Abstract
Human-Induced Rapid Environmental Change (HIREC), particularly climate change and habitat conversion, affects species distributions worldwide. Here, we aimed to (i) assess the factors that determine range patterns of European badger (Meles meles) at the southwestern edge of their distribution and (ii) forecast the possible impacts of future climate and landcover changes on those patterns. We surveyed 272 cells of 5 × 5 km, to assess badger presence and confirmed its occurrence in 95 cells (35%). Our models estimate that badger’s presence is promoted by the occurrence of herbaceous fields and shrublands (5%–10%), and low proportions of Eucalyptus plantations (<~15%). Regions with >50% of podzols and eruptive rocks, higher sheep/goat density (>4 ind/km2), an absence of cattle, intermediate precipitation regimes (800–1000 mm/year) and mild mean temperatures (15–16 °C) are also more likely to host badgers. We predict a decrease in favourability of southern areas for hosting badgers under forecasted climate and landcover change scenarios, which may lead to a northwards retraction of the species southern distribution limit, but the overall landscape favourability is predicted to slightly increase. The forecasted retraction may affect community functional integrity, as its role in southern ecological networks will be vacant.
Similar content being viewed by others
Introduction
The conservation of rear-edge populations (i.e. low latitude populations living on species range limits) is crucial for maintaining species evolutionary potential1, but since such populations face different ecological conditions and potentially distinct population dynamics from populations in core distribution areas, conservation measures should be context-specific2. Rear-edge populations are often small and fragmented/isolated (e.g.,3), more vulnerable4, and highly sensitive to climate change2. Climate change has been acknowledged as a key driver of change affecting the phenology, invasiveness and range of a wide variety of species5. However, recent data showed that climate change is not the only determinant of distribution boundaries for many terrestrial species6,7, especially for those inhabiting regions long influenced by human activities, such as Mediterranean landscapes8,9.
Drivers of Human-Induced Rapid Environmental Change (HIREC)—namely farming, pollution, harvesting or the introduction of exotic species10—affect the fundamental relationships between species and ecosystems worldwide, contributing to population declines and range shifts, and ultimately lead to evolutionary changes11,12. Such drivers can have a determinant role in wildlife ecological patterns in Mediterranean landscapes due to the long history of human influence on those landscapes13. At a regional/country scale, topography drives finer-scale discrepancies in climate and landscape composition14, which influences demographic patterns and ecological processes (e.g. survival, competition) that can be especially important at species’ distribution edges. Thus, in a changing world it is increasingly important to understand how species distributions change in response to different types of HIREC factors. This is particularly important in biodiversity hotspots where the scenarios of climate change and socio-economic modifications of the landscapes are especially acute, such as in the Mediterranean15,16. Decreased rainfall and higher temperatures are forecasted for this region, with resulting higher levels of aridity17,18. Together with land abandonment and woodland encroachment onto grasslands18 that impose landcover changes throughout the region, climate change will create new challenges for wildlife inhabiting the Mediterranean that need to be anticipated19.
Iberian populations of the European badger (Meles meles) constitute a good model for assessing the combined effects of different types of HIREC on edge populations in changing landscapes, since Iberia is currently affected by multifactorial global change18 and represents the southwestern extent of the species range20. Badger demography and foraging patterns are strongly linked to climate- and agro-ecosystem-induced variations in food availability21,22. Climate change and extreme weather events can induce seasonal food shortages that influence reproductive success (e.g. cub survival22).
At the core of the European badger’s range, its distribution is mostly constrained by disturbance factors (e.g. Netherlands23), such as human population density at lower altitudes and lack of vegetation cover at higher grounds in Moravia (Czech Republic) (e.g.24), while in England higher altitudes and afforested areas, especially shrub heath and heather moorland restrict its distribution (e.g.,25). However, in its northwestern range (Scotland), badger occurrence is mostly affected by an interaction between climatic and topographic factors (minimum winter temperature) that influences the type of land use (e.g. presence of agricultural patches), as well as by human disturbance (e.g. distance to settlements26). In the northernmost part of its range (Finland), badger distribution is limited by climatic conditions that restrict food availability and, consequently, cub survival27, as well as by steep topography28. Conversely, previous studies suggest that badger distribution in its southwestern limit is constrained by dry and closed landscapes, often linked to low food availability in such areas (e.g. earthworms29), and availability of suitable sites for setts30,31. Western Mediterranean regions are considered poor badger habitats due to spatio-temporal inconstancies in environmental conditions (especially in drier areas) and resource availability29,32, which will be exacerbated by the forecasted increased aridity for this region33. This variability in environmental conditions, together with the predicted changes in climate and landcover18, raise the need to assess how the rear-edge badger population inhabiting the most southwestern corner of its range may be distributed in future decades. This is critical information to understand badger resilience, guide possible management actions to maintain population connectivity, and ultimately comprehend how ecological processes may be affected in regions where this predator’s distribution may shift.
Based on the variety of factors already recognized as crucial in determining badger distributions throughout its range (see above), we aimed to evaluate which type of HIREC factors might be the most influential drivers determining badger distribution in its southwestern range. Our results allowed us to hypothesize how future climate and/or landcover changes envisaged for this region may impact population range. Using a Species Distribution Model approach (SDM34), we first tested five hypotheses regarding drivers of badger distribution in Portugal, i.e. if its range is determined by: (H1) Landcover composition (e.g. afforested environments and highly human-modified landcover have a negative effect25,35); (H2) Anthropogenic disturbance (i.e. a high prevalence of human-associated disturbance factors constrain badger distribution23,24); (H3) Topography and geology (i.e. higher altitudes associated with colder and less vegetated areas, as well as harder rocks making it more difficult to dig setts, limit badger range25,36); and (H4) Climate (i.e. climate variations restrict food availability and, therefore, badger distribution27,29). Due to the spatial and temporal environmental complexity and heterogeneity of the Mediterranean biogeographical region13, which encompasses most of the Portuguese mainland, badger distribution patterns might not be determined by a unifactorial mechanism but by cumulative effects of diverse factors. Therefore, we also considered a fifth hypothesis (H5) representing the combined effects of landcover, anthropogenic disturbance, and abiotic and climatic factors. Finally, we identified the most favorable regions for badgers in Portugal and predicted the evolution of the badger’s range in the country based on the factors we identified as most influential in determining its current distribution.
Results
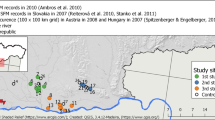
We surveyed a total of 1315 transects, representing ca. 657.5 km of walked trails, in 272 cells of 5 × 5 km (Fig. 1). Signs of badgers were detected in 95 cells of 5 × 5 km (Fig. S1 - Supplementary Material), which exhibited significant spatial autocorrelation (Moran I = 0.057, p < 0.01). We only detected collinearity in one ecogeographical variable included in the topography and geology hypothesis (Tables S1–S4 - Supplementary Material), which was excluded from subsequent analyses: Sediment (Table S3 - Supplementary Material).
The four RAC-BRT (Residuals AutoCovariate Boosted Regression Trees models) models produced for the H1–H4 hypotheses showed that the percentage cover of herbaceous vegetation, shrubland and Eucalyptus (H1); the density of goats and sheep and cattle (H2); the percentage of soils dominated by podzols and eruptive rocks (H3); and the annual mean temperature and annual precipitation (H4), were the most influential variables in each hypothesis and the only ones included in the first quarter of relative influence per hypothesis (Table 1). For this reason, we used them as candidate variables for the hybrid hypothesis (H5). Comparing the best BRT models for the first four hypotheses (H1–H4), the one based on landcover composition (H1) was the best fitting, reaching AUC values of 0.853 (SE = 0.018) and a percentage of explained deviance of 52.55% (Table 1).
We found no multicollinearity between the candidate variables for the hybrid hypothesis (H5) RAC-BRT model, so all of them were used in the modelling procedure (Table S5 - Supplementary Material). The generated model presented a better fit (AUC = 0.858; 44.36% explained deviance) than all those from the previous four hypotheses, indicating that hypothesis H5 was the most supported by our dataset. The differences in AUC values between the H5 model and the other best models produced for hypotheses H1–H4 are not high, but they indicate that the hypotheses are not completely mutually exclusive as H1–H4 included variables also present in the H5 best model. This H5 model highlighted that the percentages of podzol soils and herbaceous landcover were the most influential variables (8.53% and 8.08%, respectively; Fig. 2), with a difference of importance between both of only 5.28% (i.e. a difference of 0.45% represents 5.28% of 8.53%; Fig. 2). The difference between the relative importance of these two variables and other variables is not high, but the difference in percentage of importance reaches ~9% between the second and third most important variables (7.4%, 0.70% difference relative to % importance of podzols; Fig. 2). Furthermore, although the RAC autocovariate has a high relative importance (45.06%), the RAC-BRT model still allows for the environmental and anthropogenic disturbance variables to contribute to deviance reduction of the model while removing the model residuals responsible for spatial autocorrelation37. The RAC-BRT model residuals confirm this result, as no significant spatial autocorrelation was detected in the model residuals (Moran I = −0.017, p = 0.067).
Partial dependence of badger presence on each variable (Y-axis – Model fitted values; X-axis – variables values variation; Herbaceous, Eucalyptus and Shrubland – Percentage of area; Cattle and Goats&sheep - ind./km2; Ann_Prec – mm; Podzols and Eruptive - % of area; Ann_Temp - °C); Values within parenthesis represent variable´s relative importance in the final model). Badger predicted distribution is mostly determined by: (1) a low proportion of herbaceous fields, shrublands and Eucalyptus cover; (2) high proportions of podzols in the soil structure and eruptive rocks; (3) higher sheep/goat density but lower density/absence of cattle; as well as (4) intermediate rain regimes and mild annual mean temperatures.
The variables included in the best model showed distinct patterns regarding their relationships with badger presence (Fig. 2). Badgers have a higher probability of being present: (1) in areas with herbaceous fields and shrublands, but covering less than 20% and 15% of the landscape, respectively (ideally between 5 and 10% for both landcover types), and where Eucalyptus plantations represent <15% of the landscape; (2) in regions with more than 50% of podzols in the soil structure and at least 50% of eruptive rocks; (3) where sheep/goat density is above 4 ind/km2, but cattle are absent or exist in low densities (<0.5 ind/km2); and (4) in areas subjected to annual precipitation of between 800 and 1000 mm, and mild annual mean temperatures of between 15–16 °C (Fig. 2). Badgers avoid areas almost without goats and sheep (<2 ind/km2) (Fig. 2).
Based on our hybrid model we estimated the probability of badger presence to be high in central Portugal, particularly in the Tejo River basin (a floodplain influenced by this major river) and near the central coast of the country (Fig. 3a). The favourability map confirms this pattern, but also highlighted the importance of many inland regions near the Spanish border (Fig. 3b).
The independent badger presence data (Fig. S2, Supplementary Material) confirmed species presence in 68 of our 5 × 5 km cells. Of those, 44% (N = 30) were identified in our favourability map as moderately or very suitable to host badgers (i.e. F > 0.50). Furthermore, this percentage increased if we considered cells whose favourability almost reached the threshold of F > 0.50; 11 cells had F values in the range 0.45–0.50 (which when included enhanced detection of favourable areas to 60%). The percentage of cells containing independent badger presence data, which were identified by the best model as moderately or very suitable to host badgers (F > 0.45), was similar between areas where monitoring was more representative (Central north; 60.78% of the test cells; Fig. 1) and where sampling was less intensive (Central south; 58.82% of the test cells; Fig. 1).
We produced favourability maps of badger presence in Portugal for 2040 based on four land-use change scenarios described by Stürck and colleagues38 and an IPCC climate prediction (Scenario A1B17) (Fig. 4). All scenarios presented a similar output. By 2040, badger landscape favourability seems to increase in the country’s eastern and northeastern regions, but decreases in the North-west. Furthermore, the southern edge of the species range seems to decrease in adequacy for badgers, with a decrease of 50% in the number of cells with favourability >0.75 in area south of the Tejo River (from 35 – current situation - to 16, 15, 17 and 18 for the Libertarian Europe - A1_2040, Eurosceptic Europe - A2_2040, Social Democracy Europe - B1_2040 and European Localism - B2_2040 scenarios, respectively). The central region, which formed a core area of the most adequate territory for the species, loses some regions characterized as highly favourable (red cells), and coverage of cells representing the least suitable areas (lighter orange cells) expands in southern Portugal (evidenced by a slight reduction in this area average favourability, from 0.484 – current situation - to 0.474, 0.473, 0.470 and 0.472 for the A1_2040, A2_2040, B1_2040 and B2_2040 scenarios, respectively). Comparing the overall favourability of the country according to the four forecasted scenarios, we observed a non-significant increase (mean increase: 2.75%; χ2 = 1.392, p = 0.707) from the current situation (22.73% of the country showing a favourability >0.5) to favourability percentages of 24.71% (A1_2040), 25.17% (A2_2040), 25.86% (B1_2040) and 26.19% (B2_2040). Thus, the increase in favourability in northwestern areas compensates for the overall decrease in southern regions (Fig. 4)
Favourability maps of European badger presence in Portugal estimated for 2040, by applying the best BRT model to different land-use change scenarios, all showing a suitability decrease of the southern edge and an increase in the northeast [Libertarian Europe - A1_2040; Eurosceptic Europe - A2_2040; Social Democracy Europe - B1_2040; European Localism - B2_2040; see38 for scenario details] and scenario A1B from the Intergovernmental Panel on Climate Change (IPCC) Special Report on Emission Scenarios (SRES17).
Discussion
The estimated current distribution (i.e. range predictability and favourability) of the European badger at the limit of its southwestern range (Portugal) is widespread but patchy, and is mostly determined by a combination of landcover, environmental and anthropogenic disturbance drivers. These drivers are affected by different aspects of HIREC acting synergistically to shape the species’ distribution pattern. Badgers seem to be sensitive to changes in native (herbaceous fields and shrublands) and exotic (Eucalyptus plantations) vegetation cover, but also to soil and rock composition (preference for podzols and eruptive rocks), the intensity of pastoral activities (i.e. density of sheep/goats and cattle), and climatic conditions (i.e. temperature and precipitation). This combination of multiple drivers supports our fifth hypothesis (H5).
Badger distribution
We found that central Portugal is regionally the most favourable area for badgers, with two core critical areas, broadly corresponding to the Tejo River basin and the western coastal plains. Part of the eastern region bordering Spain also has characteristics that favour badger presence (Fig. 3b). This pattern may represent the historical distribution of the species at the southwestern edge of its range. In a study based on badger-associated toponomy (i.e. regional designations or place-names, indicating species presence in the past), Rosalino and colleagues39 identified place-names associated with badgers ranging from the south coastal region of Algarve to the northern borders of Portugal, and from the western coast to the eastern regions, near the Spanish border, suggesting that the species was also historically widespread. The patchy favourability for badgers in Portugal reveals that adequate areas for badger survival are discontinuously distributed (Fig. 3b).
Landscape drivers of badger distribution
Two major habitat factors shape badger distribution in Portugal: landcover and soil/rock structure. Our data indicates that areas showing some heterogeneity are preferred (as also found by Piza-Roca and colleagues40). Herbaceous fields and shrublands presented a positive influence on badger presence, up to a threshold of 20% and 15% coverage of the landscape, respectively, with an optimal coverage of between 5 and 10%. Heterogeneous environments can have deleterious effects on some populations, leading to decline or even extinction (e.g. prey species41), whereas others can take advantage of the multiple resources they provide. Badgers can benefit from heterogeneous habitats, using the combined resources (food and refuge) provided by such temporal and spatial heterogeneity, especially southern populations (e.g. in semi-arid environments30,42; in Mediterranean oak forests43). Herbaceous fields can provide easy access to food resources, such as insects (naturally available or derived from use of these patches by domestic ungulates, e.g. dung beetles44), earthworms (which are mainly concentrated in herbaceous areas45,46), or rodents and rabbits47,48. However, badgers are more exposed to humans in such open areas, which are characterized by having less than 20% of cover. Shrublands provide protective cover43, but since badgers can be considered more efficient food gatherers than active predators (but see47), higher shrub cover makes prey detection harder. Furthermore, high shrub cover is often associated with low abundance of some badger prey, such as earthworms49 and rabbits50, which may contribute to avoidance of those areas. Therefore, a compromise between protective cover and foraging habitat may have resulted in the selection of areas with low shrubland cover by badgers in Portugal.
Eucalyptus plantations cover ca. 9% of the Portuguese territory and represent 26% of all Portuguese forested areas51. They have been shown to have a negative effect on southern badger populations35,43,52. The effects of Eucalyptus plantations on wildlife are often associated with food scarcity and high disturbance35. However, these habitats can harbour abundant populations of prey if understory shrubs are managed properly (e.g.53,54) to provide efficient protective cover, especially in the absence of native forests55. Furthermore, their degrees of disturbance vary with harvesting phase (i.e. higher in pre-harvesting phases56) and plantation extent35. Our results support a positive effect of scarce Eucalyptus habitats (<~15% of the landscape), which likely deliver a combination of food resource availability, protective cover, and reduced anthropogenic disturbance.
We found that not only above-ground habitat characteristics drive badger presence in Portugal, since soil and rock types also emerged as being influential in our analysis. Podzols are often formed under forest ecosystems and are usually considered poor soils for agriculture as they possess low levels of moisture and lack many nutrients57,58. Although use of agricultural landscapes by badgers may be a mechanism to facilitate access to food (e.g.,30,43), agricultural fields suffer frequent soil mobilization to prepare the land for planting or when harvesting production and they are subjected to high human disturbance. This high disturbance level likely limits badger presence, with this latter being more common in areas not suitable for agriculture and where soils are covered by forests, such as those dominated by podzols (>50% of podzols in the soil structure, as detected by our analysis). Often these more “natural” habitat patches are composed of a mixture of tree cover in different successional stages (from forest with sparse understory or shrublands with sparse tree cover to more closed environments), which may provide the necessary cover/protection badgers need. This heterogeneous structure may not have been completely captured by our landcover classes (often composed of monotypic landcover types), preventing a more accurate assessment of their importance in our models. Setts are essential structures for badgers and their stability is a determining factor for population survival59. Eruptive rocks can provide such stability, although they also present badgers with a huge challenge when digging a sett. Under such conditions, geological discontinuities may facilitate sett-building31. However, no data was available at a national scale to allow us to test this hypothesis.
Apart from Eucalyptus plantations, other anthropogenic factors contribute to shaping badger distribution in Portugal. Our results suggest that badgers avoid areas with higher cattle density (>0.5 ind/km2), but high densities of sheep/goats (>4 ind/km2) promote their presence. Although there is some previous data showing that badgers use agroforestry systems devoted to cattle-raising in Iberia (usually in low density regimes60), badgers are also known to avoid cattle in many other regions61,62 due to disturbance. In Portugal, sheep and goats are mostly raised in flocks that move around the landscape, perhaps promoting higher concentrations of dung that increase dung beetle availability, a badger prey48. Finally, these livestock may also control shrub coverage63, which may prevent woodland encroachment onto badger-preferred herbaceous habitat.
Climatic drivers of badger distribution
Our data showed that climate is an important driver shaping the distribution of rear-edge badger populations. Badgers have a higher probability of using areas with mild climatic characteristics, namely with intermediate precipitation regimes (between 800 and 1000 mm rainfall annually), and mild annual mean temperatures (between 15–16 °C) (Fig. 2).
There are two critical periods for badger survival during their lifetimes, i.e. the first months of life for cubs and over-winter survival for all age classes22,64. The first period is mostly affected by extended summer drought, whereas the second is predominantly dependent on winter frost, low temperatures and heavy rainfall leading to floods. In northwestern badger populations, mild winters enhance over-winter survival (e.g.,64), as animals (especially juveniles) have a better likelihood of maintaining their body weight and energy reserves. In the UK, Noonan and colleagues65 found that badgers reduce their activity (and probably foraging bout length and frequency) when temperatures are lower, jeopardizing their efficiency in accumulating over-winter reserves. Other mesocarnivores and small carnivore species, such as raccoon dogs (Nyctereutes procyonoides) or least weasel (Mustela nivalis), exhibit similar strategies to cope with critical winter temperatures, due to the high costs of thermoregulation during activity66,67. Winter temperatures often drop below 0 °C in many regions of Portugal, perhaps inducing southwestern badger populations to adopt a strategy similar to that of their conspecifics at higher latitudes and reduce their over-winter activity. The biological costs of employing this behavioural strategy are: (i) reduced survival68 or (ii) avoidance of areas with particularly harsh winters, i.e. those with lower annual temperatures. Both consequences would result in the same spatial pattern. Our results suggest that lower temperatures are more constraining for Portuguese badger populations than higher ones. Temperatures in Portugal can exceed 35 °C, contributing to mean annual temperatures around 15–16 °C, which we identified as promoting badger presence. Thus, it is possible that badger populations inhabiting this region may have developed local adaptations that allow them to benefit from more temperate conditions. The mechanistic basis for this spatial pattern may also be linked to food resource availability. Milder climates can influence the availability of two of the foods most consumed by badgers in Portugal, i.e. coleopterans and olives48. Mild temperatures may increase winter coleopteran survival (e.g.69), thereby promoting higher species abundances. Furthermore, the reproductive structures and fruits of olive trees are sensitive to low temperatures, particularly frost70, so they present higher productivity in areas less affected by frost.
At this edge of the species distribution, the amount of rain may also be a more important driver of badger presence. Portugal is mostly characterized by a Mediterranean climate where rain mainly falls in winter and cyclic droughts occur71, with annual precipitation ranging from <400 mm in southeastern regions to >3000 mm in northwestern ones71. Badgers seem to prefer areas with mild rainy conditions (between 800 and 1000 mm of annual precipitation), likely due to the consequently higher availability of some feeding resources (e.g.72). Intermediate levels of rainfall mean greater likelihood of avoiding food shortages associated with drought or arid environments46,73. Nouvellet and colleagues68 showed that cub and juvenile survival were highest under conditions of intermediate levels of rainfall, but adult survival was mostly affected by the driest years, probably due to a decrease in food availability and quality in dry years64,74. However, excessive rainfall can affect thermoregulation during winter and early spring, especially when associated with lower temperatures, and it may constrain cub survival during the critical first months outside the sett68. Hypothermic stress associated with wetter conditions can debilitate a cub’s immune system, often promoting endoparasitic infections22 that may compromise cub survival and recruitment22,68. Moreover, depending on the soil and geological structure of the area, high rainfall can also compromise sett stability (e.g. in sandy soils) and habitability (e.g. flooding when sited in valleys or in clay-dominated soils75,76). Setts are crucial structures for central-place foragers such as badgers, where animals rest during the day, interact to maintain social cohesion, and where cubs are born and reared77,78. Poor sett-building conditions affect species density79, especially in Mediterranean areas where sett sites are the main limiting factor constraining distribution31.
Future impact of climate and landcover change on badger distribution
Badgers are central place foragers, whose ecology is intrinsically linked to sett locations78. They do not exhibit migratory movements that can lead to changes in their range limits, and so are more vulnerable to climate change21. However, badger populations at the northern limit of the species range might benefit from climate change that creates conditions for badgers to colonize environments historically inaccessible to them due to extreme weather (e.g. longer snow-free periods with consequently higher food availability27,28). However, at its southern rear-edge range, badgers might experience the inverse pattern due to the combined effects of HIREC factors. As mentioned before, climate change scenarios for Portugal estimate a generalized reduction in annual mean precipitation (by 10% up to 204017) and an increase in temperature (~1.5 °C17). This forecasted climate change, together with the predicted changes in landscape composition38, will decrease the favourability of areas of southern Portugal for badgers, potentially leading to a range retraction northwards. This retraction will likely be matched by an increase in favourability for northeastern Portugal, where badgers will find better conditions to survive (Fig. 4). Although we forecast a contraction of the badger’s southern range limit in Portugal, we estimate no overall difference in the percentage of areas in the country with higher favourability. Thus, loss of favourable areas in the south will be compensated for by more beneficial environmental conditions in the North-east.
These forecasted changes in distribution for the species in its southwestern rear-edge range can probably occur throughout the badger’s southern distribution range (i.e. the Mediterranean), since the climate change predicted for Portugal is similar to that foreseen for the entire Mediterranean region17. Moreover, landscape changes seem likely to follow the same patterns across the region38. Nevertheless, further broad-range studies targeting the drivers of distribution of central and eastern Mediterranean badger populations should be prioritized, especially to confirm if the drivers we identified for Portuguese populations have a broader effect and to assess how badger distributions will evolve in those areas. Information covering the entire southern range limit will allow us to understand the ecological strategies badger populations need to adopt to survive in the environmentally challenging landscape of Mediterranean Europe and provide data that can make conservation strategies more effective.
Methods
Study area and sampling design
To avoid the limitations associated with studies of local or even regional extent (i.e. calibration of models with limited variation of environmental and anthropogenic disturbance factors affecting model performance and predictability robustness for wider extents14), we extended our analysis across all mainland Portugal. This is the first study to evaluate badger distribution and the factors determining it at a nationwide scale in the Mediterranean region. Furthermore, our study encompasses the high bioclimatic variability characteristic of Western Iberia, where Atlantic and Mediterranean biogeographical regions interconnect80, as well as high variation in landcover, topography and disturbance conditions.
Western Iberia is characterized by a wide variety of climatic conditions typical of the two distinct bioclimatic regions covering the area: Atlantic (Cantabroatlantic sub-region) and Mediterranean (Sado-Divisorian, Luso-Extremaduran and Carpetano-Leonese sub-regions)80. Within the Mediterranean region, the climate is usually hot and dry in summer and humid cool in winter. Heavy rain occurs often, and summer droughts are common and sometimes prolonged81. In the Atlantic region, the climate is typically oceanic, with mild temperatures and high precipitation and humidity82 (see Table 2 for details). Landcover is diverse, associated with the altitudinal variation (0–1993 m), and includes deciduous (e.g. Quercus spp.) and conifer (e.g. Pinus spp.) forests and exotic plantations (e.g. Eucalyptus sp.), scrublands, natural pastures and agricultural patches (e.g. orchards, olive groves, vineyards, agroforestry, etc.16; see Table 2 for details). Mean population density for Portugal is 111.8 inhabitants/km2 (2016 data83), and the country has a fair coverage of highways (totaling 3,065 km) and 2-lane national, regional and municipal paved roads (totalling 14,313 km) (2016 data; www.pordata.pt/; see Table 2 for details). Cattle, sheep and goats are raised extensively in many regions, reaching average densities of 12.8, 24.0 and 4.4 ind./km2, respectively (National Statistics Institute, https://www.ine.pt/; see Table 2 for details).
We divided mainland Portugal (89,060 km2) into 987 cells of 10 × 10 km, using the UTM reference system, fuse 29, on WGS84 datum (EPSG code: 32629) (Fig. 1). We then selected 180 regularly distributed cells, using the chess knight movement pattern (i.e. L-shaped in any direction), starting at the northwest corner of the country (Fig. 1). Cells located in the L tips were selected for badger sampling and were subdivided into four 5 × 5 km cells, of which we randomly selected two and defined five 500 m line transects/itineraries in each. Transects were not set in areas where badger presence is highly unlikely (e.g. rivers, dams, inside estuaries, beaches) or where much of the landscape is humanized (e.g. villages, industrial compounds). The spatial allocation of sampling transects was defined to proportionally represent, as much as possible, the landscape composition of each 5 × 5 km sampled cell based on landcover characteristics. They were defined manually over a landcover map, within a Geographical Information System, and we tried to correlate the transect length within a specific landcover unit with the approximate proportion of that unit within the sampled cell.
Survey of badger presence
We surveyed all transects located in the 272 5 × 5 km cells (located in 136 10 × 10 km cells) between June 2014 and January 2017 to detect signs of badger presence. However, 38 of the 10 × 10 km cells, located in the southeastern part of the country, could not be sampled due to logistical limitations (i.e. we could sample ca. 78% of the pre-selected cells; Fig. 1). The remaining six 10 × 10 km cells were not sampled because they encompassed >75% of its area covered by sea, dams or were located within Spanish territory. Badger presence in each transect was confirmed based on signs of species presence, such as footprints, latrines, setts or fur samples found, for example, on barbed wire. Although expert-based identification of carnivore scats is prone to false positive and false negative errors84,85, badger scent-marking behaviour minimizes this bias and makes sign identification highly accurate as scats are mostly deposited in ground pits called latrines78. Badger setts—underground dens where badgers rest during the day and where their cubs are born and reared59—were identified based on the existence of other signs of badger presence in their vicinity and burrow size and structure59. When badger presence was confirmed in any transect, the corresponding 5 × 5 km cell was classified as positive.
Compilation of environmental and anthropogenic disturbance data
Each 5 × 5 km cell was characterized regarding its environmental and anthropogenic disturbance features, mostly based on remote sensing data. We first built a Geographical Information System (GIS) using several software tools (ArcGIS 10.4.186; Quantum GIS 2.14.987) and incorporating the following digital layers: landcover, road and highway network, human density, protected areas and hunting reserves, domestic ungulate densities, topography, soil types, lithology, and climatic data (Table 2).
Data analysis
We first assessed the spatial autocorrelation of badger presence data in the 5 × 5 km cells and model residuals using the Moran I index88, available in the R package “ape”89, to prevent poor inference and enhance the predictive ability of the models90. We then tested for data multicollinearity between all co-variates using the Variance Inflation Factor (VIF), to identify candidate variables that are collinear91. As there is no VIF factual cut off level, we used the values suggested by Zuur and colleagues92, and excluded all variables with VIF > 5. The VIF was recalculated for the remaining variables. The process was repeated until none of the retained variables reached VIF > 5.
We tested the factors potentially shaping badger distribution in Portugal using a Boosted Regression Trees (BRT) approach for each hypothesis (H1–H5, see Introduction). BRT combines decision trees and boosting93,94. It is based on the average of many prediction rules, achieved in a forward stage-wise procedure (a kind of additive regression model, wherein individual trees act as individual terms95), instead of a single-rule prediction. The BRT procedure starts with a regression tree that focuses on minimizing the loss function. Then a new regression tree, containing (or not) variables and nodes distinct from the first tree, is fitted to the prediction residuals of the first tree. At this stage the model comprises two trees, and its residuals are estimated. The overall BRT model is a linear combination of all the generated trees94, so global fit is improved by encompassing the predictions of previous trees (weak learners) and focusing on observations incorrectly classified by those trees93. We selected this approach because BRT is insensitive to outliers, can fit nonlinear relationships, allows use of different types of variables (e.g. continuous and binary), and automatically models interactions between predictors94,95.
Following the recommendations of Elith and colleagues94 and Elith and Leathwick96, we used a 10-fold cross-validation procedure and selected the largest learning rate (lr) and the smallest tree complexity (tc) to enable us to achieve a minimum of 1000 trees in the BRT fitting process (see Elith and colleagues94 for more details regarding the BRT fitting procedure and lr and tc). When fitting the consecutive trees, non-informative variables were removed (i.e. the least important variables were excluded and the model was re-fitted in a process we repeated sequentially until no change was achieved in either the % deviance explained or Area Under the Curve - AUC; see below), leading to simplification of the set of variables94. The relative contributions of predictors (% importance - the frequency that a variable is selected in the BRT fitting procedure, scaled to sum 100) were calculated, and partial dependence plots were produced for the most important predictors, showing their effects on probability of badger occurrence after accounting for the average effects of other variables. BRT models were fitted using the R-package ‘gbm’97.
Since we detected significant spatial autocorrelation among residuals (see Results), we adopted the methodology of Crase and collaborators37 to incorporate this spatial structure in our BRT to minimize its effects. We added an autocovariate term into the BRT that already contained the environmental and anthropogenic disturbance variables, which accounted for the influence of neighbouring observations by specifying the relationship between the value of a cell and those located in its vicinity, thereby representing the spatial autocorrelation in the residuals37. We produced residuals autocovariate (RAC) models for each hypothesis, incorporating into BRT an autocovariate estimated from the residuals of BRTs produced using only the variables described in Table 2. The BRT procedure was the same as that described above and the RAC was estimated using the R package “raster”98.
To test the first four of our pre-defined hypotheses (H1–H4), we produced residuals autocovariate BRT models (RAC-BRT) for each hypothesis by combining all the non-correlated variables related to that hypothesis (see Table 2). We then identified the variables representing the 50% more influential predictors in each hypothesis (H1–H4, excluding the autocorrelation correction factor), based on the variables relative influence (i.e. a percentage representing the average number of times a variable is used to define a split of a tree branch, weighted by the improvement of the model fit due to that split99). These variables were used to produce a hybrid hypothesis (H5), which was tested using the same methodological approach. Selection of the best hypothesis explaining the pattern of badger distribution was based on the Area Under the Curve (AUC, derived from the Receiver Operating Characteristic curve, or ROC) of the ensemble RAC-BRT models of each hypothesis93. The best model structural fit (i.e. % deviance explained) was also estimated.
Based on our modelling results, we first implemented a model-based interpolation process (i.e. a prediction of species presence in new cells that present a similar range of environmental characteristics to those of the sampled cells within the same time-period evaluated34) to estimate badger distribution in the species southwestern range limit (i.e. Portugal). As RAC was introduced into the BRT models as a variables, we needed to assign RAC values to those 5 × 5 km cells not sampled to produce a predictability map. To do this, we assigned a residual mean value for each non-sampled cell to generate a predictability estimate. Badger presence predictability for mainland Portugal was estimated using the “predict.gbm” function available in the R package “gbm”97, which allows estimating predicted values from generalized boosted models. A raster layer was produced based on those predicted values and using the “raster”98 package, which was exported to a Geographical Information System (Quantum GIS 2.14.987), where we created a predictability map.
Although producing a predictability map is important to understand species distribution and to define effective conservation plans, in regions were species presence may be more scattered or irregular (due to temporal variation of resource availability), as at the limits of a species’ distribution, predictability may be less informative. In contrast, favourability, defined by Real and colleagues100 as the “variation in the probability of occurrence of an event in certain conditions with respect to the overall prevalence of the event”, may be a more adequate index to assess which regions might be more adequate to support a badger population given the regional context of the species’ distribution. Since a favourability index of 0.5 indicates that cells/conditions have a probability of harbouring badgers equivalent to the overall presence of badgers in the entire dataset, the use of the favourability function allowed us to discriminate between cells that favour badger presence (F ≥ 0.50) and those that possess deleterious characteristics for badgers (F < 0.5)101. Thus, favourability is an important index in conservation biology and particularly for identifying routes of expansion or retraction. Favourability was estimated as described by Real and colleagues100 and Acevedo and Real101, based on the predictability results and the number of presences (n1) and number of absences (n0).
We validated the predicted performance of the favourability map by matching it with previously recorded badger presence data [obtained non-systematically from other published or unpublished studies; mostly roadkills (e.g.,102), captures (e.g.,43) and camera-trapping (e.g.,103) data; Fig. S2, Supplementary Material], and estimated the percentage of false negatives, i.e. cells with a favourability less than 0.50 but where badger presence was confirmed. All statistical analysis were implement using R software104.
Finally, we forecasted the evolution of badger distribution in Portugal up to 2040. Based on our model results, we predicted the favourability of a territory to harbour badgers based on variables with the potential for change: landcover and climate (see Results). Soil and rock types were assumed to stay unchanged. We also opted to keep constant the densities of domestic ungulates because there are no available national predictions on how these variables may evolve up to 2040.
Land-use change scenarios for 2040 were based on predictions and data provided by Stürck and colleagues (38; http://labs.kh.hercules-landscapes.eu/labs/themeLD.html). These authors developed four scenarios [Libertarian Europe - A1; Eurosceptic Europe - A2; Social Democracy Europe - B1; European Localism - B2; see38 for a detailed description of the scenarios]. As the landcover categories used in our study varied slightly from those described by Stürck and colleagues38, we grouped categories of their study that could be assigned according to those identified in our study as influential: Shrublands (semi-natural vegetation, recently abandoned arable land, and Heatland and moorlands38); Herbaceous (Pasture and recently abandoned pasture land38). Eucalyptus cover was assumed to stay constant since current Portuguese legislation prohibits an increase of these exotic plantations and wood/paper production is likely to continue.
Climate data predictions (i.e. annual precipitation and annual mean temperature; see Results) were obtained from the Intergovernmental Panel on Climate Change (IPCC) climatic dataset17, based on scenario A1B from the IPCC Special Report on Emission Scenarios (SRES17), which assumes a global economy with a balanced use of energy systems (fossil and non-fossil105). Climate change scenarios for Portugal estimate that in 2040 there will be a generalized reduction in annual mean precipitation (an average of 10% for the entire country17). Inversely, temperatures are expected to rise, with most of the country showing an average increase of 1.5 °C17.
Based on these predicted scenarios, we estimated the values of the variables included in the best model for every 10 × 10 km grid cell in 2040 and created a favourability map based on the assessed model parameters, using the same methodology as detailed above.
Data Availability
All badger’s presence data will be available at the “Atlas of Portuguese mammals” website database: http://atlas-mamiferos.uevora.pt/index.php/downloads/.
References
Provan, J. & Maggs, C. A. Unique genetic variation at a species’ rear edge is under threat from global climate change. Proc. Roy. Soc. Lond. B Biol. Sci. 279, 39–47, https://doi.org/10.1098/rspb.2011.0536 (2012).
Hampe, A. & Petit, R. J. Conserving biodiversity under climate change: the rear edge matters. Ecol. Lett. 8(5), 461–467, https://doi.org/10.1111/j.1461-0248.2005.00739.x (2005).
Pearson, G. A., Lago-Leston, A. & Mota, C. Frayed at the edges: Selective pressure and adaptive response to abiotic stressors are mismatched in low diversity edge populations. J. Ecol. 97, 450–462, https://doi.org/10.1111/j.1365-2745.2009.01481.x (2009).
Yackulic, C. B., Sanderson, E. W. & Uriarte, M. Anthropogenic and environmental drivers of modern range loss in large mammals. PNAS 108, 4024–4029, https://doi.org/10.1073/pnas.1015097108 (2011).
Walther, G. R. et al. Ecological responses to recent climate change. Nature 416, 389–395, https://doi.org/10.1038/416389a (2002).
Thomas, C. D. Climate, climate change and range boundaries. Divers. Distrib. 16, 488–495, https://doi.org/10.1111/j.1472-4642.2010.00642.x (2010).
Tucker, M. A. et al. Moving in the Anthropocene: Global reductions in terrestrial mammalian movements. Science 359, 466–469, https://doi.org/10.1126/science.aam9712 (2018).
Doblas-Miranda, E. et al. A review of the combination among global change factors in forests, shrublands and pastures of the Mediterranean region: Beyond drought effects. Global Planet. Change 148, 42–54, https://doi.org/10.1016/j.gloplacha.2016.11.012 (2017).
Rehm, E. M., Olivas, P., Stroud, J. & Feeley, K. J. Losing your edge: climate change and the conservation value of range-edge populations. Ecol. Evol. 5, 4315–4326, https://doi.org/10.1002/ece3.1645 (2015).
Sih, A. Understanding variation in behavioural responses to human-induced rapid environmental change: a conceptual overview. Anim. Behav. 85, 1077–1088, https://doi.org/10.1016/j.anbehav.2013.02.017 (2013).
Sih, A., Ferrari, M. C. O. & Harris, D. J. Evolution and behavioural responses to human-induced rapid environmental change. Evol. Appl. 4, 367–387, https://doi.org/10.1111/j.1752-4571.2010.00166.x (2011).
Tuomainen, U. & Candolin, U. Behavioural responses to human-induced environmental change. Biol. Rev. 86, 640–657, https://doi.org/10.1111/j.1469-185X.2010.00164.x (2011).
Blondel, J. The “design” of Mediterranean landscapes: a millennial story of humans and ecological systems during the historic period. Hum. Ecol. 34, 713–729, https://doi.org/10.1007/s10745-006-9030-4 (2006).
Vale, C. G., Tarroso, P. & Brito, J. C. Predicting species distribution at range margins: testing the effects of study area extent, resolution and threshold selection in the Sahara-Sahel transition zone. Divers. Distrib. 20, 20–33, https://doi.org/10.1111/ddi.12115 (2014).
Mittermeier, R. A. et al. Hotspots Revisited: Earth’s Biologically Richest and Most Threatened Ecoregions. (CEMEX, 2004).
Pereira, H. M., Domingos, T., Vicente, L. & Proença, V. Ecossistemas e Bem-estar Humano: Avaliação Para Portugal do Millennium Ecosystem Assessment. (Escolar Editora, 2009).
IPCC. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. (Cambridge University Press, 2007).
Peñuelas, J. et al. Impacts of global change on Mediterranean forests and their services. Forests 8(12), 463, https://doi.org/10.3390/f8120463 (2017).
Guiot, J. & Cramer, W. Climate change: The 2015 Paris Agreement thresholds and Mediterranean basin ecosystems. Science 354, 465–468, https://doi.org/10.1126/science.aah5015 (2016).
Proulx, G. et al. In Badgers: Systematics, Biology, Conservation and Research Techniques. (eds G. Prouxl & E. Do Linh San) 31–116 (Alpha Wildlife Publications, 2016).
Byrne, A. W., Fogarty, U., O’Keeffe, J. & Newman, C. In situ adaptive response to climate and habitat quality variation: spatial and temporal variation in European badger (Meles meles) body weight. Global Change Biol. 21, 3336–3346, https://doi.org/10.1111/gcb.12939 (2015).
Macdonald, D. W., Newman, C., Buesching, C. D. & Nouvellet, P. Are badgers ‘Under the weather’? Direct and indirect impacts of climate variation on European badger (Meles meles) population dynamics. Global Change Biol. 16, 2913–2922, https://doi.org/10.1111/j.1365-2486.2010.02208.x (2010).
Van Der Zee, F. F., Wiertz, J., Ter Braak, C. J. F. & Van Apeldoorn, R. C. Landscape changes as a possible cause of the badger Meles meles L. decline in the Netherlands. Biol. Conserv. 61, 17–22, https://doi.org/10.1016/0006-3207(92)91203-5 (1992).
Bičik, V., Foldynová, S. & Matyáštik, T. Distribution and habitat selection of badger (Meles meles) in Southern Moravia. Acta Univ. Palack. Olomuc. Fac. Rerum Natur. 38, 29–40 (2000).
Newton-Cross, G., White, P. C. L. & Harris, S. Modelling the distribution of badgers Meles meles: comparing predictions from field-based and remotely derived habitat data. Mamm. Rev. 37, 54–70, https://doi.org/10.1111/j.1365-2907.2007.00103.x (2007).
Silva, A. P. et al. Climate and anthropogenic factors determine site occupancy in Scotland’s Northern-range badger population: implications of context-dependent responses under environmental change. Divers. Distribut. 23, 627–639, https://doi.org/10.1111/ddi.12564 (2017).
Kauhala, K. Changes in distribution of the European badger Meles meles in Finland during the rapid colonization of the raccoon dog. Ann. Zool. Fennici 32, 183–191 (1995).
Bevanger, K. & Lindstrom, E. R. Distributional history of the European badger Meles meles in Scandinavia during the 20th century. Ann. Zool. Fennici 32, 5–9 (1995).
Virgós, E. & Casanovas, J. G. Environmental constraints at the edge of a species distribution, the Eurasian badger (Meles meles L.): a biogeographic approach. J. Biogeogr. 26, 539–564, https://doi.org/10.1046/j.1365-2699.1999.00320.x (1999).
Lara-Romero, C. et al. Habitat selection by European badgers in Mediterranean semi-arid ecosystems. J. Arid Environ. 76, 43–48, https://doi.org/10.1016/j.jaridenv.2011.08.004 (2012).
Rosalino, L. M., Macdonald, D. W. & Santos-Reis, M. Resource dispersion and badger population density in Mediterranean woodlands: is food, water or geology the limiting factor? Oikos 110, 441–452, https://doi.org/10.1111/j.0030-1299.2005.13755.x (2005).
Requena-Mullor, J. M., López, E., Castro, A. J., Virgós, E. & Castro, H. Landscape influence on the feeding habits of European badger (Meles meles) in arid Spain. Mammal. Research 61, 197–207, https://doi.org/10.1007/s13364-016-0269-x (2016).
Gao, X. & Giorgi, F. Increased aridity in the Mediterranean region under greenhouse gas forcing estimated from high resolution simulations with a regional climate model. Global Planet. Change 62, 195–209 (2008).
Elith, J. & Leathwick, J. R. Species distribution models: Ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Systemat. 40, 677–697, https://doi.org/10.1146/annurev.ecolsys.110308.120159 (2009).
Cruz, J., Sarmento, P. & White, P. C. L. Influence of exotic forest plantations on occupancy and co-occurrence patterns in a Mediterranean carnivore guild. J. Mammal. 96, 854–865, https://doi.org/10.1093/jmammal/gyv109 (2015).
Hipólito, D. et al. Drivers of sett site location by European badgers in Portugal. Biodivers. Conserv. 27, 2951–2970, https://doi.org/10.1007/s10531-018-1580-4 (2018).
Crase, B., Liedloff, A. C. & Wintle, B. A. A new method for dealing with residual spatial autocorrelation in species distribution models. Ecography 35, 879–888, https://doi.org/10.1111/j.1600-0587.2011.07138.x (2012).
Stürck, J. et al. Simulating and delineating future land change trajectories across Europe. Reg. Environ. Change 18, 733–749, https://doi.org/10.1007/s10113-015-0876-0 (2018).
Rosalino, L. M., Verdade, L. M. & Santos-Reis, M. IN Badgers: Systematics, Biology, Conservation and Research Technique (eds Prouxl, G. & Do Linh San, E.) 151–160 (Alpha Wildlife Publications, 2016).
Piza-Roca, C., Haye, M. J. J. L. & Jongejans, E. Environmental drivers of the distribution and density of the European badger (Meles meles): a review. Lutra 57, 87–109 (2014).
Crooks, K. R. Relative sensitivities of mammalian carnivores to habitat fragmentation. Conserv. Biol. 16, 488–502, https://doi.org/10.1046/j.1523-1739.2002.00386.x (2002).
Requena-Mullor, J. et al. Modeling spatial distribution of European badger in arid landscapes: an ecosystem functioning approach. Landsc. Ecol. 29, 843–855, https://doi.org/10.1007/s10980-014-0020-4 (2014).
Rosalino, L. M., Macdonald, D. W. & Santos-Reis, M. Spatial structure and land-cover use in a low density Mediterranean population of Eurasian badgers. Can. J. Zool. 82, 1493–1502, https://doi.org/10.1017/S0952836901001388 (2004).
Galante, E., Mena, J. & Lumbreras, C. Dung beetles (Coleoptera: Scarabaeidae, Geotrupidae) attracted to fresh cattle dung in wooded and open pasture. Environ. Entomol. 24, 1063–1068 (1995).
Kruuk, H., Parish, T., Brown, C. A. J. & Carrera, J. The use of pasture by the European badger (Meles meles). J. Appl. Ecol. 16, 453–459 (1979).
Virgós, E. et al. Food habits of European badgers (Meles meles) along an altitudinal gradient of Mediterranean environments: a field test of the earthworm specialization hypothesis. Can. J. Zool. 82, 41–51, https://doi.org/10.1139/z03-205 (2004).
Revilla, E. & Palomares, F. Does local feeding specialization exist in Eurasian badgers? Can. J. Zool. 80, 83–93, https://doi.org/10.1139/z01-208 (2002).
Rosalino, L. M., Loureiro, F., Macdonald, D. W. & Santos-Reis, M. Dietary shifts of the badger (Meles meles) in Mediterranean woodlands: an opportunistic forager with seasonal specialisms. Mamm. Biol. 70, 12–23, https://doi.org/10.1017/S0952836901001388 (2005).
Hofer, H. Variation in resource presence, utilization and reproductive success within a population of European badgers (Meles meles). Mamm. Rev. 18, 25–36 (1988).
Virgós, E., Cabezas-Diaz, S., Malo, A., Lozano, J. & Lopez-Huertas, D. Factors shaping European rabbit abundance in continuous and fragmented populations of central Spain. Acta Theriol. 48, 113–122 (2003).
Silva, J. S. Sequeira, E., Catry, F. & Aguiar, C. In Pinhais e Eucaliptais. A Floresta Cultivada (ed. Silva, J. S.) 221–239 (Publico, Comunicação Social SA, 2007).
Revilla, E., Palomares, F. & Delibes, M. Defining key habitats for low density populations of Eurasian badgers in Mediterranean environments. Biol. Conserv. 95, 269–277, https://doi.org/10.1016/S0006-3207(00)00043-4 (2000).
Carrilho, M., Teixeira, D., Santos-Reis, M. & Rosalino, L. M. Small mammal abundance in Mediterranean Eucalyptus plantations: how shrub cover can really make a difference. For. Ecol. Manag. 391, 256–263, https://doi.org/10.1016/j.foreco.2017.01.032 (2017).
Teixeira, D. et al. Management of Eucalyptus plantations influences small mammal density: Evidence from Southern Europe. For. Ecol. Manag. 385, 25–34, https://doi.org/10.1016/j.foreco.2016.11.009 (2017).
Santos, M. J. & Beier, P. Habitat selection by European badgers at multiple spatial scales in Portuguese Mediterranean ecosystems. Wildl. Res. 35, 835–843, https://doi.org/10.1071/WR08009 (2008).
Timo, T. P. C., Lyra-Jorge, M. C., Gheler-Costa, C. & Verdade, L. M. Effect of the plantation age on the use of Eucalyptus stands by medium to large-sized wild mammals in south-eastern Brazil. Iforest 8, 108–113, https://doi.org/10.3832/ifor1237-008 (2015).
Joffe, J. S. Morphology and genesis of podzols. Soil Sci. Soc. Am. J. 14, 22–29 (1933).
Sanborn, P., Lamontagne, L. & Hendershot, W. Podzolic soils of Canada: Genesis, distribution, and classification. Can. J. Soil Sci. 91, 843–880, https://doi.org/10.4141/cjss10024 (2011).
Roper, T. J. B. Meles meles setts - architecture, internal environment and function. Mamm. Rev. 22, 43–53 (1992).
Hipólito, D., Santos-Reis, M. & Rosalino, L. M. European badger (Meles meles) diet in an agroforestry and cattle ranching area of Central-West Portugal. Wildlife Biology in Practice 12, 1–13 (2016).
Benham, P. F. J. & Broom, D. M. Interactions between cattle and badgers at pasture with reference to bovine tuberculosis transmission. Br. Vet. J. 145, 226–241 (1989).
Mullen, E. M. et al. Foraging Eurasian badgers Meles meles and the presence of cattle in pastures. Do badgers avoid cattle? Appl. Anim. Behav. Sci. 144, 130–137, https://doi.org/10.1016/j.applanim.2013.01.013 (2013).
Perevolotsky, A. & Haimov, Y. The effect of thinning and goat browsing on the structure and development of Mediterranean woodland in Israel. For. Ecol. Manag. 49, 61–74, https://doi.org/10.1016/0378-1127(92)90160-B (1992).
Macdonald, D. W. & Newman, C. Population dynamics of badgers (Meles meles) in Oxfordshire, U.K.: numbers, density and cohort life histories, and a possible role of climate change in population growth. J. Zool., Lond. 256, 128–138, https://doi.org/10.1017/S0952836902000158 (2002).
Noonan, M. J. et al. Climate and the individual: Inter-annual variation in the autumnal activity of the European badger (Meles meles). PLoS ONE 9, e83156, https://doi.org/10.1371/journal.pone.0083156 (2014).
Kitao, N., Fukui, D., Hashimoto, M. & Osborne, P. G. Overwintering strategy of wild free-ranging and enclosure-housed Japanese raccoon dogs (Nyctereutes procyonoides albus). Int J. Biometeorology 53(2), 159–165, https://doi.org/10.1002/joc.1276 (2009).
Zub, K., Szafrańska, P. A., Konarzewski, M., Redman, P. & Speakman, J. R. Trade-offs between activity and thermoregulation in a small carnivore, the least weasel Mustela nivalis. Proc. Roy. Soc. Lond. B 276, 1921–1927, https://doi.org/10.1098/rspb.2008.1936 (2009).
Nouvellet, P., Newman, C., Buesching, C. D. & Macdonald, D. W. A multi-metric approach to investigate the effects of weather conditions on the demographic of a terrestrial mammal, the European badger (Meles meles). PLOS ONE 8, e68116, https://doi.org/10.1371/journal.pone.0068116 (2013).
Bale, J. S. et al. Herbivory in global climate change research: direct effects of rising temperature on insect herbivores. Global Change Biol. 8, 1–16, https://doi.org/10.1046/j.1365-2486.2002.00451.x (2002).
Connor, D. J., Gómez-del-Campo, M., Rousseaux, M. C. & Searles, P. S. Structure, management and productivity of hedgerow olive orchards: a review. Scientia Horticulturae 169, 71–93 (2014).
Santos, F. D., Forbes, K. & Moita, R. Climate Change in Portugal. Scenarios, Impacts and Adaptation Measures. SIAM Project. (Gradiva, 2002).
Kruuk, H. Foraging and spatial organisation of the European badger, Meles meles L. Behav. Ecol. Sociobiol. 4, 75–89 (1978).
Loureiro, F., Bissonette, J. A., Macdonald, D. W. & Santos-Reis, M. Temporal variation in the availability of Mediterranean food resources: do badgers Meles meles track them? Wildl. Biol. 15, 197–206, https://doi.org/10.2981/07-046 (2009).
Woodroffe, R. & Macdonald, D. W. Female/female competition in European badger Meles meles: effects on breeding success. J. Anim. Ecol. 64, 12–20, https://doi.org/10.2307/5823 (1995).
Dunwell, M. R. & Killingley, A. The distribution of badger setts in relation to the geology of the Chilterns. J. Zool., Lond. 158, 204–208, https://doi.org/10.1111/j.1469-7998.1969.tb02136.x (1969).
Revilla, E., Palomares, F. & Fernández, N. Characteristics, location and selection of diurnal resting dens by Eurasian badgers (Meles meles) in a low-density area. J. Zool., Lond. 255, 291–299, https://doi.org/10.1017/S0952836901001388 (2001).
Neal, E. & Cheeseman, C. Badgers. (T & A Poyser. 1996).
Roper, T. J. Badger. London: Harper Collins Publishers. (2010)
Lara-Romero, C., Virgós, E. & Revilla, E. Sett density as an estimator of population density in the European badger Meles meles. Mamm. Rev. 42, 78–84, https://doi.org/10.1111/j.1365-2907.2011.00194.x (2012).
Rivas-Martínez, R., Penas, A. & Díaz T. E. Biogeographic Map of Europe. (Cartographic Service, University of Leon, 2004).
Condé, S. & Richard, D. (Coordinators) The Mediterranean biogeographical region. EEA, Europe’s Biodiversity - Biogeographical regions and seas. EEA Report N° 1/2002. http://www.eea.europa.eu/publications/report_2002_0524_154909 (2002).
Condé, S., & Richard, D. (Coordinators) The Atlantic Region. EEA, Europe’s Biodiversity - Biogeographical Regions and Seas. EEA Report No 1/2002. http://www.eea.europa.eu/publications/report_2002_0524_154909 (2002).
INE Portugal in Figures 2016. (Instituto Nacional de Estatística, I.P., 2018).
Monterroso, P. et al. Factors affecting the (in)accuracy of mammalian mesocarnivore scat identification in South-western Europe. J. Zool. 289, 243–250, https://doi.org/10.1111/j.1365-2486.2010.02208.x (2012).
Morin, D. J. et al. Bias in carnivore diet analysis resulting from misclassification of predator scats based on field identification. Wildl. Soc. Bull. 40, 669–677, https://doi.org/10.1002/wsb.723 (2016).
ESRI. ArcGIS Desktop: Release 10. (Environmental Systems Research Institute, 2011).
Quantum GIS Development Team. Quantum GIS Geographic Information System. (Open Source Geospatial Foundation Project. http://qgis.osgeo.org, 2016).
Legendre, P. Spatial autocorrelation: trouble or new paradigm? Ecology 74, 1659–1673 (1993).
Paradis, E., Claude, J. & Strimmer, K. Analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290, https://doi.org/10.1093/bioinformatics/btg412 (2004).
Record, S., Fitzpatrick, M. C., Finley, A. O., Veloz, S. & Ellison, A. M. Should species distribution models account for spatial autocorrelation? A test of model projections across eight millennia of climate change. Global Ecol. Biogeogr. 22, 760–771, https://doi.org/10.1111/geb.12017 (2013).
Zar, J. H. Biostatistical Analysis. (Pearson Prentice Hall, 2010).
Zuur, A. F., Ieno, E. N. & Smith, G. M. Analysing Ecological Data. (Springer, 2007).
Carvalho, J., Santos, J. P. V., Torres, R., Santarém, F. & Fonseca, C. Tree-based methods: concepts, uses and limitations under the framework of resource selection models. J. Environ. Inform. 0, 1–13 (Retrieved from, http://www.jeionline.org/index.php?journal=mys&page=article&op=view&path%5B%5D=201600352). https://doi.org/10.3808/jei.201600352 (2017).
Elith, J., Leathwick, J. R. & Hastie, T. A working guide to boosted regression trees. J. Anim. Ecol. 77, 802–813, https://doi.org/10.1111/j.1365-2656.2008.01390.x (2008).
Harris, D. B. et al. The influence of non-climate predictors at local and landscape resolutions depends on the autecology of the species. Austral Ecol. 39, 710–721, https://doi.org/10.1111/aec.12134 (2014).
Elith, J. & Leathwick, J. R. Boosted regression trees for ecological modelling. Retrieved from, https://cran.r-project.org/web/packages/dismo/vignettes/brt.pdf (2017).
Ridgeway, G. gbm: Generalized Boosted Regression Models. R package version 2.1. Retrieved from from, http://CRAN.R-project.org/package=gbm (2013).
Hijmans, R. J. raster: Geographic data analysis and modeling. R package version 2.5-8. Retrieved from, https://CRAN.R-project.org/package=raster (2016).
Buston, P. M. & Elith, J. Determinants of reproductive success in dominant pairs of clownfish: a boosted regression tree analysis. J. Anim. Ecol. 80, 528–538, https://doi.org/10.1111/j.1365-2656.2011.01803.x (2011).
Real, R., Barbosa, A. M. & Vargas, J. M. Obtaining environmental favourability functions from logistic regression. Environ. Ecol. Stat. 13, 237–245, https://doi.org/10.1007/s10651-005-0003-3 (2006).
Acevedo, P. & Real, R. Favourability: concept, distinctive characteristics and potential usefulness. Naturwissenschaften 99, 515–522, https://doi.org/10.1007/s00114-012-0926-0 (2012).
Grilo, C., Bissonette, J. A. & Santos-Reis, M. Spatial–temporal patterns in Mediterranean carnivore road casualties: Consequences for mitigation. Biol. Conserv. 142, 301–313, https://doi.org/10.1016/j.biocon.2008.10.026 (2009).
Monterroso, P., Alves, P. C. & Ferreras, P. Plasticity in circadian activity patterns of mesocarnivores in Southwestern Europe: implications for species coexistence. Behav Ecol Sociobiol. 68, 1403–1417, https://doi.org/10.1007/s00265-014-1748-1 (2014).
R Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. http://www.R-project.org/ (2014).
EEA. Trends and Projections in Europe 2017. Tracking Progress Towards Europe’s Climate and Energy Targets. (European Environment Agency, 2017).
DGT - Direcção Geral do Território. Carta de Uso e Ocupação do Solo de Portugal Continental para 2007 (COS 2007 N5) WMS. (Direção de Serviços de Geodesia, Cartografia e Informação Geográfica, Direção-Geral do Território, 2010).
OpenStreetMap contributors. Planet Dump. retrieved from https://planet.openstreetmap.org (2015).
DGA. Atlas digital do ambiente. (Direcção-Geral do Ambiente, Accessed at http://sniamb.apambiente.pt/Home/Default.htm, 2017).
Hijmans, R. J., Cameron, S. E., Parra, J. L., Jones, P. G. & Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978, https://doi.org/10.1002/joc.1276 (2005).
Acknowledgements
Thanks for financial support are due to University of Aveiro (Department of Biology), to CESAM (UID/AMB/50017 - POCI-01-0145-FEDER-007638), to cE3c (UID/BIA/00329/2013), to FCT/MCTES through national funds (PIDDAC), and the co-funding by the FEDER within the PT2020 Partnership Agreement and Compete 2020. JC was supported by a PhD Grant (SFRH/BD/98387/2013) from Fundação para a Ciência e a Tecnologia (FCT), Portugal.
Author information
Authors and Affiliations
Contributions
L.M.R., C.G., M.S.-R., P.M., C.F., X.P., E.V., J.C. and D.H. conceived the ideas; D.H., D.G., D.C., A.S. and L.M.R. collected the data; L.M.R., D.H. and J.C. analysed the data; All authors contributed to the writing.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rosalino, L.M., Guedes, D., Cabecinha, D. et al. Climate and landscape changes as driving forces for future range shift in southern populations of the European badger. Sci Rep 9, 3155 (2019). https://doi.org/10.1038/s41598-019-39713-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-39713-1
This article is cited by
-
Can Eucalyptus plantations influence the distribution range of mesocarnivores?
Landscape Ecology (2023)
-
Soil δ13C and δ15N baselines clarify biogeographic heterogeneity in isotopic discrimination of European badgers (Meles meles)
Scientific Reports (2022)
-
How far from the road should land cover be assessed? A case study on mesopredator mortality on roads
European Journal of Wildlife Research (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.