Abstract
Late Embryogenesis Abundant (LEA) proteins are mostly predicted to be intrinsically disordered proteins (IDPs) that are induced under conditions of cellular dehydration. Their functions, however, are largely unexplored and also their structure and interactions with potential target molecules have only recently been investigated in a small number of proteins. Here, we have characterized the wheat LEA protein TdLEA3, which has sequence homology with the group of LEA_4 proteins that are characterized by the 11-mer repeat motif TAQAAKEKAXE. TdLEA3 has five repeats of this imperfectly conserved 11-mer amino acid motif. To investigate the structure of the protein, we used circular dichroism (CD) and Fourier-transform infrared (FTIR) spectroscopy. The data show that TdLEA3 was largely disordered under fully hydrated conditions and acquired α-helical structure upon drying and in the presence of trifluoroethanol (TFE). Moreover, the addition of increasing glycerol concentrations to the protein solution induced a progressive gain in α-helix content. Activity assays indicated that TdLEA3 was able to prevent the inactivation of lactate dehydrogenase (LDH) under heat, dehydration-rehydration and freeze-thaw treatments. In addition, TdLEA3 reduced aggregate formation in the enzyme during these treatments.
Similar content being viewed by others
Introduction
During their maturation phase, orthodox seeds lose most of their water content as part of their normal development. This maturation drying induces the accumulation of Late Embryogenesis Abundant (LEA) proteins. LEA proteins were first described more than 35 years ago, when they were found to be highly abundant during the late stages of cotton seed development, when the embryo becomes desiccation tolerant1. Subsequently, related proteins were found not only in the seeds of all other investigated plant species, but also in other plant tissues, in some bacterial species and in invertebrate animals such as nematodes, rotifers and brine shrimp2. According to their amino acid sequences, LEA proteins are classified into eight Pfam families based on conserved motifs3,4. The LEA_4 (PF02987) proteins, also referred to as Group 3 proteins5, are accumulated by plants, microorganisms and invertebrates in response to dehydration stress6,7,8,9.
LEA_4 proteins are hydrophilic and have been predicted to belong to the group of intrinsically disordered proteins (IDPs)7,8. This prediction has been confirmed for all LEA_4 proteins investigated experimentally8,10. However, their flexibility allows them to adopt mainly α-helical structure upon drying8,10,11. Recent studies on LEA proteins have focused on their structural transitions upon dehydration and the relationship of these transitions to possible functions. Structural analyses by circular dichroism (CD), nuclear magnetic resonance (NMR) and Fourier-transform infrared (FTIR) spectroscopy methods showed that several LEA proteins fold into α-helices in the presence of the chemical helix inducer trifluoroethanol (TFE) and when subjected to molecular crowding in the presence of glycerol12,13,14,15 or partial dehydration at low relative humidity16. Moreover, the addition of sodium dodecyl sulfate (SDS) induced α-helicity in several LEA proteins7,8 and NMR analyses indicated direct interactions of a dehydrin with this membrane-mimicking detergent17. Also, LEA18 from Arabidopsis thaliana showed partial folding into β-sheet conformation in the presence of liposomes18, while LEA7 showed partial folding into α-helices19. COR15A and two other LEA_4 proteins, on the other hand, only showed increased α-helicity in the presence of high concentrations of glycerol, indicating that partial folding was necessary for membrane interaction15.
LEA_4 proteins are able to function as protectants for enzymes during freezing or drying in addition to protecting membranes20,21,22,23,24,25,26,27. The ability of LEA proteins to protect the activity of desiccation sensitive enzymes from the deleterious effects of dehydration can, at least partially, be attributed to an ability to prevent enzyme aggregation21,28,29. It has been proposed that LEA proteins may act as a molecular shield to prevent protein aggregation. Their disordered and flexible structure could allow them to function as a physical barrier between enzyme molecules and thus prevent their aggregation under conditions that induce (partial) unfolding. In agreement with this proposed mechanism, it has been shown that several LEA proteins are able to reduce the aggregation of polyglutamine (polyQ) or amyloid ß-peptides when co-expressed in mammalian cells30,31,32.
Here, we have investigated the structural and functional properties of the LEA_4 protein TdLEA3 from durum wheat (Triticum turgidum L. subsp. durum). This protein shows 83% and 98% amino acid sequence identity to TaLEA2 and TaLEA3, respectively, from bread wheat (Triticum aestivum L.), which have, however, not been structurally and functionally characterized33. Here, we show that TdLEA3 is an IDP in dilute aqueous solution and folds into α-helices during drying and under conditions of glycerol-induced crowding. TdLEA3 was able to prevent the inactivation and aggregation of the enzyme lactate dehydrogenase (LDH) under heat, dehydration or freeze-thaw treatments indicating its potential function in cellular stress tolerance. To the best of our knowledge, TdLEA3 is the first LEA_4 protein for which chaperone activity, i.e. stabilization of an enzyme during heat stress in solution, has been shown.
Results
Production and Purification of Recombinant TdLEA3
The open reading frame of TdLEA3 was cloned in frame with the polyhistidine tag of the pET28a expression vector. Recombinant TdLEA3 protein was expressed in E. coli cells and purified by affinity chromatography. The purity of the protein was assessed by SDS-PAGE and western blotting (Fig. 1a). The protein migrated at a position in the gel corresponding to a higher molecular mass (35 kDa) than calculated from the amino acid sequence (21.9 kDa; Table 1), which has been frequently observed for LEA proteins34. The identity of TdLEA3 was verified by western blot analysis using an anti-His6 antibody (Fig. 1a).
Purity and properties of the TdLEA3 protein. (a) Molecular mass and purity of TdLEA3. M. Size markers. A. SDS-PAGE analysis of recombinant TdLEA3. B. Western blot of TdLEA3 protein identified using a His6-tag specific antibody. (b) Hydropathy analysis of TdLEA3 using the Kyte-Doolittle scale. Regions with values above 0 are hydrophobic in character. (c) Alignment of the repeating 11-mer motifs found in TdLEA3 protein sequence using the RADAR software. Numbers refer to the amino acid position of the first residue in the motif. Green indicates polar amino acids, red hydrophobic residues, blue negatively charged residues and pink positively charged residues. (d) Helical wheel projection of the 11-mer repeat 68–87 region of TdLEA3. Red indicates negatively charged, blue positively charged, grey hydrophilic amino acids, (µH) is the mean hydrophobic moment and (H) the mean hydrophobicity of the sequence domain.
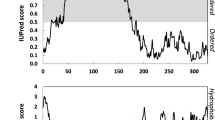
TdLEA3 amino acid composition and properties
In silico analysis of TdLEA3 using IUPRED indicated a high predicted level of disorder (76%), while the SOPMA tool predicted an 81% content of α-helices (Table 1). The amino acid composition of TdLEA3 is dominated by hydrophilic and charged amino acids. Consequently, the protein is highly hydrophilic, with a GRAVY of −1.096 and has a pI of 9.02. Also, a hydropathy analysis (Fig. 1b) based on the Kyte–Doolittle scale35 showed that the N-terminal 168 amino acids of the protein and the C-terminus (amino acids 185–208) are hydrophilic. However, there is an intervening hydrophobic domain that is rather atypical of LEA_4 proteins. Typical LEA_4 proteins contain one or several 11-mer repeat sequences TAQAAKEKAXE. TdLEA3 has five repeats of an imperfect 11-mer amino acid motif that are not identical to the consensus sequence (Fig. 1c). HELIQUEST predicts that the 11-mer repeat located in the region between amino acids 68 and 87 forms an amphipathic α-helix (Fig. 1d). Such helices have previously been suggested to be involved in interactions of LEA_4 proteins with target structures such as membranes15.
TdLEA3 has an intrinsic propensity to fold into an α-helical conformation
Given the diverging structural predictions obtained from IUPRED and SOPMA (Table 1), the structural features of TdLEA3 were further investigated by CD spectroscopy. The far UV-CD spectrum of TdLEA3 under fully hydrated conditions showed a negative ellipticity around 200 nm, indicating a largely unstructured conformation, typical for IDPs (Fig. 2a). However, after drying, the spectrum changed drastically and showed two minima at 208 and 222 nm, typical of a mainly α-helical conformation. A similar spectrum was also obtained in the presence a chemical inducer of α-helicity, trifluoroethanol (TFE). Secondary structure estimates obtained from these spectra indicated that TdLEA3 was approximately 84% random coil in the fully hydrated state, while both drying and the presence of 50% TFE induced over 80% α-helix content in the protein (Fig. 2b).
Far UV-CD analysis of the secondary structure of TdLEA3. (a) CD spectra of the protein in the hydrated state, in the dry state and in the presence of 50% of trifluoroethanol (TFE), (b) Secondary structure composition of TdLEA3, as calculated from the respective CD data for the hydrated protein, the dried protein and the protein exposed to 50% TFE. Error bars represent ± SEM from three different samples.
FTIR spectroscopy was used to further probe the secondary structure of TdLEA3. H2O gives rise to a strong absorbance peak in the FTIR spectrum around 1645 cm−1 that overlaps with the Amide I peak, which is indicative of protein secondary structure. Therefore, the protein was dissolved in D2O to avoid this interference. The broad Amide I peak (between approximately 1700 and 1600 cm−1) is composed of several component peaks that originate from different secondary structures. Bands in the region between 1660–1650 cm−1 are assigned to α-helix, while a peak in the region between 1640 cm−1 and 1650 cm−1 indicates unordered regions in a protein. A peak at around 1620 cm−1 is associated with intermolecular β-sheet aggregates36,37,38. The Amide I peak of the hydrated protein was centered at 1648 cm−1, indicating a mainly unstructured protein. Upon drying, this maximum was shifted to 1657 cm−1 (Fig. 3), indicating a gain in α-helix conformation during drying, in agreement with the CD spectra. We did not observe any indication for β-sheet aggregates.
FTIR analysis of the secondary structure of TdLEA3. The protein was investigated in the hydrated (D2O) and in the dry state and the Amide I region of the FTIR spectra is shown as a mean of three samples. The Amide I peak of the fully hydrated protein was located at 1648 cm−1 indicating a highly disordered protein, while in the dry state the peak was shifted to 1657 cm−1 indicating largely α-helical conformation.
Low water availability due to glycerol-induced crowding or dehydration leads to folding of TdLEA3
It has been shown previously that some intrinsically disordered LEA proteins fold into α-helices in the presence of high concentrations of glycerol12,13,15. CD spectroscopy showed that TdLEA3 also folded in the presence of glycerol, from about 7% α-helix in 10% glycerol to 85% α-helix in 80% glycerol (Fig. 4).
We further explored the protein conformational transition by rehydrating the dry protein under conditions of different relative humidity. The secondary structure of TdLEA3 was recorded by FTIR spectroscopy under increasing humidity as previously reported16. In the dry state, the protein was characterized by a peak centered at about 1657 cm−1, indicating a largely α-helical protein, as indicated in Fig. 3. Equilibration of the protein at 11% RH only induced a minor shift in the Amide I peak. With further increases in RH, the Amide I peak broadened on the low wavenumber side, indicating partial unfolding. In addition, a shoulder at about 1624 cm−1 suggested partial aggregation under these conditions. At the two highest RH (93% and 100%) aggregation was no longer visible and the spectra were centered at about 1647 cm−1, in agreement with a disordered protein (Fig. 5).
FTIR analysis of the secondary structure of TdLEA3 at different relative humidities (RH). Dry protein samples were equilibrated over different saturated salt solutions or over D2O (100% RH) producing the indicated RH. The Amide I peaks of TdLEA3 at different RH are shown as means of three samples each and are vertically off-set to improve the visibility of the curves.
TdLEA3 stabilizes the enzyme LDH under different stress conditions
LEA proteins confer in vitro stabilization of several enzymes under stress conditions (see2,4 for reviews). Therefore, we tested the ability of TdLEA3 to prevent the loss of LDH activity after heating, dehydration and freezing. We compared the effects of TdLEA3 with the effects of BSA as an example of a non-specific protectant and with LDH treated in buffer without additional protein. Under all stress conditions and at all concentrations, TdLEA3 provided a higher degree of protection for LDH than BSA, indicating that the LEA protein had a protective activity that went beyond the unspecific effects of having a second protein present (Fig. 6). After heating at 48 °C for 10 min, LDH had lost about half of its activity in buffer and activity was further reduced with longer incubation times, to about 20% after 30 min. In contrast, the enzyme activity was completely preserved after 10 or 20 min of heat treatment in the presence of TdLEA3 at mass ratios (LDH: TdLEA3) of 1:20 and 1:40. At the highest mass ratio TdLEA3 preserved more than 90% of the enzyme activity after 30 min at 48 °C.
TdLEA3 protects lactate dehydrogenase (LDH) against inactivation under stress. (a–c) recovery of LDH activity during heating at 48 °C for the indicated times in the presence of TdLEA3, BSA, or buffer at the indicated mass ratios. (d) The recovery of LDH activity after dehydration in the presence of TdLEA3, BSA, or buffer at the indicated mass ratios. (e,f) Recovery of LDH activity following 2–4 freeze–thaw cycles in the presence of TdLEA3, BSA, or buffer at the indicated mass ratios. Throughout the experiments, addition of buffer was used as a negative control to assess the dilution effect. Data are expressed as % activity measured before the stress in the presence of the corresponding protein and are the averages ± SEM of three to five replicates from two independent experiments. Different letters indicate a significant difference (p-value < 0.05) evaluated with the Student’s t test.
After dehydration and rehydration, LDH activity was reduced to about 35% of its initial value. TdLEA3 provided strong stabilization to the enzyme at all mass ratios, reaching 93% of the fresh activity at a mass ratio (LDH: TdLEA3) of 1:40 (Fig. 6d). Two cycles of freezing and thawing were sufficient to reduce LDH activity massively and after four cycles, residual activity was below 20%. In the presence of the TdLEA3, LDH activity was partially protected. While at a mass ratio of 1:40 residual activity was close to 90% after two freeze-thaw cycles, it still reached 62% after four cycles (Fig. 6e,f).
TdLEA3 inhibits LDH aggregation during stress treatments
LDH is well known to form aggregates when subjected to dehydration, heating or freeze-thaw treatments (see39 for a review). We therefore investigated the ability of TdLEA3 to reduce LDH aggregation during stress treatments by measuring apparent light scattering of protein solutions as absorbance. We used two mass ratios of enzyme: protein (1:1 and 1:2). After heating for 20 min at 80 °C, LDH showed massive aggregation. The presence of TdLEA3 reduced the aggregation of LDH at both investigated mass ratios to a larger extent than BSA (Fig. 7a). In the dehydration-rehydration assay, the absorbance in the presence of TdLEA3 and BSA was very similar at a mass ratio 1:1, but at a mass ratio 1:2 TdLEA3 reduced aggregate formation more strongly than BSA (Fig. 7b). During freezing and thawing, both BSA and TdLEA3 provided strong protection for LDH after both two and three cycles of freezing-thawing treatment at both mass ratios (Fig. 7c,d). However, LDH aggregation was consistently lower in the presence of the LEA protein than in the presence of the non-specific protectant BSA.
Anti-aggregation activity of TdLEA3. Heating (a), dehydration-rehydration (b), and two (c) or three freeze-thaw cycles (d) were used. Aggregation was measured in a spectrophotometer as the absorbance at 340 nm. TdLEA3 and BSA were added to the LDH at the indicated mass ratios. The data are the averages ± SEM from three independent experiments. Different letters indicate a significant difference (p-value < 0.05) evaluated with the Student’s t test.
Discussion
Both prediction (e.g.40) and spectroscopic investigations of recombinant proteins7,8,10 have indicated that LEA_4 proteins from different organisms lack stable secondary structure in dilute solution. This lack of structure places LEA proteins within a large class of proteins most commonly called intrinsically disordered proteins, or IDPs41. Among the most intensively studied plant IDPs are LEA proteins42. In an effort to provide additional insights into the structure and function of LEA_4 family proteins, we report the characterization of the wheat LEA protein TdLEA3. In agreement with its likely nature as an IDP, the protein is largely hydrophilic, with the exception of a small stretch of amino acids close to the C-terminus. To reveal the functional relevance of this unusual feature in a LEA_4 protein, mutational studies will be necessary. Also, the functional role of the amphipathic α-helix in TdLEA3 will require further research.
Bioinformatic prediction of TdLEA3 secondary structure resulted in widely diverging results in that IUPRED predicted the protein to be 76% disordered, while SOPMA predicted 81% α-helix content. Our CD and FTIR data clearly showed that recombinant TdLEA3 was predominantly disordered in dilute solution, but folded into α-helices during dehydration or under crowded conditions induced by high concentrations of glycerol. In addition, TdLEA3 gained α-helical structure in the presence of 50% TFE. This chemical agent induces protein folding by desolvating the protein backbone43, similar to drying or high concentrations of glycerol14. This also explains the prediction of a high degree of helicity in TdLEA3 by SOPMA, as this tool predicts structure in vacuo and therefore tends to predict the structure of dry LEA proteins rather than the hydrated disorder15,19,44,45.
Physiologically, it may be argued that LEA proteins such as TdLEA3 that are involved in the freezing or dehydration tolerance of plants should be inactive under non-stress, fully hydrated conditions, where they are unstructured, but may become activated through folding when water is lost from the cells due to evaporation or extracellular ice crystal formation. Increasing solute concentrations during dehydration were simulated in vitro with glycerol. Concentration-dependent folding in the presence of glycerol has also been shown for intrinsically disordered LEA proteins from other families13,46 and other osmolytes such as TMAO, sucrose and proline may have similar folding effects47,48. In line with this argument, our FTIR spectroscopy data showed a clear influence of relative air humidity on TdLEA3 folding. Such an effect has been shown previously for the LEA_4 protein COR15A from Arabidopsis thaliana16 which showed significant α-helicity at 97% RH. TdLEA3, on the other hand, was completely unfolded at 93% RH and only showed secondary structure at 85% RH or lower, indicating that different LEA_4 proteins differ in their propensity for dehydration-induced folding. This has not been observed previously as TdLEA3 is only the second LEA protein that has been investigated in this way and may open an interesting area of future research.
We have shown that TdLEA3 is able to protect the stress-sensitive target enzyme LDH from damage during drying, freezing and heat treatment. The protection afforded by the LEA protein was better than that provided by BSA, which is a well-known unspecific enzyme stabilizer. TdLEA3 did not only protect LDH from loss of catalytic activity, but also prevented enzyme aggregation. Such a reduction of enzyme aggregation, in particular during drying, has been observed for other LEA_4 proteins before and has been explained on the basis of the “molecular shield” hypothesis21,27,28,49. It should be stressed, however, that aggregation of LDH during drying depends strongly on the experimental conditions and that LDH can be completely inactivated by drying in the absence of any aggregation50. LDH activity was nevertheless protected under these conditions by the LEA_4 protein LEA7, indicating that protection of enzymes by LEA_4 proteins may be due to more than one mechanism.
Quite strikingly, TdLEA3 protected LDH not only during drying and freezing, activities that have been reported frequently for LEA proteins in the literature, but also under heat stress. To the best of our knowledge, such a chaperone activity has previously only been reported for two dehydrins from Arabidopsis34, while a LEA_4 protein from an anhydrobiotic nematode was not able to stabilize enzymes during heating21,30. Our data indicate that it will be well worth testing further LEA_4 proteins for potential chaperone activity and to characterize this interesting function further. It has been proposed34 that the chaperone function of dehydrins may be due to a “molecular shield” mechanism that would prevent aggregation, however, experimental evidence for such a mechanism is still lacking. Also, since LDH is a tetramer in its functional form, TdLEA3 may also be able to prevent dissociation of the monomers under heat stress or allow functional reassembly upon cooling. Further research will be necessary to resolve this question.
Materials and Methods
Plant material and stress treatment
Seeds of durum wheat (Triticum turgidum L. subsp. durum) cultivar OmRabia3 were provided by the Tunisian Agronomic Research Institute. After sterilization and germination, four days-old seedlings were transferred to containers with modified half-strength Hoagland’s solution51 to grow for 10 days under greenhouse conditions (25 ± 5 °C, 16 h photoperiod at 280 µmol photons m−2 s−1 and 60 ± 10% relative humidity). For stress treatments, seedlings were transferred to a solution containing 200 mM NaCl. After three days of stress application, plants were immersed in liquid nitrogen and then stored at −80 °C for RNA isolation.
Isolation, expression and purification of durum wheat TdLEA3 protein
Total RNA was isolated from the durum wheat seedlings using Trizol Reagent (TaKaRa, China). cDNA was synthesized by M-MLV reverse transcriptase (Invitrogen) at 37 °C for 1 h using oligo-dT18 primers. The TdLEA3 cDNA was subsequently amplified using PFU polymerase (Fermentas) and primers designed on the basis of the gene sequence of Triticum aestivum LEA3 (AY148492): 5′-ATGGCCTCCAACCAGAACCA-3′ and 5′-CTAGTGATTCCTGGTGGTGGT-3′. Amplified products were purified and cloned into the pGEM-T Easy vector system (Promega) and successful isolation of TdLEA3 DNA was confirmed by restriction analysis and sequencing. The 5′ and 3′ regions of TdLEA3 cDNA were verified with the 5′- and 3′-RACE technique using the ‘First choice RLM Race kit’ according to the instructions of the manufacturer (Ambion). The following primers were used (5′ to 3′): R5-TdLEA3: ATCACCTGCCCGGTCTTCT and R3-TdLEA3: ACAACGCCACCAAGGACAC. The full-length open reading frame of TdLEA3 (GenBank accession: KR698795) was amplified from the durum wheat variety OmRabia3 cDNA using the following primers corresponding to the 5′ and 3′ ends and containing EcoRI restriction sites at their ends: TdLEA3: 5′-GAATTCATGGCCTCCAACCAGAAC-3′ and TdLEA3: 5′-GAATTCGTGATTCCTGGTGGTGGT-3′.The TdLEA3 ORF was cloned into the EcoRI site of the Escherichia coli expression vector Pet28a. Recombinant protein production of TdLEA3 in E. coli BL21 (DE3) was induced by 1 mM IPTG at 37 °C, cells were grown for 4 h, lysed in NENT buffer (100 mM NaCl, 1 mM EDTA, 0.5% NP40, 20 mM Tris-HCl, pH 7.9) containing the protease inhibitor phenylmethylsulfonyl fluoride and purified by HisLink Protein Purification Resin according to the manufacturer’s (Promega) instructions. The concentration of the recombinant protein was determined spectrophotometrically at 280 nm using a molar extinction coefficient calculated from the amino acid sequence using an online tool (http://encorbio.com/protocols/Prot-MW-Abs.htm).
In-silico analysis
Molecular mass, theoretical pI and grand average of hydropathy (GRAVY) of TdLEA3 were assessed by the ExPASy-ProtParam tool (http://web.expasy.org/protparam/). Sequence identities were determined using BLAST on the NCBI web server (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The Kyte–Doolittle scale48 was used for hydrophobicity plots. Multiple sequence alignment was performed using Clustal Omega with default parameters. The tendency of disorder of the investigated protein was predicted by IUPRED (http://iupred.enzim.hu/). Secondary structure was predicted using the SOPMA tool (https://npsa-prabi.ibcp.fr/cgi-bin/npsa_automat.pl?page=/NPSA/npsa_sopma.html). Protein conserved motif analysis was conducted using RADAR (https://www.ebi.ac.uk/Tools/pfa/radar/). The resulting α-helical domain of TdLEA3 was visualized in a helical wheel projection using the HELIQUEST web tool (http://heliquest.ipmc.cnrs.fr/cgi-bin/ComputParams.py).
SDS-PAGE and western blot analysis
Five µg of protein per sample were separated by SDS-PAGE52. Protein bands were either visualized by Coomassie staining or electroblotted onto a nitrocellulose membrane (Schleicher and Schuell) using a semi-dry blotting system (Bio-Rad). Nonspecific binding sites were blocked with PBST (Tween 20 diluted 1000-fold in 10x PBS + 5% non-fat dry milk powder). The blot was probed with peroxidase-coupled anti-His tag antibody (Abcam) in a 1:2000 dilution in PBST with 1% non-fat dry milk.
CD spectroscopy
CD spectra were obtained with a Jasco-815 spectropolarimeter (Jasco Instruments). Protein solutions containing approximately 0.5 mg/ml protein in H2O were measured in a 0.1 mm path-length cuvette. Protein concentrations were estimated from the absorbance at 193 nm as described previously53. For the measurement of dry samples, 20 µl of 2 mg/ml protein dissolved in H2O were spread on CaF2 windows and dried in a desiccator over silica gel over night at 28 °C. We have previously verified the accuracy of our secondary structure determination of proteins in the dry state using reference proteins of known structure15. Measurements and data analysis were performed as described in detail previously15.
FTIR spectroscopy
FTIR measurements were performed on a Perkin Elmer GX2000 FTIR spectrometer equipped with a vacuum cuvette in the IR beam (Specac, Worthington, UK, 54). Samples (2 mg protein/ml) dissolved in D2O were analyzed between two CaF2 windows separated by a 0.01 mm Teflon spacer at ambient pressure55. Dry samples were prepared by spreading 50 µl of protein solution in H2O on a CaF2 window and drying them as described above for CD spectroscopy. These samples were measured under vacuum as described before54,56. To measure FTIR spectra of TdLEA3 at different relative humidities, dry protein samples on CaF2 windows prepared as described above were incubated for 24 h over different saturated salt solutions or over D2O. Samples were then covered by a second CaF2 window and measured under the same conditions as the fully hydrated samples16. Three samples per condition were measured and 32 spectra were coadded per sample. Changes in the secondary structure of the protein were estimated by analyzing the Amide I peak (1700–1600 cm−1) of the FTIR spectra using Spectrum 10.4.3 software (PerkinElmer, Rodgau, Germany). Spectra from the three replicates were averaged and smoothed in Kaleidagraph 4.5.0 (Synergy Software, Reading, PA, USA) using a 10 point sliding window.
LDH protection assay
Lactate dehydrogenase (LDH) from bovine heart was obtained from Sigma–Aldrich and diluted in 10 mM sodium phosphate, pH 7.4 following the manufacturer’s recommendations. One µl of 20 µg/µl LDH was added to a total of 20 µl of buffer with or without TdLEA3 or BSA at a mass ratio of 1:1, 1:10, 1:20 and 1:40 (LDH: LEA/BSA). Samples were vacuum-dried in a Speed Vac (Savant Speed Vac Plus Model SC110A Concentrator) to a final volume of 6 µl and rehydrated by the addition of 14 µl of buffer. To test the effect of freezing on LDH activity, samples were frozen in liquid nitrogen for 1.5 min and thawed at room temperature. Freeze-thaw cycles were performed up to four times. In heating assays, samples were treated at 48 °C for up to 30 min. To determine enzyme activity, 1 ml of freshly prepared assay buffer (10 mM sodium phosphate (pH 7.4), 2 mM NADH, and 10 mM pyruvic acid) was added to the LDH samples. NADH oxidation was monitored by measuring the absorbance at 340 nm over 3 min, during which the reaction rate was linear. The rate of absorbance decrease was then used to calculate activity (∆OD/min) × 8095 = U/l (Biomaghreb kit). All samples were assayed in triplicate.
In vitro protein aggregation assay
LDH was chosen as an example of an aggregation-prone protein during desiccation–rehydration, freezing-thawing and upon heating. Aggregation of LDH was monitored in a spectrophotometer (JENWAY, 7305) as the change in absorbance at 340 nm. Drying was performed as described above with the following modifications. One hundred µl of LDH (1 mg/ml) were partially dried in the absence or presence of TdLEA3 or BSA at 1:1 and 1:2 (enzyme: protein mass ratio) for 2.5 h until reaching 30% of the initial sample volume. Subsequently, samples were rehydrated to the original volume with water. In heating assays, samples were treated at 80 °C for 20 min. To test the effect of freezing on LDH activity, samples were frozen in liquid nitrogen for 1.5 min and thawed at room temperature. Freeze-thaw cycles were performed up to three times. All samples were assayed in triplicates.
Data Availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
References
Dure, L. III., Greenway, S. C. & Galau, G. A. Developmental biochemistry of cotton seed embryogenesis and germination: changing messenger ribonucleic acid populations as shown by in vitro and in vivo protein synthesis. Biochemistry 20, 4162–4168 (1981).
Battaglia, M., Olvera-Carrillo, Y., Garciarrubio, A., Campos, F. & Covarrubias, A. A. The enigmatic LEA proteins and other hydrophilins. Plant Physiol. 148, 6–24 (2008).
Hunault, G. & Jaspard, E. LEAPdb: a database for the late embryogenesis abundant proteins. BMC Genomics 11, 221 (2010).
Jaspard, E., Macherel, D. & Hunault, G. Computational and statistical analyses of amino acid usage and physico-chemical properties of the twelve late embryogenesis abundant protein classes. Plos One 7, e36968 (2012).
Bray, E. A. Molecular responses to water deficit. Plant Physiol. 103, 1035–1040 (1993).
Tunnacliffe, A. & Wise, M. J. The continuing conundrum of the LEA proteins. Naturwissenschaften 94, 791–812 (2007).
Tunnacliffe, A., Hincha, D. K., Leprince, O. & Macherel, D. LEA proteins: versatility of form and function. In Sleeping Beauties: Dormancy and Resistance in Harsh Environments. Lubzens, E. Cerda, J. & Clark, M. eds. (Berlin: Springer) pp. 91–108 (2010).
Hand, S. C., Menze, M. A., Toner, M., Boswell, l. & Moore, D. LEA proteins during water stress: not just for plants anymore. Annu. Rev. Physiol. 73, 115–134 (2011).
Liu, Y. et al. The effect of phosphorylation on the salt-tolerance-related functions of the soybean protein PM18, a member of the Group-3 LEA protein family. Biochim. Biophys. Acta 1865, 1291–1303 (2017).
Hincha, D. K. & Thalhammer, A. LEA proteins: IDPs with versatile functions in cellular dehydration tolerance. Biochem. Soc. Trans. 40, 1000–1003 (2012).
Goyal, K. et al. Transition from natively unfolded to folded state induced by desiccation in an anhydrobiotic nematode protein. J. Biol. Chem. 278, 12977–12984 (2003).
Thalhammer, A., Bryant, G., Sulpice, R. & Hincha, D. K. Disordered Cold Regulated 15 proteins protect chloroplast membranes during freezing through binding and folding, but do not stabilize chloroplast enzymes in vivo. Plant Physiol. 166, 190–201 (2014).
Cuevas-Velazquez, C. L., Saab-Rincon, G., Reyes, J. L. & Covarrubias, A. A. The unstructured N-terminal region of Arabidopsis Group 4 late embryogenesis abundant (LEA) proteins is required for folding and for chaperone-like activity under water deficit. J. Biol. Chem. 291, 10893–10903 (2016).
Navarro-Retamal, C. et al. Molecular Dynamics simulations and CD spectroscopy reveal hydration-induced unfolding of the intrinsically disordered LEA proteins COR15A and COR15B from Arabidopsis thaliana. Phys. Chem. Chem. Phys. 18, 25806–25816 (2016).
Bremer, A., Thalhammer, A., Wolff, M. & Hincha, D. K. Folding of intrinsically disordered plant LEA proteins is driven by glycerol-induced crowding and the presence of membranes. Febs J. 284, 919–936 (2017).
Bremer, A. et al. Intrinsically disordered stress protein COR15A resides at the membrane surface during dehydration. Biophys J. 113, 572–579 (2017).
Atkinson, J., Clarke, M. W., Warnica, J. M., Boddington, K. F. & Graether, S. P. Structure of an intrinsically disordered stress protein alone and bound to a membrane surface. Biophys J. 111, 480–491 (2016).
Hundertmark, M., Dimova, R., Lengefeld, J., Seckler., R. & Hincha, D. K. The intrinsically disordered late embryogenesis abundant protein LEA18 from Arabidopsis thaliana modulates membrane stability through binding and folding. Biochim. Biophys. Acta 1808, 446–453 (2011).
Popova, A. V., Hundertmark, M., Seckler, R. & Hincha, D. K. Structural transitions in the intrinsically disordered plant dehydration stress protein LEA7 upon drying are modulated by the presence of membranes. Biochim. Biophys. Acta 1808, 1879–1887 (2011).
Sanchez-Ballesta, M. T., Rodrigo, M. J., Lafuente, M. T., Granell, A. & Zacarias, L. Dehydrin from citrus, which confers in vitro dehydration and freezing protection activity, is constitutive and highly expressed in the flavedo of fruit but responsive to cold and water stress in leaves. J. Agric. Food Chem. 52, 1950–1957 (2004).
Goyal, K., Walton, L. J. & Tunnacliffe, A. LEA proteins prevent protein aggregation due to water stress. Biochem. J. 388, 151–157 (2005).
Grelet, J. et al. Identification in pea seed mitochondria of a late-embryogenesis abundant protein able to protect enzymes from drying. Plant Physiol. 137, 157–167 (2005).
Reyes, J. L. et al. Hydrophilins from distant organisms can protect enzymatic activities from water limitation effects in vitro. Plant Cell Environ. 28, 709–718 (2005).
Liu, Y. et al. ZmLEA3, a multifunctional Group 3 protein from maize (Zea mays L.), is involved in biotic and abiotic stresses. Plant Cell Physiol. 54, 944–954 (2013).
Boswell, L., Menze, M. A. & Hand, S. C. Group 3 late embryogenesis abundant proteins from embryos of Artemia franciscana: structural properties and protective abilities during desiccation. Physiol. Biochem. Zool. 87, 640–651 (2014).
Hatanaka, R. et al. An abundant LEA protein in the anhydrobiotic midge, PvLEA4, acts as a molecular shield by limiting growth of aggregation particles. Insect Biochem. Mol. Biol. 11, 1055–1067 (2013).
Furuki, T. & Sakurai, M. Group 3 LEA protein model peptides protect enzymes against desiccation stress. Biochim. Biophys. Acta 1864, 1237–1243 (2016).
Boucher, V. et al. MtPM25 is an atypical hydrophobic late embryogenesis-abundant protein that dissociates cold and desiccation-aggregated proteins. Plant Cell Environ. 33, 418–430 (2010).
Furuki, T. et al. Effects of group 3 LEA protein model peptides on desiccation‐induced protein aggregation. Biochim. Biophys. Acta 1824, 891–897 (2012).
Chakrabortee, S. et al. Hydrophilic protein associated with desiccation tolerance exhibits broad protein stabilization function. Proc. Natl. Acad. Sci. USA 104, 18073–18078 (2007).
Chakrabortee, S. et al. Intrinsically disordered proteins as molecular shields. Mol. Biosyst. 8, 210–219 (2012).
Liu, G., Xu, H., Zhang, L. & Zheng, Y. Fe binding properties of two soybean (Glycine max L.) LEA4 proteins associated with antioxidant activity. Plant Cell Physiol. 52, 994–1002 (2011).
Yu, J.-N., Zhang, J.-S., Shan, L. & Chen, S.-Y. Two new group 3 LEA genes of wheat and their functional analysis in yeast. J. Integr. Plant Biol. 47, 1372–1381 (2005).
Kovacs, D., Kalmar, E., Torok, Z. & Tompa, P. Chaperone activity of ERD10 and ERD14, two disordered stress-related plant proteins. Plant Physiol. 147, 381–390 (2008).
Kyte, J. & Doolittle, R. F. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157, 105–132 (1982).
Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta 1767, 1073–1101 (2007).
Byler, D. M. & Susi, H. Examination of the secondary structure of proteins by deconvolved FTIR spectra. Biopolymers 25, 2507–2511 (1986).
Dong, A., Matsuura, J., Manning, M. C. & Carpenter, J. F. Intermolecular β-sheet results from trifluoroethanol-induced nonnative α-helical structure in β-sheet predominant proteins: infrared and circular dichroism spectroscopic study. Arch. Biochem. Biophys. 355, 275–281 (1998).
Graether, S. P. & Boddington, K. F. Disorder and function: a review of the dehydrin protein family. Front. Plant Sci. 5, 576 (2014).
Hundertmark, M. & Hincha, D. K. LEA (Late Embryogenesis Abundant) proteins and their encoding genes in Arabidopsis thaliana. BMC Genomics 9, 118 (2008).
Uversky, V. N. & Dunker, A. K. Understanding protein non-folding. Biochim. Biophys. Acta 1804, 1231–1264 (2010).
Sun, X. L., Rikkerink, E. H. A., Jones, W. T. & Uversky, V. N. Multifarious roles of intrinsic disorder in proteins illustrate its broad impact on plant biology. Plant Cell 25, 38–55 (2013).
Kentsis, A. & Sosnick, T. R. Trifluoroethanol promotes helix formation by destabilizing backbone exposure: desolvation rather than native hydrogen bonding defines the kinetic pathway of dimeric coiled coil folding. Biochemistry 37, 14613–14622 (1998).
Tolleter, D., Hincha, D. K. & Macherel, D. A mitochondrial late embryogenesis abundant protein stabilizes model membranes in the dry state. Biochim. Biophys. Acta 1798, 1926–1933 (2010).
Moore, D. S., Hansen, R. & Hand, S. C. Liposomes with diverse compositions are protected during desiccation by LEA proteins from Artemia franciscana and trehalose. Biochim. Biophys. Acta 1858, 104–115 (2016).
Rivera-Najera, L. Y. et al. A group 6 late embryogenesis abundant protein from common bean is a disordered protein with extended helical structure and oligomer-forming properties. J. Biol. Chem. 289, 31995–32009 (2014).
Baskakov, I. & Bolen, D. W. Forcing thermodynamically unfolded proteins to fold. J. Biol. Chem. 273, 4831–4834 (1998).
Qu, Y., Bolen, C. L. & Bolen, D. W. Osmolyte-driven contraction of a random coil protein. Proc. Natl. Acad. Sci. USA 95, 9268–9273 (1998).
Cuevas-Velazquez, C. L., Rendon-Luna, D. F. & Covarrubias, A. A. Dissecting the cryoprotection mechanisms for dehydrins. Front. Plant Sci. 5, 583 (2014).
Popova, A. V., Rausch, S., Hundertmark, M., Gibon, Y. & Hincha, D. K. The intrinsically disordered protein LEA7 from Arabidopsis thaliana protects the isolated enzyme lactate dehydrogenase and enzymes in a soluble leaf proteome during freezing and drying. Biochim. Biophys. Acta 1854, 1517–1525 (2015).
Epstein, E. Mineral nutrition of plants: principles and perspectives. John Wiley & Sons, New York, NY. (1972).
Schägger, H. & von Jagow, G. Tricine-sodiumdodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166, 368–379 (1987).
Thalhammer, A., Hundertmark, M., Popova, A. V., Seckler, R. & Hincha, D. K. Interaction of two intrinsically disordered plant stress proteins (COR15A and COR15B) with lipid membranes in the dry state. Biochim. Biophys. Acta 1798, 1812–1820 (2010).
Popova, A. V. & Hincha, D. K. Intermolecular interactions in dry and rehydrated pure and mixed bilayers of phosphatidylcholine and digalactosyldiacylglycerol: a Fourier-transform infrared spectroscopy study. Biophys. J. 85, 1682–1690 (2003).
Hundertmark, M., Popova, A. V., Rausch, S., Seckler, R. & Hincha, D. K. Influence of drying on the secondary structure of intrinsically disordered and globular proteins. Biochem. Biophys. Res. Commun. 417, 122–128 (2012).
Popova, A. V. & Hincha, D. K. Effects of cholesterol on dry bilayers: interactions between phosphatidylcholine unsaturation and glycolipid or free sugar. Biophys. J. 93, 1204–1214 (2007).
Acknowledgements
This study was supported by a grant from the Ministry of Higher Education and Scientific Research of Tunisia and the Max-Planck Society.
Author information
Authors and Affiliations
Contributions
S.K., D.K.H. and F.B. designed the experiments; S.K. performed the experiments with contributions of A.B. to the CD spectroscopy and FTIR measurements; S.K., A.B., D.K.H. and F.B. analyzed the data; S.K., D.K.H. and F.B., wrote the manuscript; all authors read the manuscript, edited, and commented on it before submission.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Koubaa, S., Bremer, A., Hincha, D.K. et al. Structural properties and enzyme stabilization function of the intrinsically disordered LEA_4 protein TdLEA3 from wheat. Sci Rep 9, 3720 (2019). https://doi.org/10.1038/s41598-019-39823-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-39823-w
This article is cited by
-
The thioredoxin h-type TdTrxh2 protein of durum wheat confers abiotic stress tolerance of the transformant Arabidopsis plants through its protective role and the regulation of redox homoeostasis
Protoplasma (2024)
-
Enzyme stabilization and thermotolerance function of the intrinsically disordered LEA2 proteins from date palm
Scientific Reports (2023)
-
The tardigrade protein CAHS D interacts with, but does not retain, water in hydrated and desiccated systems
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.