up, year of publication, degree of adjustment for confounding variables, variance and NOS score.
We assessed for presence of heterogeneity using Cochran’s Q test with p value significance set at <0.1 and quantified heterogeneity with the I2 statistic42,43. We assessed for the presence of publication bias by visual inspection of funnel plots and Egger’s regression test with p value significance set at <0.144.
All analyses were undertaken in R version 3.3.1 using the “metafor” package45,46. All data is available in original manuscripts.
References
World Health Organisation. World Health Statistics 2017: Monitoring Health for the SDGs, Sustainable Development Goals. Tech. Rep., World Health Organisation, Geneva (2017).
Lozano, R. et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet 380, 2095–2128 (2012).
Office for National Statistics. Deaths registered in England and Wales - Office for National Statistics. Bulletin, Office for National Statistics (2017).
American Heart Association & American Stroke Association. A.S.A Cardiovascular disease: a costly burden for America, projections through 2035 (2017). Tech. Rep., The American Heart Association, Connecticut (2017).
Gaziano, T. A. Cardiovascular Disease in the Developing World and Its Cost-Effective Management. Circulation 112, 3547–3553 (2005).
Harrison, E. M. et al. Hospital volume and patient outcomes after cholecystectomy in Scotland: retrospective, national population based study. BMJ 344, e3330 (2012).
Portincasa, P., Moschetta, A. & Palasciano, G. Cholesterol gallstone disease. The Lancet 368, 230–239 (2006).
Everhart, J. E., Khare, M., Hill, M. & Maurer, K. R. Prevalence and ethnic differences in gallbladder disease in the United States. Gastroenterology 117, 632–639 (1999).
Chavez-Tapia, N. C. et al. Association between cholecystectomy for gallstone disease and risk factors for cardiovascular disease. Annals Hepatol. 11, 85–89 (2012).
Chen, L.-Y. et al. Metabolic syndrome and gallstone disease. World J. Gastroenterol.: WJG 18, 4215–4220 (2012).
Jiang, Z.-Y. et al. Gallbladder Gallstone Disease Is Associated with Newly Diagnosed Coronary Artery Atherosclerotic Disease: A Cross-Sectional Study. PLOS ONE 8, e75400 (2013).
Paul, O. et al. A Longitudinal Study of Coronary Heart Disease. Circulation 28, 20–31 (1963).
Petitti, D. B., Wingerd, J., Pellegrin, F. & Ramcharan, S. Risk of Vascular Disease inWomen: Smoking, Oral Contraceptives, Noncontraceptive Estrogens, and Other Factors. JAMA 242, 1150–1154 (1979).
Bortnichak, E. A. et al. The Association Between Cholesterol Cholelithiasis and Coronary Heart Disease in Framingham, Massachusetts. Am. J. Epidemiol. 121, 19–30 (1985).
Grimaldi, C. H. Increased Mortality with Gallstone Disease: Results of a 20-Year Population-Based Survey in Pima Indians. Annals Intern. Medicine 118, 185 (1993).
Ruhl, C. E. & Everhart, J. E. Gallstone Disease is Associated with Increased Mortality in the United States. Gastroenterology 140, 508–516 (2011).
Olaiya, M. T., Chiou, H.-Y., Jeng, J.-S., Lien, L.-M. & Hsieh, F.-I. Significantly Increased Risk of Cardiovascular Disease among Patients with Gallstone Disease: A Population-Based Cohort Study. PLOS ONE 8, e76448 (2013).
Wei, C.-Y. et al. Gallstone Disease and the Risk of Stroke: A Nationwide Population-based Study. J. Stroke Cerebrovasc. Dis. 23, 1813–1820 (2014).
Lv, J. et al. Gallstone Disease and the Risk of Ischemic Heart Disease. Arter. Thromb. Vasc. Biol. 35, 2232–2237 (2015).
Wirth, J. et al. Presence of gallstones and the risk of cardiovascular diseases: The EPIC-Germany cohort study. Eur. J. Prev. Cardiol. 22, 326–334 (2015).
Zheng, Y. et al. Gallstones and Risk of Coronary Heart Disease: Prospective Analysis of 270 000 Men and Women From 3 US Cohorts and Meta-Analysis. Arter. Thromb. Vasc. Biol. ATVBAHA. 116.307507 (2016).
Shabanzadeh, D. M., Skaaby, T., Sorensen, L. T. & Jorgensen, T. Screen-detected gallstone disease and cardiovascular disease. Eur. journal epidemiology 32, 501–510 (2017).
Upala, S., Sanguankeo, A. & Jaruvongvanich, V. Gallstone Disease and the Risk of Cardiovascular Disease: A Systematic Review and Meta-Analysis of Observational Studies. Scand. J. Surg. 106, 21–27 (2017).
Fan, Ll, Chen, B. H. & Dai, Z. J. The relation between gallstone disease and cardiovascular disease. Sci. Reports 7, 15104 (2017).
Rocha, V. Z. & Libby, P. Obesity, inflammation, and atherosclerosis. Nat. Rev. Cardiol. 6, 399–409 (2009).
Hansson, G. K., Libby, P. & Tabas, I. Inflammation and plaque vulnerability. J. Intern. Medicine 278, 483–493 (2015).
Buch, S. et al. A genome-wide association scan identifies the hepatic cholesterol transporter ABCG8 as a susceptibility factor for human gallstone disease. Nat. Genet. 39, 995–999 (2007).
Rudkowska, I. & Jones, P. J. H. Polymorphisms in ABCG5/G8 transporters linked to hypercholesterolemia and gallstone disease. Nutr. Rev. 66, 343–348 (2008).
Koeijvoets, K. C. M. C. et al. ABCG8 gene polymorphisms, plasma cholesterol concentrations, and risk of cardiovascular disease in familial hypercholesterolemia. Atherosclerosis 204, 453–458 (2009).
Beukers, N. G. F. M., Heijden, G. J. M. G. v. d., Wijk, A. J. v. & Loos, B. G. Periodontitis is an independent risk indicator for atherosclerotic cardiovascular diseases among 60 174 participants in a large dental school in the Netherlands. J Epidemiol Community Heal. 71, 37–42 (2017).
Ridker, P. M. From C-Reactive Protein to Interleukin-6 to Interleukin-1: Moving Upstream To Identify Novel Targets for Atheroprotection. Circ. Res. 118, 145–156 (2016).
Zhu, Q. et al. The association between gallstones and metabolic syndrome in urban Han Chinese: a longitudinal cohort study. Sci. Reports 6, 29937 (2016).
Wu, T. et al. Gut microbiota dysbiosis and bacterial community assembly associated with cholesterol gallstones in large-scale study. BMC genomics 14, 669 (2013).
Miele, L. et al. Impact of Gut Microbiota on Obesity. Diabetes, and Cardiovascular Disease Risk. Curr. Cardiol. Reports 17, 120 (2015).
Moschetta, A. et al. Effects of growth hormone deficiency and recombinant growth hormone therapy on postprandial gallbladder motility and cholecystokinin release. Dig. Dis. Sci. 49, 529–534 (2004).
Juul, A., Scheike, T., Davidsen, M., Gyllenborg, J. & Jørgensen, T. Low serum insulin-like growth factor I is associated with increased risk of ischemic heart disease: a population-based case-control study. Circulation 106, 939–944 (2002).
Stroup, D. F. et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283, 2008–2012 (2000).
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G. & Group, T. P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLOS Medicine 6, e1000097 (2009).
Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25, 603–605 (2010).
DerSimonian, R. & Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 7, 177–188 (1986).
Knapp, G. & Joachim, H. Improved tests for a random effects meta-regression with a single covariate. Stat. Medicine 22, 2693–2710 (2003).
Higgins, J. P. T., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. BMJ: Br. Med. J. 327, 557–560 (2003).
Higgins, J. P. T. & Thompson, S. G. Quantifying heterogeneity in a meta-analysis. Stat. Medicine 21, 1539–1558 (2002).
Egger, M., Smith, G. D., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 (1997).
R Core Team. R: A Language and Environment for Statistical Computing (2017).
Viechtbauer, W. Conducting Meta-Analyses in R with the metafor Package. J. Stat. Softw. 36, 1–48 (2010).
Acknowledgements
This research was undertaken as part of the third year of the MSc in Surgical Sciences (The University of Edinburgh).
Author information
Authors and Affiliations
Contributions
S.J.W. developed the research question, C.J.F. collected the data, C.J.F. and E.M.H. analysed the data. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fairfield, C.J., Wigmore, S.J. & Harrison, E.M. Gallstone Disease and the Risk of Cardiovascular Disease. Sci Rep 9, 5830 (2019). https://doi.org/10.1038/s41598-019-42327-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-42327-2
This article is cited by
-
Associations of cholecystectomy with metabolic health changes and incident cardiovascular disease: a retrospective cohort study
Scientific Reports (2024)
-
Impact of gallstone disease on the risk of stroke and coronary artery disease: evidence from prospective observational studies and genetic analyses
BMC Medicine (2023)
-
Gallbladder disease is associated with the risk of cardiovascular disease among Uyghurs in Xinjiang: a prospective cohort study
BMC Public Health (2023)
-
Impact of cholecystectomy on acute coronary syndrome according to metabolic condition: a nationwide population-based cohort study
Scientific Reports (2023)
-
The association between hypertension and the risk of gallstone disease: a cross-sectional study
BMC Gastroenterology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.