Abstract
Sessile serrated adenomas/polyps (SSA/Ps) are precancerous lesions that account for one-third of colorectal cancers. The endoscopic and pathologic differentiation between SSA/Ps without dysplasia (SSA/POs) and SSA/Ps with dysplasia or adenocarcinoma (SSA/PDAs) can be difficult. This study aimed to assess the clinical characteristics of SSA/PDs. This multicenter retrospective cohort study included 532 patients who underwent endoscopic resection and were pathologically diagnosed with SSA/POs and SSA/PDAs. Initially, medical, endoscopic, and histopathological records of patients who underwent endoscopic resection of SSA/POs and SSA/PDAs at eight university hospitals in Korea between January 2005 and December 2015 were reviewed. A total of 307 (57.7%) patients were detected in men and 319 (60.0%) were located in the proximal colon. Most SSA/Ps had a flat, slightly elevated, or sessile morphology. The most prevalent endoscopic findings of SSA/Ps were nodular surface (244, 45.9%), disrupted vascular pattern (232, 43.6%), altered fold contour (141, 26.5%), dome-shaped morphology (135, 25.4%), and pale color (115, 21.6%). SSA/POs were more commonly found in the proximal colon, compared to SSA/PDAs. SSA/PDAs displayed 0-Ip, Isp, IIb or IIa + IIc morphologies more frequently, while SSA/POs displayed 0-Is or IIa morphology more frequently. The frequency of a rim of debris/bubbles was significantly higher in SSA/POs, while nodular surface and disrupted vascular pattern were significantly higher in SSA/PDAs. In the univariate analysis of endoscopic features, SSA/PDAs were significantly associated with the distal colon location, 0-Isp and IIb morphologies, nodular surface, and disrupted vascular pattern. In the multivariate analysis, 0-IIb, nodular surface, and disrupted vascular pattern were significantly associated with SSA/PDAs. SSA/Ps with 0-IIb morphology, nodular surface and disrupted vascular pattern are associated with an increased risk of dysplasia or adenocarcinoma.
Similar content being viewed by others
Introduction
Previously, two main groups of colorectal polyps were widely recognized: adenomatous polyps and non-adenomatous polyps, which include hyperplastic polyps (HPs). Conventional adenomatous polyps were traditionally considered the only precursor lesions of colorectal cancer, while non-adenomatous polyps were considered benign1,2. However, recent discoveries showed that colorectal serrated lesions with characteristic saw-tooth morphology of crypts, previously called HPs, lead to colorectal cancer via the serrated neoplasia pathway, which is different from the adenoma-carcinoma sequence pathway for conventional adenomatous polyps3,4,5,6,7. This pathway accounts for about 15–30% of sporadic colorectal cancers3,4,5,6,7. Molecular features of the serrated neoplasia pathway, including point mutations in the BRAF oncogene and methylation of CpG islands (CIMP) in the promoter regions of key regulatory and tumor suppressor genes, lead to epigenetic silencing of mismatch repair of genes such as MLH1, resulting in microsatellite instability (MSI)3,4,5,6,7.
Histopathologically, colorectal serrated lesions are classified into the following three general types according to the 2010 World Health Organization (WHO) classification: HPs, traditional serrated adenomas (TSAs), and sessile serrated adenomas/polyps (SSA/Ps)8. HPs are considered harmless with no malignant potential, while both TSAs and SSA/Ps are considered precursors of colorectal cancer3,4,5,6,7,8.
An interval colorectal cancer is defined as a cancer diagnosed prior to the date of the next recommended examination after a previous negative colonoscopy result. This cancer is thought to be resulted from previously missed lesions, rapid progression, or incomplete resection of colorectal precancerous lesions. Moreover, it is more likely to be associated with CIMP and MSI compared to non-interval colorectal cancer9,10,11.
SSA/Ps have more pale, flat, sessile, or indistinct borders compared to conventional adenomatous polyps. Therefore, they can be difficult to detect during colonoscopy and are commonly missed or incompletely resected12,13,14,15,16,17,18,19,20. Moreover, SSA/Ps are considered to have the potential of rapid progression owing to the development of MSI, especially after the development of cytological dysplasia3,4,5,6,7,8. These findings suggest that SSA/Ps are important contributors to interval colorectal cancers9,10,11. Therefore, SSA/Ps merit special attention to ensure optimal detection, complete resection, and appropriate surveillance. Previous studies have investigated the clinical and endoscopic features of SSA/Ps12,13,14,15,16,17,18,19,20; however, the clinical and endoscopic features of SSA/Ps with cytological dysplasia or adenocarcinoma have not yet been fully elucidated21,22,23,24,25,26,27,28,29,30.
This study aimed to evaluate the clinical and endoscopic characteristics of SSA/Ps and compare the characteristics of SSA/Ps without dysplasia with those of SSA/Ps with dysplasia or adenocarcinoma.
Materials and Methods
Study design and population
This retrospective, multicenter cohort study assessed consecutive patients with endoscopically resected and pathologically diagnosed lesions as either SSA/Ps without dysplasia (SSA/POs) or SSA/Ps with low-grade dysplasia, high-grade dysplasia, or adenocarcinoma (SSA/PDAs) at eight university hospitals throughout Korea affiliated with the Korean Association for the Study of Intestinal Disease between January 2005 and December 2015. One board-certified gastrointestinal endoscopist with extensive experience in endoscopic resections such as polypectomy, endoscopic mucosal resection (EMR), endoscopic piecemeal mucosal resection (EPMR), endoscopic submucosal dissection (ESD) at each hospital was responsible for data collection, and the completeness of the data collection was monitored by one of the authors (Y.E.J.). We excluded patients with lack of complete clinicopathological data and inflammatory bowel diseases, familial adenomatous polyposis, or non-epithelial neoplasms as carcinoid or lymphoma, and the presence of non-neoplastic histology such as chronic colitis. A total of 532 SSA/P lesions were retrospectively analyzed for various clinicopathological characteristics by reviewing medical, endoscopic, and histopathological records of enrolled patients. The patient-related factors including age, sex, smoking, alcohol drinking, smoking, body mass index (BMI), and use of aspirin and nonsteroidal anti-inflammatory drugs (NSAIDs), lesion-related factors including size, location, endoscopic morphology and feature, associated lesion, procedure-related factors including removal method and post-procedure complication and histologic factors were obtained by medical record reviews, pathologists, and gastroenterologists contact when necessary. Informed consent was obtained from every patients. The study was performed in accordance with the ethical principles of the Declaration of Helsinki and was approved by the Chonnam National University Hwasun hospital Institutional Review Board as well as by each Institutional Review Board at 7 hospitals (Yeungnam University Hospital Institutional Review Board, Soonchunhyang University Hospital Institutional Review Board, Chosun University Hospital Institutional Review Board, Chonbuk University Hospital Institutional Review Board, Kosin University Hospital Institutional Review Board, Samsung Medical Center Institutional Review Board, Seoul St. Mary’s Hospital Institutional Review Board).
Endoscopic and histologic analysis of SSA/Ps
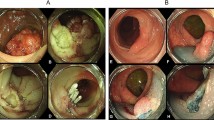
All patients were examined using video colonoscopes (Olympus CF-240I or CF-H260; Olympus, Tokyo, Japan). Bowel preparation was performed with polyethylene glycol electrolyte solution in all hospitals and classified according to endoscopist estimation into adequate in all cases. Two endoscopists (K.H.K. and Y.E.J.) reviewed the endoscopic findings of SSA/Ps and evaluated conventional white-light colonoscopic images. Endoscopic characteristics were evaluated using previously validated criteria defined by Tadepalli et al.31: nodular surface, disrupted vascular pattern, altered fold contour, dome-shaped morphology, pale color, mucus cap, and rim of debris/bubbles (Fig. 1). According to the Paris classification, the endoscopic morphologies of superficial lesions are divided into three categories: protruding (0-I), non-protruding and non-excavated (0-II), and excavated (0-III). Type 0-I lesions are further subdivided into pedunculated (0-I), sessile (0-Is), or mixed (0-Isp); Type 0-II lesions are subdivided into slightly elevated (0-IIa), flat (0-IIb), or depressed (0-IIc)32. The locations of the adenomas were classified as follows: the proximal colon (cecum, ascending colon, hepatic flexure, and transverse colon) and distal colon (splenic flexure of the colon, descending colon, sigmoid, and rectum). Histologic diagnoses of SSA/POs and SSA/PDAs were evaluated separately by gastrointestinal pathologists who were blinded to the knowledge of original pathologic report at each institute on the basis of the 2010 WHO classification for the presence of serrated crypts, irregularly dilated and/or branching crypts, and horizontally and/or laterally arranged basal crypts8. SSA/Ps with cytological dysplastic changes were graded as low-grade dysplasia, high-grade dysplasia, or adenocarcinoma (Fig. 2). In case of any difference in histologic diagnosis, the two pathologists discussed the case until consensus was achieved.
Histopatholgic findings with hematoxylin-eosin staining of the resected specimens of sessile serrated adenomas/polyps (SSA/Ps) (x100). (a) SSA/P without cytologic dysplasia shows the presence of serrated crypts, irregularly dilated and branching crypts, and horizontally and laterally arranged basal crypts. (b) SSA/P with low grade dysplasia (arrowhead). (c) SSA/P with high grade dysplasia (short arrow). (d) SSA/P with adenocarcinoma (long arrow).
Statistical analysis
Clinical and endoscopic characteristics of SSA/POs and SSA/PDAs were compared using the chi-square test, Student’s t-test, or analysis of variance, as appropriate. Descriptive analyses included proportions for categorical data, as well as mean ± standard deviation (SD) for continuous data. Furthermore, a binary logistic regression model was used to identify the risk factors of SSA/PDs. All statistical analyses were performed with the Statistical Packages for the Social Sciences (SPSS, version 18.0; SPSS Inc., Chicago, IL, USA). A difference with P < 0.05 was considered statistically significant.
Results
Baseline characteristics of patients with SSA/P
The baseline characteristics of patients with SSA/P are summarized in Table 1. The mean age of enrolled patients was 58.0 ± 12.5 years (range, 23.0–90.0 years). The study group included 307 men (57.7%) and 225 women (42.3%). The mean SSA/P size was 10.8 ± 7.5 (range, 2.0–50.0) mm. Of the five hundred thirty two detected SSA/P lesions, three hundred nineteen lesions (60.0%) were localized in the proximal colon. According to Paris classification32, the numbers of subjects (percentage) in 0-Ip, 0-Isp, 0-Is, 0-IIa, 0-IIb, and 0-IIa + IIc classes were 52 (9.8%), 76 (14.3%), 234 (44.0%), 151 (28.4%), 5 (0.9%), and 14 (2.6%), respectively. No class 0-III lesion was detected. The frequencies of SSA/Ps endoscopic features were as follows: nodular surface (244, 45.9%), disrupted vascular pattern (232, 43.6%), altered fold contour (141, 26.5%), dome-shaped morphology (135, 25.4%), pale color (115, 21.6%), mucus cap (62, 11.7%), and rim of debris/bubbles (32, 6.0%). In the prevalence of synchronous colorectal neoplasms in patients with SSA/P, 210 (39.5%) patients had conventional adenoma with low-grade dysplasia, 129 (24.2%) had hyperplastic polyp, 31 (5.8%) had conventional adenoma with high-grade dysplasia, 30 (5.6%) had traditional serrated adenoma, and 24 (4.5%) had colorectal adenocarcinoma. SSA/Ps were resected by polypectomy such as cold biopsy or snare (79, 15.2%), EMR (412, 79.1%), EPMR (5, 1.0%), or ESD (20, 3.8%). The post-procedural bleeding rate was 6.3% (33/532) and the perforation rate was 0.4% (2/532). In histologic examination, 370 (69.6%) lesions were SSA/POs and 162 (30.4%) lesions were SSA/PDAs [139 low-grade dysplasia (26.1%), 16 were high-grade dysplasia (3.0%), and 7 were adenocarcinoma (1.3%)] (Table 1).
Comparison of clinical characteristics of SSA/POs and SSA/PDAs
SSA/Ps included 370 SSA/POs and 162 SSA/PDAs. With regard to patient-related factors, no statistically significant differences were found in age, sex, smoking, alcohol, or use of NSAIDs between SSA/POs and SSA/PDAs groups. The mean BMI value was significantly higher in SSA/POs than that of SSA/PDAs (P = 0.019). With regard to lesion-related factors, SSA/PDAs were more commonly found in the distal colon than in the proximal colon (P = 0.002). The distribution of SSA/POs and SSA/PDAs were also different according to location by subsites (P = 0.004). The proportion of rectosigmoid lesions among the subjects with SSA/PDAs (45%) was relatively higher than that among the subjects with SSA/POs (27.6%). No statistically significant differences were found in tumor size between SSA/POs and SSA/PDAs groups. The frequency of endoscopic morphology by Paris classification was different between SSA/POs and SSA/PDAs (P < 0.001). The analysis of endoscopic features showed that nodular surface and disrupted vascular pattern were more commonly found in SSA/PDAs (P < 0.001 and P = 0.006, respectively), and a rim of debris was more commonly found in SSA/POs (P = 0.023). Conventional adenomas with low-grade dysplasia were more commonly detected in SSA/PDAs than in SSA/POs (P < 0.001). Conventional adenomas with high-grade dysplasia and adenocarcinoma tended to be found more frequently in SSA/PDAs than in SSA/POs, but the difference was not statistically significant (P = 0.090). Traditional serrated adenomas were more commonly found in SSA/POs than in SSA/PDAs (P = 0.036). With regard to procedure-related factors, polypectomy, such as cold biopsy or snare, was more frequently performed in SSA/POs, while EMR was more frequently performed in SSA/PDAs (P = 0.001). Post-procedural bleeding was more commonly found in SSA/PDAs than in SSA/POs (P = 0.006) (Table 2).
Univariate analysis of risk factors associated with SSA/PDAs
The results of univariate analysis of risk factors associated with SSA/PDAs are summarized in Table 3. With regard to patient-related factors, no significant association with the risk of dysplasia was detected in terms of age, sex, smoking, alcohol drinking, BMI, and use of NSAIDs. With regard to lesion-related factors, SSA/PDAs were less commonly found in proximal colon, [odds ratio (OR) 0.555, 95% confidence interval (CI) 0.381–0.806, P = 0.002]. No significant association was found with tumor size. According to the Paris classification of endoscopic morphology, the risk of dysplasia was higher in 0-Isp (OR 2.295 95% CI 1.252–4.204, P = 0.007) and 0-IIb (OR 4.016, 95% CI 2.056–7.845, P < 0.001) morphologies compared to 0-IIa morphology. The analysis of endoscopic features showed that nodular surface and disrupted vascular pattern were positively associated with the risk of dysplasia (OR 4.975, 95% CI 3.317–7.461, P < 0.001; and OR 1.675, 95% CI 1.154–2.429, P = 0.007, respectively), while the rim of debris/bubbles was reversely associated with the risk of dysplasia (OR 0.309, 95% CI 0.107–0.897, P = 0.031). The risk of dysplasia was increased in subjects with conventional adenomas with low-grade dysplasia (OR 2.085, 95% CI 1.432–3.037, P < 0.001) and decreased in subjects with traditional serrated adenomas (OR 0.335, 95% CI 0.115–0.976, P = 0.045) (Table 3).
Multivariate analysis of risk factors associated with SSA/PDAs
The results of the multivariate analysis of risk factors associated with SSA/PDAs are summarized in Table 4. On multivariate logistic regression analysis, SSA/Ps with 0-IIb morphology, nodular surface, or disrupted vascular pattern showed a significant association with the risk of dysplasia (OR 3.107, 95% CI 1.447–6.671, P = 0.004; OR 4.686, 95% CI 2.962–7.414, P < 0.001; and OR 1.770, 95% CI 1.150–2.724, P = 0.009, respectively) (Table 4).
Comparison of tumor size between SSA/POs and SSA/PDAs groups according to removal method and age groups
The results of the comparison of tumor size between SSA/POs and SSA/PDAs groups according to removal methods are summarized in Table 5. The tumor size of SSA/Ps treated by polypectomy, such as cold biopsy or snare, was 6.2 ± 5.9 mm, by EMR, 10.6 ± 6.0 mm, by EPMR, 27.0 ± 8.3 mm, and by ESD, 27.5 ± 12.6 mm. The comparison of tumor size between SSA/POs and SSA/PDAs groups according to removal method and age groups showed statistically non-significant differences among applied removal methods (Table 5).
Discussion
SSA/Ps are considered major precursor lesions of the serrated neoplasia pathway, which account for one-third of all sporadic colorectal cancers3,4,5,6,7,8. SSA/Ps comprise approximately 15–25% of colorectal serrated lesions and 2–9% of all colorectal polyps. SSA/Ps have a marked predilection for the proximal colon and have predominantly a sessile or flat morphology12,13,14,15,16,17,18,19,20. In our study, 60.0% of SSA/Ps were localized in the proximal colon and 75.9% had sessile (0-Is) or flat morphologies (0-IIa, 0-IIb, and 0-IIa + IIc). These results are similar to those revealed by previous12,13,14,15,16,17,18,19,20 and our studies. Histopathologically, SSA/Ps are subclassified as SSA/POs and SSA/PDAs, and SSA/PDAs accounted for about 15.1% of SSA/Ps and 0.18% of all colorectal polyps in a large cohort study33. In our study, the incidence of SSA/PDs was 30.4% of SSA/Ps. This result is inconsistent with that of another report33 and may be related to the variable sample size of the current study and the previous report and the unavoidable selection bias of the present retrospective study.
Endoscopic detection of SSA/Ps is difficult because of their subtle features compared to conventional adenomas12,13,14,15,16,17,18,19,20. Previously, the most prevalent endoscopic features of SSA/Ps according to the criteria defined by Tadefalli et al. were mucous cap, rim of debris/bubbles, altered fold contour, and disrupted vascular pattern31. Another study showed that the disrupted vascular pattern, altered fold contour, or rim of debris/bubbles were most prevalent endoscopic features of SSA/Ps21. In our study, the main endoscopic features of SSA/Ps were nodular surface, disrupted vascular pattern, or altered fold contour.
Before the development of cancer, SSA/Ps progress indolently; however, they are believed to progress rapidly to cancer after the beginning of cytological dysplasia3,4,5,6,7,8. Therefore, the differentiation between SSA/POs and SSA/PDAs is clinically important. Especially, accurate endoscopic differentiation is very important and can eventually result in complete resection via immediate decision-making of the resection of target lesions. Moreover, the complete en bloc resection may be basically indicated for SSA/PDAs, regardless of tumor size12,13,14,15,16,17,18,19,20.
SSA/PDAs occur frequently in older female patients and in the proximal colon21,22,23,24,25,26,27,28,29,30. In our study, no significant differences were detected between SSA/POs and SSA/PDAs in terms of age and sex, but SSA/PDAs affected predominantly the distal colon.
SSA/PDAs are clinically and endoscopically similar to SSA/POs, making endoscopic differentiation difficult. A previous study showed that endoscopic features of SSA/POs tended to present as more often altered fold contour and SSA/PDAs tended to be more often characterized by a pale color and dome-shaped morphology than SSA/POs21. Another study reported that the incidence of any 0-Is morphologies or nodular components within the lesions was higher for SSA/PDAs than for SSA/POs29. Moreover, in SSA/PDAs with nodule/protrusion, the nodule/protrusion detected by endoscopy corresponded to the portion of dysplasia or carcinoma on histology28. Furthermore, SSA/PDAs displayed pedunculated (0-Ip) or semipedunculated (0-Isp) morphologies more frequently than SSA/POs30. In our study, nodular surface, disrupted vascular pattern, and 0-Isp or 0-IIb morphologies were more commonly found in SSA/PDAs, while rim of debris/bubbles was more commonly found in SSA/POs in analysis of endoscopic features. On multivariate analysis, SSA/Ps with 0-IIb, nodular surface, or disrupted vascular pattern showed the significant association with the risk of dysplasia or adenocarcinoma. The analyses of sensitivity and specificity of each characteristic for SSA/PDs showed the results as follow; SSA/Ps with 0-IIb (sensitivity 1.9% and specificity 99.5%), nodular surface (sensitivity 72.2% and specificity 65.7%), and disruption of vascular pattern (sensitivity 52.5% and specificity 60.3%). Nodular surface showed a better predictive performance than others. Therefore, because SSA/Ps with these findings are likely to be accompanied by dysplasia or adenocarcinoma, they should be removed en bloc for accurate histopathological assessment and prevention of interval cancer development.
The presence of SSA/Ps was associated with the presence of synchronous advanced colorectal neoplasia34,35,36. In our study, 49.8% of patients with SSA/Ps had conventional adenoma with low-grade dysplasia, high-grade dysplasia, or adenocarcinoma. Furthermore, conventional adenomas with low-grade dysplasia were more commonly found in SSA/PDAs than in SSA/POs. Advanced adenomas with high-grade dysplasia and adenocarcinoma tended to be found more frequently in SSA/PDAs compared to SSA/POs. Therefore, the recognition of SSA/Ps should alert the endoscopist to meticulously inspect the remaining part of the colonic mucosa for more lesions.
In our study, SSA/Ps were resected by various techniques, including polypectomy, such as cold biopsy or snare, EMR, EPMR, or ESD as endoscopic resection of conventional adenoma37,38,39,40. Moreover, treatment was applied according to the tumor size, namely polypectomy was usually used for resection of SSA/Ps < 10 mm, EMR was used for SSA/Ps 10–20 mm, and ESD or EPMR was used for SSA/Ps ≥ 20 mm. In addition, the comparison of tumor size between SSA/POs and SSA/PDAs groups according to removal method and age groups showed no statistically significant differences between applied removal methods. Although the treatment strategy for SSA/Ps has not yet been established, our results showed that the principles for the management of SSA/Ps may be similar to those for conventional adenomas in clinical practice. Post-procedural bleeding was more commonly found in SSA/PDAs than in SSA/POs. This may be related to the fact that the lesions with dysplasia or adenocarcinoma present rapid growth and increased neovascularization toward the submucosa.
However, our study has some limitations. First, the study design was retrospective and nonrandomized; therefore, selection biases were unavoidable. Second, the heterogeneity of the SSA/PO and SSA/PDA groups was inevitable. For these reasons, large prospective, multicenter studies evaluating the clinical and endoscopic characteristics and outcomes of SSA/PDAs for optimal detection, complete resection, and appropriate surveillance of SSA/Ps are needed to provide more definitive evidence.
In conclusion, SSA/Ps with 0-IIb, nodular surface and disrupted vascular pattern are associated with an increased risk of dysplasia or adenocarcinoma. Therefore, these findings can be considered useful indicators in the management of SSA/Ps.
References
Vogelstein, B. M. et al. Genetic alterations during colorectal-tumor development. N Engl J Med. 319, 525–532 (1988).
Jass, J. R. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology. 50, 113–130 (2007).
Snover, D. C. Update on the serrated pathway to colorectal carcinoma. Hum Pathol. 42, 1–10 (2011).
Zhu, H. et al. Histology subtypes and polyp size are associated with synchronous colorectal carcinoma of colorectal serrated polyps: a study of 499 serrated polyps. Am J Cancer Res. 5, 363–374 (2014).
O’Brien, M. J. et al. Comparison of microsatellite instability, CpG island methylation phenotype, BRAF and KRAS status in serrated polyps and traditional adenomas indicates separate pathways to distinct colorectal carcinoma end points. Am J Surg Pathol. 30, 1491–1501 (2006).
Kim, K. M. et al. Molecular features of colorectal hyperplastic polyps and sessile serrated adenoma/polyps from Korea. Am J Surg Pathol. 35, 1274–1286 (2011).
Fujita, K. et al. Sessile serrated adenoma with early neoplastic progression: a clinicopathologic and molecular study. Am J Surg Pathol. 35, 295–304 (2011).
Snover, D. C. et al. Serrated polyps of the colon and rectum and serrated polyposis in WHO Classification of Tumors of the Digestive System (eds Bosman, F. T., Carneiro, F. & Hruban, R.) 160–165 (Stylus Publishing 2010).
Singh, S., Singh, P. P., Murad, M. H., Singh, H. & Samadder, N. J. Prevalence, risk factors, and outcomes of interval colorectal cancers: a systematic review and meta-analysis. Am J Gastroenterol. 109, 1375–1389 (2014).
Samadder, N. J. et al. Characteristics of missed or interval colorectal cancer and patient survival: a population-based study. Gastroenterology. 146, 950–960 (2014).
Dong, S. H., Huang, J. Q. & Chen, J. S. Interval colorectal cancer: a challenging field in colorectal cancer. Future Oncol. 14, 1307–1316 (2018).
Rex, D. K. et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol. 107, 1315–1329 (2012).
Rosty, C., Hewett, D. G., Brown, I. S., Leggett, B. A. & Whitehall, V. L. Serrated polyps of the large intestine: current understanding of diagnosis, pathogenesis, and clinical management. J Gastroenterol. 48, 287–302 (2013).
Hetzel, J. T. et al. Variation in the detection of serrated polyps in an average risk colorectal cancer screening cohort. Am J Gastroenterol. 105, 2656–2664 (2010).
Kolb, J. M. et al. Detection, Diagnosis, and Resection of Sessile Serrated Adenomas and Polyps. Gastroenterology. 153, 646–648 (2017).
Fan, C., Younis, A., Bookhout, C. E. & Crockett, S. D. Management of Serrated Polyps of the Colon. Curr Treat Options Gastroenterol. 16, (182–202 (2018).
Okamoto, K. et al. Clinicopathological characteristics of serrated polyps as precursors to colorectal cancer: Current status and management. J Gastroenterol Hepatol. 32, 358–367 (2017).
Thorlacius, H. et al. Serrated polyps - a concealed but prevalent precursor of colorectal cancer. Scand J Gastroenterol. 52, 654–661 (2017).
Crockett, S. D., Snover, D. C., Ahnen, D. J. & Baron, J. A. Sessile serrated adenomas: an evidence-based guide to management. Clin Gastroenterol Hepatol. 13, 11–26 (2015).
Bordaçahar, B. et al. Sessile serrated adenoma: from identification to resection. Dig Liver Dis. 47, 95–102 (2015).
Bouwens, M. W. et al. Endoscopic characterization of sessile serrated adenomas/polyps with and without dysplasia. Endoscopy. 46, 225–235 (2014).
Sano, W. et al. Clinical and endoscopic evaluations of sessile serrated adenoma/polyps with cytological dysplasia. J Gastroenterol Hepatol. 33, 1454–1460 (2018).
Tanaka, Y. et al. Endoscopic and molecular characterization of colorectal sessile serrated adenoma/polyps with cytologic dysplasia. Gastrointest Endosc. 86, 1131–1138 (2017).
Bettington, M. et al. Clinicopathological and molecular features of sessile serrated adenomas with dysplasia or carcinoma. Gut. 66, 97–106 (2017).
Yang, J. F. et al. Anatomic distribution of sessile serrated adenoma/polyp with and without cytologic dysplasia. Arch Pathol Lab Med. 139, 388–393 (2015).
Murakami, T., Sakamoto, N. & Nagahara, A. Endoscopic diagnosis of sessile serrated adenoma/polyp with and without dysplasia/carcinoma. World J Gastroenterol. 24, 3250–3259 (2018).
Liu, T. Y. et al. Clinicopathological features of advanced colorectal serrated lesions: A single-center study in China. J Dig Dis. 19, 235–241 (2018).
Tate, D. J. et al. A standardized imaging protocol for the endoscopic prediction of dysplasia within sessile serrated polyps (with video). Gastrointest Endosc. 87, 222–231 (2018).
Burgess, N. G. et al. Clinical and endoscopic predictors of cytological dysplasia or cancer in a prospective multicentre study of large sessile serrated adenomas/polyps. Gut. 65, 437–446 (2016).
Murakami, T. et al. Distinct endoscopic characteristics of sessile serrated adenoma/polyp with and without dysplasia/carcinoma. Gastrointest Endosc. 85, 590–600 (2017).
Tadepalli, U. S. et al. A morphologic analysis of sessile serrated polyps observed during routine colonoscopy (with video). Gastrointest Endosc. 74, 1360–1368 (2011).
Endoscopic Classification Review Group. Update on the paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy. 37, 570–578 (2005).
Lash, R. H., Genta, R. M. & Schuler, C. M. Sessile serrated adenomas: prevalence of dysplasia and carcinoma in 2139 patients. J Clin Pathol. 63, 681–686 (2010).
IJspeert, J. E. et al. Prevalence, distribution and risk of sessile serrated adenomas/polyps at a center with a high adenoma detection rate and experienced pathologists. Endoscopy. 48, 740–746 (2016).
Hazewinkel, Y. et al. Prevalence of serrated polyps and association with synchronous advanced neoplasia in screening colonoscopy. Endoscopy. 46, 219–224 (2014).
Álvarez, C. et al. Relationship of colonoscopy-detected serrated polyps with synchronous advanced neoplasia in average-risk individuals. Gastrointest Endosc. 78, 333–341 (2013).
Tanaka, S. et al. Evidence-based clinical practice guidelines for management of colorectal polyps. J. Gastroenterol. 50, 252–260 (2015).
De Ceglie, A. et al. Endoscopic mucosal resection and endoscopic submucosal dissection for colorectal lesions: A systematic review. Crit Rev Oncol Hematol. 104, 138–155 (2016).
Ma, M. X. & Bourke, M. J. Complications of endoscopic polypectomy, endoscopic mucosal resection and endoscopic submucosal dissection in the colon. Best Pract Res Clin Gastroenterol. 30, 749–767 (2016).
Lee, E. J., Lee, J. B., Lee, S. H. & Youk, E. G. Endoscopic treatment of large colorectal tumors: comparison of endoscopic mucosal resection, endoscopic mucosal resection-precutting, and endoscopic submucosal dissection. Surg Endosc. 26, 2220–2230 (2012).
Author information
Authors and Affiliations
Contributions
Conceptualization: Y.E.J. Data curation: K.H.K., K.O.K., Y.H.J., J.L., S.W.K., J.H.K., T.J.K., Y.S.C. Formal analysis: K.H.K., K.O.K., Y.H.J., J.L., S.W.K., J.H.K., T.J.K., Y.S.C. Investigation: K.H.K., Y.E.J. Methodology: Y.E.J. Project administration: Y.E.J. Resources: K.H.K., K.O.K., Y.H.J., J.L., S.W.K., J.H.K., T.J.K., Y.S.C. Writing-original draft: Y.E.J. Writing-review & editing: Y.E.J.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, KH., Kim, KO., Jung, Y. et al. Clinical and endoscopic characteristics of sessile serrated adenomas/polyps with dysplasia/adenocarcinoma in a Korean population: A Korean Association for the Study of Intestinal Diseases (KASID) multicenter study. Sci Rep 9, 3946 (2019). https://doi.org/10.1038/s41598-019-40559-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-40559-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.