Abstract
Mind-wandering or the spontaneous, uncontrolled changes in the allocation of attention resources (lapses) may cause variability in performance. In childhood, the relationship between the activation state of the brain, such as in attentional performance, and sleep has not been explored in detail. We investigated the role of sleep in attentional performance, and explored the most important parameters of their relationship. We objectively measured momentary lapses of attention of 522 children and correlated them with sleep schedules. In the subgroup of young children (age 7.1 ± 0.6 years; 60.8% girls), increasing age, long sleep duration and assessment closer to the previous night’s sleep period was associated with impaired performance speed and consistency. From pre-adolescence (age 9.4 ± 0.8 years; 50.5% girls) onwards somno-typologies may develop. As a result, in adolescence (age 13.4 ± 1.2 years; 51.3% girls) not only sleep duration but also sleep midpoint and sleep regularity influence the individual speed and stability of attention. Across development, regularity of sleep, individual sleep midpoint and bedtime become increasingly important for optimal performance throughout the day. Attentional performance and sleep shared almost half of their variance, and performance was sleep-driven across childhood. Future studies should focus on intra- and inter-individual differences in sleep-wake behavior to improve performance or decrease mind-wandering in youth by targeting sleep habits.
Similar content being viewed by others
Introduction
In the scientific literature, terms such as arousal, alertness, vigilance and attention are often interchangeably used. Despite their varied associations and definitions, these different terms describe the ‘activation states’ of the cerebral cortex. Attention, for instance, is considered to have a significant impact on a variety of neurobehavioral task performances; i.e., it functions as a filter to select stimuli and is therefore an important step in a cascade of interacting neural and neurotransmitter systems. Alternatively, the inhibition of neural networks involved in the regulation of ‘activation states’ of the brain such as the ascending reticular activating system in the brainstem, allows sleep. Sleep and waking, and their characteristic states, therefore depend on activation and inhibition of neural networks1.
Pediatric sleep research is a rapidly growing field, with increasing interest shown to the interface of sleep-wake behavior during childhood. For example, polls2,3 indicate that nowadays children sleep much less than what is appropriate for their stage of development. Sleep duration of 5–18 year olds has been consistently declining over the last 100 years. Yet this fact remains vigorously debated4. In addition, attention problems affect 5.29% of children and adolescents worldwide5. Foremost, sleep problems are common in children with attention deficit hyperactivity disorder6,7,8,9. Both sleep problems and attention problems have been predictive of scholastic underperformance and behavioral maladjustment. Despite a variety of studies investigating the relationship between sleep and attentional behaviors, their association is complex across childhood. A recent meta-analysis reported that sleep loss was not associated with processing speed and attention in childhood10; in contradistinction, other studies reported that attention appears to be sensitive to sleep deprivation in adolescents but attentional components of tasks showed inconsistent results to no effects11.
Objective evidence of impaired attentional performance due to poor sleep in children is indeed limited (i.e., about 20 studies)12,13,14,15,16,17,18. Studies often applied an AB design involving ‘an’ experimental change in sleep duration during childhood. Several examples are: one-night sleep loss12,19; an average of 30-min sleep extension or restriction successfully accomplished in 60% of 4th and 6th grade children20; a 3 week-long sleep schedule manipulation consisting of baseline (self-selected), optimized, and restricted while attending school14,15; sleep restriction for 1 week of 1-hour per day in 6 school-aged children16; a 5-hour restricted time in bed in 14 school-aged girls21; and more recently a randomly counterbalanced within-subjects cross-over design of a 6.5 hour sleep duration in adolescents22. Each of these studies suggests that performance is adversely affected by experimentally manipulated sleep schedule conditions, across a variety of neurobehavioral tasks assessing, for example, attention, cognition, school performance and others.
Small effect sizes have been reported regarding the impact of sleep quality, sleep duration and sleepiness on school performance in children and adolescents23. Amongst these, sleepiness showed the strongest relation to school performance. Alternatively, a meta-analysis in late adolescence and adulthood yielded small overall effect sizes ascribed to chronotypes, suggesting that evening orientation is associated with poorer academic performance24. Although, change towards eveningness is considered to occur mainly at the age of 12–13 years25. Arbabi et al.26 furthermore showed that already at the age of 10 years, evening orientation adversely affected academic achievement.
Other sleep researchers27 have proposed that the timing of sleep and wakefulness is more strongly correlated to performance than total sleep time with the addition, that a time of day effect, denoted by a performance increase during the morning, a post-lunch fall, and then an increase in the afternoon, was observed28,29. This notion of time of day has been put to practice in terms of school starting times in France, and elsewhere29,30,31,32,33. Although authors did not consistently incorporate bedtime, sleep duration or chronotype in their design, they did observe inter-individual patterns in sleep-wake behavior and suggested a flexibility to adjust to different schedules around the age of 1032,33.
In other words, pediatric sleep is a complex behavior to study (e.g., inferred from sleep duration, chronotype, bedtime, sleepiness, and other sleep parameters)34. Given the diversity in the applied methodologies in the aforementioned studies, and despite all being suggestive of poor(er) performance, neither the overlap with sleep nor the key determinants in performance, are conclusive. To date, neuroscience has focused mostly on daytime functioning (e.g. attention, learning) while the critically important role of sleep in this context has not always been sufficiently considered. At the same time, sleep researchers may have overlooked the shared variance between ‘activation states’ of the brain35.
Since the 1960’s it became evident that any cognitive task requires not only the activation of specific brain regions relevant to the task, but also the deactivation of brain regions irrelevant to the task, that might interfere with task-related regions. Lately, accumulating evidence suggests that within the same individual everyday task performance can vary tremendously36,37. As a result, the concept of “default-mode” brain activity state or wakeful rest of the brain is gaining empirical support38,39,40,41,42,43. Studies further suggest that some operations of this default system might continue during sleep, might be modulated by sleep, or might be maintained due to sleep38,39. At the basis of abnormal resting-state brain activity might be momentary lapses of attention; that is, this wandering of the mind possibly generates variability in performance. Gujar et al.44 showed that one-night sleep deprivation in adults impaired stability and balance of task-related deactivation in key default-mode regions. That is, subjects showed unsuccessful memory encoding attempts. Such findings concur with conceptions explaining time on task difficulties45, difficulties on tasks with higher cognitive load or processing demands16, that primarily occur in sleep deprivation experiments. This mind-wandering also coincides with Carskadon’s et al.12 pertinent observation in children that ‘sleepiness’ is the clearest and most consistent result of sleep restriction. To date, the objective investigation of this “default-mode” brain activity state in relation to sleep and attentional performance across childhood remains unexplored.
The main aim of the current study was to investigate the relationship between sleep and attentional performance in children aged 6 to 18 years old in their natural habitat. We were additionally interested in which of the parameters of sleep and performance where most prominent in this relationship. We hypothesized that impaired attentional performance depends on sleep duration, sleep irregularity with a shift, around pre-adolescence, towards the importance of sleep midpoint. These parameters may further amplify the individual variability in performance.
Methods
Procedure
This study was approved by the Institutional Review Board of Rhône-Alpes, Lyon (France), and participating schools. The study was performed in accordance with relevant guidelines and regulations. We obtained informed consent from parents and assent from children. Subjects were 6 to 18 years of age and lived in Lyon. Typically developing children from the 1st to 12th grade were recruited in schools through simple random sampling. That is, none of the children was receiving an Individual Education Plan at school, indicative of significant learning or other difficulties. Children were assessed in small groups of 3 to 5 whenever convenient in their school schedule, i.e., per child the time of assessment was random yet recorded. Assessments were performed during the school year (i.e., no assessments during summer holiday that is July and August).
Measures
Sleep schedule
During the assessment period in the schools, parents filled out health-related questionnaires which provided the bedtime (BT) and wake up time (WU) during the week and weekend (i.e., Saturday and Sunday). We calculated the sleep period time (SPT) for week and weekend. The difference in SPT between weekend and week, approximating sleep irregularity or social jetlag, was also calculated. A higher positive difference means longer SPT in weekend. Chronotype was expressed as midpoint of sleep (SM) which represents the actual timing of daily sleep as a tendency of morningness-eveningness, and is considered as a biological marker in adolescence46.
Stabilo
Stabilo is an attention test performed on a tablet (several papers in preparation, www.agence-nationale-recherche.fr/Projet-ANR-10-BLAN-1409). It was designed to measure spontaneous, uncontrolled changes in the allocation of attention resources or momentary lapses of attention (MLA). More specifically, subjects perform an attention-demanding task47 involving the presentation of a letter at a central fixation point (the target) for 250 ms. The mask (i.e., 500 ms–4 dots) is replaced with a 2 × 2 array of letters that will stay on screen until the subject presses a button. The response can be ‘target letter was present’ button or ‘the target letter was absent’ button. Response buttons were reversed to the subject’s hand laterality (e.g., for the right-handed child, the ‘yes’ button was on the left side of the tablet). The target was present in 50% of the trials. Its presence and position were randomized across trails. Subjects are encouraged to answer as fast as possible due to a challenge-mode design (i.e., improving past performance). Reaction time (RT) is defined as the latency between the onset of the target and the button press. This task involves the following neuropsychological functions48,49: a letter encoding, visual search and motor transform.
Four versions have been developed. The standard version includes 50 trials or 2 minutes having a right/wrong auditory feedback with 5 seconds penalty for mistakes. A similar version for children younger than 8 years old, consists of having the first letter of the array always being a V or T. In the colored version, similar to the standard version, the target is always colored in red. The long version includes 200 trials or 10 minutes without auditory feedback or coloring.
Momentary Lapses of Attention (MLA) indexes: Several indexes assessing MLA, or the speed and stability of attention are calculated. A pre-test establishing a subject’s baseline performance; i.e., the fastest response five times in a row without mistake generates a norm index. Below this index, a subject is considered guessing. A higher norm index means slower RT. The speed of attention is inferred from two indexes: a best speed index: a subject’s best RT and a consistent speed index: a subjects best RT on 5 consecutive correct sets, i.e., the subject can’t go faster without making mistakes. Higher scores on the speed of attention indexes suggest slower RT and slower RT for a prolonged period, respectively. The stability of attention is based on a moving curve of RT (correct) performance. In short, a precision index (expressed in percentage) at 3000 ms (good precision index) and at 1500 ms (very good precision index) can be calculated. For example, to score a 100% subjects need to make no mistakes and respond each time below 3000 ms. Higher scores reflect few mistakes under fast RT (subject’s performance is precise) and faster RT (subject’s performance is very precise), respectively. A regularity index is also calculated on a moving curve of (correct) response performance. A good regularity index is based on the cut-off of 40%, and a very good regularity index uses the cut-off of 20%. That is, subjects scoring higher than for instance 40% succeeded in performing long sets with little RT variability. Higher scores mean long sets with little (subject’s performance is regular) and very little variation in RT (subject’s performance is very regular), respectively. The error index represents the percentage of mistakes made during the task.
Parameters calculated based on sleep schedule and daytime assessment: The time of assessment during the day (HRtest) was collected. We calculated the time up to HRtest from WU (i.e., Time since WU), SM (i.e., Time since SM) and BT (i.e., Time since BT). That is, larger values are indicative of a later time of assessment during the day.
Statistical Analysis
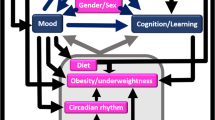
Descriptive analyses were performed to describe the sample. Canonical correlation analyses (CCA) were applied to investigate the concurrent relationship between the group of sleep variables and the group of attention variables. CCA needs to be understood as “multiple-multiple” correlations or extracted roots whereby sets of variables are associated. Figure 1 is a scheme of the variables used for the CCA and a guideline to their interpretation. In addition to the shared variance, the canonical factor loadings (i.e., pertaining to the overall correlation of the variables with the canonical variate or root), lambda prime (i.e., the proportion of unexplained variance) are reported. Loadings could be perceived as expressing the contribution to the shared variance, whereas a canonical score is the individual weighted z-transformed score on the set of parameters studied (Fig. 2). The redundancy index is an index to measure the degree to which one set of variables can predict another set of variables. Inspection of scatterplots (not printed) generated from the CCA’s assists in a more detailed interpretation of the “multiple-multiple” correlations. In addition, correlograms (Fig. 3) may aid the interpretation but caution is warranted since a root is their aggregated ‘result’. Statistical analyses were performed with Statistica version 13 (StatSoft, Inc. (2009), STATISTICA, Tulsa, OK). P-values are determined.
Scheme of variables used. You can think of canonical variates as describing some underlying “latent” variable. The shared variance is the average proportion of variance extracted by the roots. The specific loadings can be found in Table 2. Higher loadings will have more importance within the canonical variates and especially in the root. The redundancy coefficients (i.e., how redundant one set of variables is, given the other set of variables) should be interpreted as a measure of possible predictive ability.
Canonical scores across the age-ranges studied. Y- axis: A canonical score is the individual weighted z-transformed score on the set of parameters studied, i.e., sleep and attentional performance; X- axis: increasing age; Purple dots: 2 minutes standard version for <8 year olds; Orange dots: 2 minutes standard version; Yellow dots: 10 minutes version of Stabilo; Grey dots: 2 minutes standard version for a posteriori regrouping of ≥8 year olds.
Statement of Significance
The concept of the brain’s default-mode activity, or wakeful rest, is gaining empirical support. We investigated the degree of commonality between attentional performance and sleep, and explored their determinants by assessing momentary lapses of attention. During childhood, performance was found to be sleep-driven. Especially from pre-adolescence onwards intra- and inter-individual differences in sleep-wake habits may promote mind-wandering, i.e. the spontaneous, uncontrolled changes in the allocation of attention resources. That is, regularity of sleep, individual sleep midpoint and bedtime become increasingly important for optimal performance throughout the day. When non-optimized to the individual need, they may amplify variability in performance. Somnotypology may lead to better understanding of an interdependence between brain activity in the sleep and waking-states.
Results
Based on the assessment with different versions of Stabilo across the age-ranges included in the study, we report below the results of several subsamples. For reference, we show in Table 1 the sleep parameters of these subsamples. CCA results are included in Table 2, expressing the loadings between the variate and the variables in each set (or correlograms are printing the first-order correlations in Fig. 3a–d). Figure 2 shows the associative trend between the sleep and attention variable sets, expressed as canonical scores, across the age-ranges studied.
In young children (age 7.1 ± 0.6 years [min. 6 to max. 7.9]; 60.8% girls) sleep and attentional performance shared 40.3% variance [Chi²(54) = 83.5, p = 0.006, lambda prime: 35.2%]. Their association is sleep-driven with SPT and the time up to assessment influencing the speed of attention, and to some extent the ‘very good’ regularity index. In more detail, for the sleep parameters with highest contribution, findings were suggestive for longer SPT, especially for older children in this subsample, to decelerate the speed of attention (also visualized in Fig. 2). In addition, for older children in this subsample specifically, assessment far from BT decelerated their speed of attention, and assessment far from their SM improved their consistency in attention. Note for example, that an individual can have a slow reaction time (slow speed) and this for several consecutive sets (high consistency).Whereas for younger children in this subsample, speed of attention decelerated when assessment was closer to WU, SM and BT (scatterplots not shown). In terms of the ‘very good’ regularity of attention index, especially in the older children shorter SPT, and in the younger children also the assessment closer to WU, SM and BT increased the index. Interpretatively, apart from SPT, within this age-range, timing during the day of a task is relevant.
In pre-adolescents (age 9.4 ± 0.8 years [min. 8 to max. 10.9]; 50.5% girls) sleep and attentional performance as assessed with the Stabilo were unassociated.
For adolescents (age 13.4 ± 1.2 years [min. 11 to max. 18]; 51.3% girls) similar results were obtained with the standard version of the Stabilo [31.3% shared variance between sleep and attention; Chi²(54) = 128.5, p < 0.0001; lambda prime: 59.9%] and the long version of the Stabilo [31.1% shared variance between sleep and attention; Chi²(54) = 144.8, p < 0.0001; lambda prime: 56.2%]. Both associations were mostly sleep-driven. The most important contributors to their shared variances were SPT, SM, sleep irregularity (or social jetlag) as well as norm index and ‘very good’ precision index. Although the contribution to the shared variance of these parameters is nearly equivalent, their sign is opposite, and therefore suggestive that the duration of the task is another important factor in performance. In more detail (scatterplots not printed) for the sleep parameters with strongest contribution to the shared variance, the following was found: Long SPT and large positive sleep irregularities (i.e., more sleep on weekends) at any age, and especially around the age of 13–14 (i.e. crossing point Fig. 2), were associated with a higher norm index. The norm index also increased with later SM for the younger ones and earlier SM for the older ones in this subsample. Alternatively, a long SPT at any age on the standard version, and especially for the older ones on the longer version, were associated with a lower ‘very good’ precision index. On the 2-minutes version, especially earlier SM for the older ones in the subsample decreased the ‘very good’ precision index. On the 10-minutes version, earlier SM at any age was associated with a lower ‘very good’ precision index. Increased sleep irregularity at any age, and especially shorter sleep duration during weekends in the younger ones, were associated with a lower ‘very good’ precision index. Interpretatively, within this age-range, precision of attentional performance is especially relevant. The correlograms (Fig. 3a–c) may assist in the interpretation if the final root is understood as their aggregated result. An interpretative summary (Fig. 2, orange and yellow dots) would be that a non-optimized sleep to the individual need for the older ones in the subsamples adversely affects their performance on a 2-minutes attention-demanding task, whereas in younger ones it would be their 10-minutes performance.
A posteriori-stratification
The results in the adolescent age-range were suggestive for the influence of chronotype and therefore we included the pre-adolescents into the adolescent sample (i.e., ≥8 year olds, Fig. 2 grey dots). The assessment with the standard version of the Stabilo in this sample suggested a differential associative profile (although with caution, this could also be inferred from glancing at the correlograms).
Both sleep and attention variables almost equally contributed to the shared variance of 45.0% [Chi²(54) = 289.54, p < 0.00001; lambda prima: 50.1%, Table 2]. Principal determinants of this association were SPT, SM, sleep irregularity and time since BT as well as all attention variables. The norm index and the ‘very good’ precision index showed the highest factor loadings.
In more detail (Fig. 2 shows a trend by the negative canonical scores between the variables sets) for the sleep parameters with strongest contribution to the shared variance, long SPT associated with poorer precision, regularity, speed and consistency, especially in the older children (scatterplots not printed). An earlier SM also adversely affected attention performance especially in the older children. Yet for younger children (in the ≥8 years old subsample) specifically the regularity of attention was poorer with later SM. With irregular sleep, attentional performance especially deteriorated in the youngest children such that more sleep during the weekend was associated with a poorer norm index, and less sleep on the weekend worsened precision and regularity of attentional performance (scatterplots not printed). Sleep irregularity (or here social jetlag) such as sleeping more during week or more during weekends was related to slower reaction times in young children (i.e., higher best speed index). Whereas from age 10 and older, this irregularity may improve the consistency in their attentional performance. Assessment far from BT was associated with better precision and regularity especially in the older children. Whereas their speed and consistency of attention were better with assessment close to BT. Note that around the age of 10–11 years old canonical scores cross the zero-line in Fig. 2.
An interpretative summary here would be that across the age-ranges in this subsample (≥8 years old) particularly SPT, SM, sleep regularity and BT have their adverse impact on a 2-minutes attention-demanding task. The correlograms (Fig. 3d) and the gradual declining canonical scores as shown in Fig. 2 similarly depict this.
Discussion
The findings from this study provide evidence that regular sleep is imperative for fast, precise and steady attentional performance. These behavioral states share 40–45% of the variance with performance being sleep-driven. That is, not only sleep duration but also sleep regularity and sleep midpoint were significant determinants, especially from pre-adolescence onwards. In younger children sleep was important for the speed of attentional performance and, possibly, with the manifestation of chronotypes, individual differences in attentional performance emerged. As a result, the regularity of sleep, the individual sleep midpoint and bedtime become increasingly important, across developmental stages, for optimal and steady performance throughout the day. These findings have important implications for the acquisition of skill and performance in childhood, as well as for an approach to the sleep habits of youngsters.
The first main finding is that during childhood the substantial shared variance of sleep with attentional performance is sleep-driven. This underscores the importance of defining and measuring optimal sleep during childhood. That is, the field of pediatric sleep medicine should be more critical regarding the variety of methods that exist for measuring sleep34,50. Consensus guidelines and panel groups as well as critical self-reflection on applied sleep (research) methodology is indeed compulsory at the verge of a sleep health era. Despite the complexity of operationalizing the sleep behavior of an individual in their habitat, it is furthermore a societal responsibility to provide opportunities for sleep throughout childhood. Contrariwise, we should particularly remain cognizant that in the absence of objective sleep assessment, and in the realm of the dispute about adequate pediatric sleep duration4, that our sample might be (chronically) sleep deprived.
The second main finding relates to the importance of the developmental stage concerning childhood sleep, and its impact on performance. On the one hand, the proportion of unexplained variance in this sleep-attentional performance relationship almost doubles from young childhood to adolescence. In school-aged children, complex and interactive relationships among school schedules, parental sleep-wake patterns, socioeconomic status, and daytime activities exist in determining the schedules of children51. Such societal characteristics, potentially interacting with intrapersonal factors, adversely affect bedtime, sleep onset time and total sleep time, especially in adolescence52. On the other hand, our findings on the role of sleep irregularity (social jetlag) and the time elapsed since sleep midpoint suggest the emergence of diverse somnotypologies from puberty onwards. There is furthermore reasonable indication that part of the unexplained variance could be ascribed to gender-specific alterations in sleep-wake states since canonical correlation models did not converge in girls and boys separately. For example in the literature, girls were found to be more morning-oriented than boys53. In other words, which sleep parameter phenotypes your sleep the best? Together, our findings may stimulate a ‘pediatric’ interpretation of the two-process model first proposed by Borbély54,55 which has been primarily useful in describing normal sleep in adults56. Namely, the prominence of the two main processes affecting sleepiness, i.e. circadian rhythms and homeostatic sleep drive, may shift around pre-adolescence, and hence affect their performance. Gender differences have been well acknowledged in psychology or neurosciences, yet the role of sleep might have been overlooked.
The third main finding concurs with the small to moderate correlations between sleep and performance in general as reported in the literature, and with the inconsistent results on which neurocognitive and neurobehavioral performance is (mostly) affected. Namely, impaired performance might be due to such MLA as assessed in our study. MLA as a potential ‘latent’ dysfunction might act in a cumulative or synergetic manner to the assessed performance. Although in the current study we did not investigate separate neuropsychological functions, our attention-demanding task involved a letter encoding, visual search and motor transform (and auditory feedback) activation of the brain. Their combined output, however, reflected the allocation of attentional resources in terms of different indexes. In the younger children, primarily speed of attention and, in the older children, stability of attention were changed by sleep. In the aforementioned experimental designs12,14,15,16,19,20,21,22 deteriorating reaction times of the neuropsychological functions investigated, as well as behaviors suggestive of inattention and sleepiness, were reported, as in a true cause-effect relation. Our findings therefore add to the existing literature that, given the concept of a default-mode network brain activity, these experiment-derived results might be moderated and/or mediated by mind-wandering. This might be (mistakenly) perceived as fatigue, sleepiness or sleep inertia by scientists, clinicians, or significant others, or in neurosciences as inefficient or slower information processing17. Our findings may therefore further support the notion by Carskadon et al.12 that children, during the formative period of their brain networks, may not recover from sleep restriction as rapidly as adults.
The fourth main finding is that only one root consistently reflected the shared variance, and this consequently suggests the importance of considering the activation states of the cortex in their entirety. Future research may move beyond conceptualizations in terms of time-on-task or time-of-day as regards performance variables, or sleep timing, duration, and midpoint, as regards sleep variables. The association between sleep ‘parameters’ and performance might not be simplistic. Presumably, critical points could be around the age-ranges of 8–10 years and 13–14 years. Although additional studies are needed, if one root reflects the activation states of the cortex, then studies should investigate the brain’s default-mode network activity across childhood and sleep researchers may consider studying sleep longitudinally.
The present study has several limitations. Habitual sleep was not objectively recorded; moreover, SPT, as calculated in our study rather reflects the time allocated to sleep than actual sleep. Regarding the subjective report of sleep patterns, the discrepancy between parent versus adolescent reported sleep patterns is undocumented. We also lack other (self-)measures of circadian preference25 which might be different from the applied chronotype formula. Next, we have no measure of sleepiness, chronicity of poor sleep, or sleep disorders in this sample. Future studies should therefore focus on objective measurement of inter- and intra-individual differences in sleep and acrophase, in addition to self-/parental reported sleep measures. Somnotypology may lead to better understanding of the interdependence between the sleep-states and waking-states default brain activity, especially regarding performance during the next day. Another limitation might be that the Stabilo assessment was performed in small groups of 3–5 children, simultaneously during schooldays, at the time most convenient for their school schedule. Yet, Wilson et al.57 showed that a 5-minute psychomotor vigilance task in a primary school classroom setting was reliable. Apart from gender, we did not pursue subgroup analysis based on grades in school or socio-economic status. Lastly, given the lack of objective sleep recordings we did not discuss the neural substrates35,58,59 potentially underlying our findings.
The present study has also several strengths including the age-range, the inclusion of the time elapsed since known sleep determinants, and especially an objective measure sensitive to MLA. We also did not conceptualize circadian preference in a bi-dimensional construct of owls and larks, but applied an approximation of chronotype based on parental reported hours. Furthermore, the separate sleep and attentional parameters were analyzed together, capturing their interdependence. Lastly, in contrast to others we assessed a large pediatric sample in their naturalistic environment.
In summary, the findings of this study replicate and extend an established literature on the importance of acquiring adequate sleep and of maintaining a sleep schedule that is consistent with the individual biological sleep need across childhood. Alternatively, while an increasing number of studies have actively manipulated sleep in order to observe neurocognitive and behavioral consequences, they may have consistently overlooked the role of momentary lapses of attention. Therefore sleep and waking, and their characteristic states, may benefit from a more holistic research approach.
References
Lin, J. S., Anaclet, C., Sergeeva, O. A. & Haas, H. L. The waking brain: an update. Cell Mol Life Sci 68, 2499–2512, https://doi.org/10.1007/s00018-011-0631-8 (2011).
Sleep in America Poll - sleep in children survey. http://www.sleepfoundation.org/article/sleep-americapolls/2004-children-and-sleep.
Sleep in America Poll - teens and sleep. http://www.sleepfoundation.org/article/sleep-americapolls/2006-teens-and-sleep.
Matricciani, L., Olds, T. & Petkov, J. In search of lost sleep: secular trends in the sleep time of school-aged children and adolescents. Sleep medicine reviews 16, 203–211, https://doi.org/10.1016/j.smrv.2011.03.005 (2012).
Polanczyk, G., de Lima, M. S., Horta, B. L., Biederman, J. & Rohde, L. A. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. The American journal of psychiatry 164, 942–948, https://doi.org/10.1176/ajp.2007.164.6.942 (2007).
Coogan, A. N. & McGowan, N. M. A systematic review of circadian function, chronotype and chronotherapy in attention deficit hyperactivity disorder. Attention deficit and hyperactivity disorders, https://doi.org/10.1007/s12402-016-0214-5 (2017).
Cortese, S. et al. Assessment and management of sleep problems in youths with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 52, 784–796, https://doi.org/10.1016/j.jaac.2013.06.001 (2013).
Spruyt, K. & Gozal, D. Sleep disturbances in children with attention-deficit/hyperactivity disorder. Expert review of neurotherapeutics 11, 565–577, https://doi.org/10.1586/ern.11.7 (2011).
Spruyt, K., Raubuck, D. L., Grogan, K., Gozal, D. & Stein, M. A. Variable sleep schedules and outcomes in children with psychopathological problems: preliminary observations. Nature and science of sleep 4, 9–17, https://doi.org/10.2147/nss.s29299 (2012).
Short, M. A. et al. Cognition and objectively measured sleep duration in children: a systematic review and meta-analysis. Sleep Health 4, 292–300, https://doi.org/10.1016/j.sleh.2018.02.004 (2018).
de Bruin, E. J., van Run, C., Staaks, J. & Meijer, A. M. Effects of sleep manipulation on cognitive functioning of adolescents: A systematic review. Sleep medicine reviews 32, 45–57, https://doi.org/10.1016/j.smrv.2016.02.006 (2017).
Carskadon, M. A., Harvey, K. & Dement, W. C. Sleep loss in young adolescents. Sleep 4, 299–312 (1981).
Randazzo, A. C., Muehlbach, M. J., Schweitzer, P. K. & Walsh, J. K. Cognitive function following acute sleep restriction in children ages 10–14. Sleep 21, 861–868 (1998).
Fallone, G., Acebo, C., Arnedt, J. T., Seifer, R. & Carskadon, M. A. Effects of acute sleep restriction on behavior, sustained attention, and response inhibition in children. Perceptual and Motor Skills 93, 213–229 (2001).
Fallone, G., Acebo, C., Seifer, R. & Carskadon, M. A. Experimental restriction of sleep opportunity in children: effects on teacher ratings. Sleep 28, 1561–1567 (2005).
Molfese, D. L. et al. A one-hour sleep restriction impacts brain processing in young children across tasks: evidence from event-related potentials. Developmental neuropsychology 38, 317–336, https://doi.org/10.1080/87565641.2013.799169 (2013).
Hoyniak, C. P., Petersen, I. T., McQuillan, M. E., Staples, A. D. & Bates, J. E. Less Efficient Neural Processing Related to Irregular Sleep and Less Sustained Attention in Toddlers. Developmental neuropsychology 40, 155–166, https://doi.org/10.1080/87565641.2015.1016162 (2015).
Vriend, J., Davidson, F., Rusak, B. & Corkum, P. Emotional and Cognitive Impact of Sleep Restriction in Children. Sleep medicine clinics 10, 107–115, https://doi.org/10.1016/j.jsmc.2015.02.009 (2015).
Reddy, R., Palmer, C. A., Jackson, C., Farris, S. G. & Alfano, C. A. Impact of sleep restriction versus idealized sleep on emotional experience, reactivity and regulation in healthy adolescents. Journal of sleep research, https://doi.org/10.1111/jsr.12484 (2016).
Sadeh, A., Gruber, R. & Raviv, A. The effects of sleep restriction and extension on school-age children: what a difference an hour makes. Child Dev 74, 444–455 (2003).
Peters, J. D. et al. The sensitivity of a PDA-based psychomotor vigilance task to sleep restriction in 10-year-old girls. Journal of sleep research 18, 173–177, https://doi.org/10.1111/j.1365-2869.2008.00716.x (2009).
Beebe, D. W., Field, J., Milller, M. M., Miller, L. E. & LeBlond, E. Impact of Multi-Night Experimentally Induced Short Sleep on Adolescent Performance in a Simulated Classroom. Sleep 40, https://doi.org/10.1093/sleep/zsw035 (2017).
Dewald, J. F., Meijer, A. M., Oort, F. J., Kerkhof, G. A. & Bogels, S. M. The influence of sleep quality, sleep duration and sleepiness on school performance in children and adolescents: A meta-analytic review. Sleep medicine reviews 14, 179–189, https://doi.org/10.1016/j.smrv.2009.10.004 (2010).
Tonetti, L., Natale, V. & Randler, C. Association between circadian preference and academic achievement: A systematic review and meta-analysis. Chronobiology international 32, 792–801, https://doi.org/10.3109/07420528.2015.1049271 (2015).
Tonetti, L., Adan, A., Di Milia, L., Randler, C. & Natale, V. Measures of circadian preference in childhood and adolescence: A review. European psychiatry: the journal of the Association of European Psychiatrists 30, 576–582, https://doi.org/10.1016/j.eurpsy.2015.01.006 (2015).
Arbabi, T., Vollmer, C., Dorfler, T. & Randler, C. The influence of chronotype and intelligence on academic achievement in primary school is mediated by conscientiousness, midpoint of sleep and motivation. Chronobiology international 32, 349–357, https://doi.org/10.3109/07420528.2014.980508 (2015).
Eliasson, A. H., Lettieri, C. J. & Eliasson, A. H. Early to bed, early to rise! Sleep habits and academic performance in college students. Sleep & breathing=Schlaf & Atmung 14, 71–75, https://doi.org/10.1007/s11325-009-0282-2 (2010).
van der Heijden, K. B., de Sonneville, L. M. & Althaus, M. Time-of-day effects on cognition in preadolescents: a trails study. Chronobiology international 27, 1870–1894, https://doi.org/10.3109/07420528.2010.516047 (2010).
Janvier, B. & Testu, F. Age-related differences in daily attention patterns in preschool, kindergarten, first-grade, and fifth-grade pupils. Chronobiology international 24, 327–343, https://doi.org/10.1080/07420520601139839 (2007).
Montagner, H. & Testu, F. Biological, behavioral and intellectual rhythms in pupils during the school-day. Pathologie-biologie 44, 519–533 (1996).
Testu, F. Diurnal variations of performance and information processing. Chronobiologia 13, 319–326 (1986).
Testu, F. Diurnal variation in mental activities of French pupils and influence of test protocol. Chronobiology international 9, 439–443 (1992).
Testu, F. & Clarisse, R. Time-of-day and day-of-week effects on mnemonic performance. Chronobiology international 16, 491–503 (1999).
Blunden, S. & Galland, B. The complexities of defining optimal sleep: empirical and theoretical considerations with a special emphasis on children. Sleep medicine reviews 18, 371–378, https://doi.org/10.1016/j.smrv.2014.01.002 (2014).
Zhao, Y. & Lin, J.-S. 中枢组织胺 H3受体在睡眠-觉醒调节中的 功用及作为治疗睡眠障碍药物干预靶点的依据 (2014).
Smallwood, J. & Schooler, J. W. The science of mind wandering: empirically navigating the stream of consciousness. Annual review of psychology 66, 487–518, https://doi.org/10.1146/annurev-psych-010814-015331 (2015).
Smallwood, J. & Schooler, J. W. The restless mind. Psychol Bull 132, 946–958, https://doi.org/10.1037/0033-2909.132.6.946 (2006).
Fukunaga, M. et al. Large-amplitude, spatially correlated fluctuations in BOLD fMRI signals during extended rest and early sleep stages. Magnetic resonance imaging 24, 979–992, https://doi.org/10.1016/j.mri.2006.04.018 (2006).
Horovitz, S. G. et al. Low frequency BOLD fluctuations during resting wakefulness and light sleep: a simultaneous EEG-fMRI study. Human brain mapping 29, 671–682, https://doi.org/10.1002/hbm.20428 (2008).
Weissman, D. H., Roberts, K. C., Visscher, K. M. & Woldorff, M. G. The neural bases of momentary lapses in attention. Nat Neurosci 9, 971–978, https://doi.org/10.1038/nn1727 (2006).
Rosenberg, M. D., Finn, E. S., Constable, R. T. & Chun, M. M. Predicting moment-to-moment attentional state. NeuroImage 114, 249–256, https://doi.org/10.1016/j.neuroimage.2015.03.032 (2015).
Hedden, T. & Gabrieli, J. D. E. The ebb and flow of attention in the human brain. Nature Neuroscience 9, 863.
Buckner, R. L., Andrews-Hanna, J. R. & Schacter, D. L. The brain’s default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences 1124, 1–38, https://doi.org/10.1196/annals.1440.011 (2008).
Gujar, N., Yoo, S. S., Hu, P. & Walker, M. P. The unrested resting brain: sleep deprivation alters activity within the default-mode network. Journal of cognitive neuroscience 22, 1637–1648, https://doi.org/10.1162/jocn.2009.21331 (2010).
Van Dongen, H. P., Belenky, G. & Krueger, J. M. A local, bottom-up perspective on sleep deprivation and neurobehavioral performance. Current topics in medicinal chemistry 11, 2414–2422 (2011).
Roenneberg, T. et al. A marker for the end of adolescence. Curr Biol 14, R1038–1039, https://doi.org/10.1016/j.cub.2004.11.039 (2004).
Mainy, N. et al. Neural correlates of consolidation in working memory. Hum Brain Mapp 28, 183–193, https://doi.org/10.1002/hbm.20264 (2007).
Jensen, O., Kaiser, J. & Lachaux, J. P. Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci 30, 317–324, https://doi.org/10.1016/j.tins.2007.05.001 (2007).
Brovelli, A., Lachaux, J. P., Kahane, P. & Boussaoud, D. High gamma frequency oscillatory activity dissociates attention from intention in the human premotor cortex. Neuroimage 28, 154–164, https://doi.org/10.1016/j.neuroimage.2005.05.045 (2005).
Spruyt, K. & Gozal, D. Pediatric sleep questionnaires as diagnostic or epidemiological tools: a review of currently available instruments. Sleep medicine reviews 15, 19–32, https://doi.org/10.1016/j.smrv.2010.07.005 (2011).
Zhang, J., Li, A. M., Fok, T. F. & Wing, Y. K. Roles of Parental Sleep/Wake Patterns, Socioeconomic Status, and Daytime Activities in the Sleep/Wake Patterns of Children. The Journal of pediatrics 156, 606–612.e605, https://doi.org/10.1016/j.jpeds.2009.10.036 (2010).
Bartel, K. A., Gradisar, M. & Williamson, P. Protective and risk factors for adolescent sleep: a meta-analytic review. Sleep medicine reviews 21, 72–85, https://doi.org/10.1016/j.smrv.2014.08.002 (2015).
Randler, C. Chronotype in children and adolescents. Somnologie 20, 166–171, https://doi.org/10.1007/s11818-016-0073-5 (2016).
Borbely, A. A., Daan, S., Wirz-Justice, A. & Deboer, T. The two-process model of sleep regulation: a reappraisal. Journal of sleep research 25, 131–143, https://doi.org/10.1111/jsr.12371 (2016).
Borbely, A. A. A two process model of sleep regulation. Human neurobiology 1, 195–204 (1982).
Waterhouse, J., Fukuda, Y. & Morita, T. Daily rhythms of the sleep-wake cycle. Journal of physiological anthropology 31, 5, https://doi.org/10.1186/1880-6805-31-5 (2012).
Wilson, A., Dollman, J., Lushington, K. & Olds, T. Reliability of the 5-min psychomotor vigilance task in a primary school classroom setting. Behavior research methods 42, 754–758, https://doi.org/10.3758/brm.42.3.754 (2010).
Curcio, G., Ferrara, M. & De Gennaro, L. Sleep loss, learning capacity and academic performance. Sleep medicine reviews 10, 323–337, https://doi.org/10.1016/j.smrv.2005.11.001 (2006).
Maski, K. P. & Kothare, S. V. Sleep deprivation and neurobehavioral functioning in children. International journal of psychophysiology: official journal of the International Organization of Psychophysiology 89, 259–264, https://doi.org/10.1016/j.ijpsycho.2013.06.019 (2013).
Acknowledgements
We would like to thank Drs Herbillon and Putois, and their students for the extensive data-collection. We would like to thank all the children for their participation. This study was financially supported by l’Institut National de la Santé et de la Recherche Médicale (INSERM).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study design. Data-collection were performed by V. Herbillon and B. Putois. Data-analysis, interpretation and drafting of the manuscript was performed by K.Spruyt. All authors approved the final version of the manuscript for submission.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Spruyt, K., Herbillon, V., Putois, B. et al. Mind-wandering, or the allocation of attentional resources, is sleep-driven across childhood. Sci Rep 9, 1269 (2019). https://doi.org/10.1038/s41598-018-37434-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-37434-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.