Abstract
Inter-specifically, relative energy costs of terrestrial transport vary several-fold. Many pair-wise differences of locomotor costs between similarly-sized species are considerable, and are yet to be explained by morphology or gait kinematics. Foot contact time, a proxy for rate of force production, is a strong predictor of locomotor energy costs across species of different size and might predict variability between similarly sized species. We tested for a relationship between foot contact time and metabolic rate during locomotion from published data. We investigated the phylogenetic correlation between energy expenditure rate and foot contact time, conditioned on fixed effects of mass and speed. Foot contact time does not explain variance in rate of energy expenditure during locomotion, once speed and body size are accounted for. Thus, perhaps surprisingly, inter-specific differences in the mass-independent net cost of terrestrial transport (NCOT) are not explained by rates of force production. We also tested for relationships between locomotor energy costs and eco-physiological variables. NCOT did not relate to any of the tested eco-physiological variables; we thus conclude either that interspecific differences in transport cost have no influence on macroecological and macrophysiological patterns, or that NCOT is a poor indicator of animal energy expenditure beyond the treadmill.
Similar content being viewed by others
Introduction
When moving around, larger animals enjoy economies of scale over smaller animals; per unit mass they expend less energy to travel a given distance1,2,3,4,5. Yet terrestrial animals across a great range of sizes have the same mass-independent mechanical cost of transport6. Thus when running, the muscles of smaller animals must be employed less efficiently than those of larger animals7. Since at least the 1980s2 there has been a drive to explain why. Most notably, Taylor et al.8 and others sought to explain their observation that during terrestrial locomotion the energy cost to apply a unit of force to the ground is higher in smaller animals. When terrestrial animals are on the move their primary energy cost comes from the need to support their body weight9,10, which is achieved through muscular contractions generating the vertical component of the ground reaction force each time a limb makes contact with the substrate. In absolute terms smaller animals have shorter legs (Kram and Taylor 1990) and therefore higher stride frequencies than do larger animals11,12,13,14, and in turn the duration that their feet are in contact with the ground per step is lower. Thus smaller animals must apply the necessary ground reaction forces more quickly, which is believed to require the employment of muscle fibres that are less energetically efficient9,15,16.
While the relationship between the energy costs of transport and body mass is strong (e.g. r2 = 0.8517) at any given mass there is a several-fold range in metabolic costs per unit distance (net cost of transport; NCOT) between species while walking on the laboratory treadmill1,4,18,19. For example, both the wild turkey and the marabou stork weigh around 4 kg, yet the former consumes 0.41 mlO2 kg−1 per m20 while the stork consumes around eight times that amount21. Given that locomotion costs can represent a substantial proportion of an animal’s daily energy budget22,23,24,25,26,27, these large differences in energy expenditure between species are surprising. From the perspective of optimal foraging28,29 and energy allocation theories30,31, such vast variability seems counter to the concept that animals have evolved to economise their daily energy expenditure, within the constraints of their ecology, in order to maximise the energy they have remaining, which can be directed towards reproduction32. In turn, this variability calls into question the importance of energetics, or at least locomotory energy expenditure, in shaping an animal’s ecology33. Yet there has been little research into the ecological implications of these differences in locomotion cost exhibited by similarly sized species.
It has been known for a long time that penguins have a high cost of transport34,35. Initially, this was believed explicable by their waddling gait. However, Griffin and Kram36 posited that penguins expend a lot of energy when walking because of their relatively short hind limbs resulting in low foot contact times, and thus high rates of force production9. They therefore argued that the proposed explanation for the greater relative NCOT in smaller animals might also explain the greater relative NCOT in the short-legged penguins4. By extension, the inference has been made that in general, species exhibiting a high NCOT for their size might have relatively short legs and, conversely, animals that display particularly good energy economies tend to have long legs (see also11). Kram and Taylor’s (1990) function relating metabolic rate of locomotion to foot contact time has successfully predicted gait-metabolic cost relationships in various contexts37,38,39. However, there has yet to be a substantial across-species analysis to test the hypothesis of a relationship between foot contact time and the cost of transport. Thus although it is often assumed to be the case, we do not know whether rate of force production describes variation in the cost of transport inter-specifically.
Limb morphology was included as an explicit determinant of locomotion energy costs in a biomechanical model (LiMb) developed by Pontzer40. The LiMb model recognises that energy expenditure during terrestrial locomotion, while mostly explained by the costs of generating the vertical component of the ground reaction force, also includes the costs of the horizontal component associated with cyclical braking and propulsion41 and the costs to swing the limbs42,43. The model was initially applied to an intra-specific analysis of human participants and performed better at predicting running metabolic rate than did foot contact time40. A similar conclusion arose from intra-specific analyses of two quadruped species: dogs and goats44.
The LiMb model requires the input of three parameters, two of which are the energy cost of swinging the limb and the excursion angle of the limb during the stance phase. Both of these are generally only obtainable under laboratory conditions. However, the model predicts that over a wide range of body sizes the other and most easily measured input parameter, effective limb length (the functional length of the limb as a mechanical strut), will accurately estimate locomotion costs because it is the only one of the three parameters that scales with body mass. Evidence supporting this prediction came from an across-species study; log-transformed effective limb length explained 98% of the variance in log-transformed mass-specific locomotor costs (as opposed to 94% explained by log-transformed body mass)45, in turn supporting aforementioned previous work suggesting that the magnitude and frequency of muscle forces generated to counter gravitational acceleration is a key determinant of NCOT across species36,37,38,39 (cf6).
However, despite this impressively high coefficient of variation, similarly to the aforementioned relationships between cost of transport and body mass, the use of log-transformed data across a wide mass range masks the absolute size of the residuals; a third of the residuals have a magnitude greater than 20%. Moreover, many comparisons between relevant pairs of species within the dataset do not support the premise that longer effective limbs associate with lower transport costs45. This might be explained by effective limb length not relating to rate of force production, for example because it does not account for the excursion angle of the limb (which as mentioned previously is an additional variable in the LiMb model); excursion angle dominates the variation in locomotor cost at the intraspecific level40,45. This proposed explanation is indirectly evidenced by an across-species regression, based on the present dataset, of relative effective limb length against rate of force production represented by relative foot contact time; accounting for speed, this regression returned a comparatively low R2 of 0.61 (Fig. 1).
An inter-specific regression of the residuals of log(effective limb length) on log(mass) against the residuals of log(1/contact time [tc]) on log(mass) and log(speed) from the dataset of the present study returned R2 = 0.61 (r = −0.78, N = 13). Data for effective limb length are from Pontzer45 and Halsey72; data for contact time are provided online (Supplementary).
Foot contact time is mathematically more closely associated with rate of force production against the ground than is effective limb length. Assuming that rate of force production is a key driver of NCOT across species then foot contact time is perhaps the proxy likely to associate best with variations in NCOT between similarly-sized species despite the great variation in morphologies and walking gaits they exhibit. At the least, it seems reasonable to re-asses this hypothesis, drawing upon the more extensive data sets for foot contact times and metabolic rates of terrestrial animals on the move that are now available. We collated these data to investigate whether, across species, rate of force production represented by foot contact time is predictive of a species’ locomotion costs. Using a phylogenetically informed approach, we focussed on birds and mammals (since data for these taxa are by far the most prevalent) to test for an interspecific relationship between metabolic rate during locomotion and foot contact time, while also recognising and accounting for the relationships between each of these variables and both body mass and locomotion speed, using phylogenetic mixed models to formally incorporate the non-independence associated with multiple measurements of a single species. We then expanded the analysis to consider additional predictors of interspecific variation in NCOT, testing for correlations between NCOT and a host of putative eco-physiological correlates, to examine the ecological relevance of NCOT beyond the laboratory. For this analysis we focussed on mammals to take advantage of the rich data sets published by Jones et al.46 and multiple other sources24,47,48,49,50,51,52.
Materials and Methods
A total of 288 observations of mass-specific rate of oxygen consumption (\({\dot{V}}_{{O}_{2}}\), ml kg−1 min−1) and 126 observations of foot contact time (tc, s) during pedestrian locomotion were obtained for 21 species (combining data for large leghorn and bantam leghorn chickens, and for musculus and musculus longshanks mice; Table 1) mainly from published studies9,15,34,36,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71. The species in this study represent a mass range of 32 g to 467 kg. Previously unpublished kinematic data for king penguins are included as part of the present study. In most cases collation of data from the literature required data extraction from digitised versions of published figures. We used data for \({\dot{V}}_{{O}_{2}}\) (i.e. rather than NCOT) to test for the relationship between transport costs and 1/tc because the relationship between speed and 1/tc is not always linear, and it is therefore not possible to calculate species-specific speed-independent values for foot contact times. Because the y-intercept of the relationship between \({\dot{V}}_{{O}_{2}}\) and speed is greater than zero and typically also greater than resting metabolic rates e.g.72, for each data set we calculated a linear regression relating \({\dot{V}}_{{O}_{2}}\) and speed, and subtracted the y-intercept of this relationship from each value of \({\dot{V}}_{{O}_{2}}\) in the data set, following previous studies (e.g15.). Our data for \({\dot{V}}_{{O}_{2}}\) therefore represent only the incremental increases in \({\dot{V}}_{{O}_{2}}\) associated with increases in speed.
Data for NCOT were obtained from a published compilation4. Data for putative eco-physiological correlates of NCOT were obtained from the PanTheria database46, namely: home range size, geographical range, group size, terrestriality, diet breadth, trophic level and habitat breadth. Additional variables were obtained from other sources: basal metabolic rate47, field metabolic rate48, maximum aerobic metabolic rate49, daily movement distance24, maximum running speed50, and body fat51,52. Absolute aerobic scope was calculated as the difference between maximum aerobic metabolic rate and basal metabolic rate. Factorial aerobic scope was calculated as the ratio of maximum aerobic metabolic rate to basal metabolic rate. Activity metabolic rate was calculated as the difference between field metabolic rate and basal metabolic rate73,74.
The speed- and size-independent relationship between log10-transformed mass-specific rate of oxygen consumption (\({\dot{V}}_{{O}_{2}}\), ml kg−1 min−1) and log10-transformed 1/tc (s−1) was analysed using multivariate phylogenetic mixed models75,76,77. Phylogenetic mixed models were implemented in the ASReml-R v3.078 package of R v3.0.279, with inverse relatedness matrices calculated from phylogenetic covariance matrices using the MCMCglmm package v2.2180. Phylogenetic mixed models were selected over the more commonly used methods of independent contrasts81,82 and phylogenetic generalised least squares82,83 because the former can formally incorporate phylogenetic non-independence as well as non-independence associated with multiple measurements of single species (i.e. measurements of \({\dot{V}}_{{O}_{2}}\) and tc for a single species running at multiple speeds). Phylogenetic mixed models are an analogue of the mixed model from quantitative genetics, which partitions phenotypes of related individuals into heritable (additive genetic) and non-heritable components to estimate inter-specific variances and covariances between traits77.
We used multivariate phylogenetic mixed models to examine the phylogenetic correlation among the traits \({\dot{V}}_{{O}_{2}}\) and 1/tc, accounting for either body mass or speed, or accounting for both body mass and speed (a phylogenetic correlation between two traits represents the proportion of variance that these two traits share due to phylogenetic relatedness, after accounting for any fixed effects in the model). The multivariate models included log10(\({\dot{V}}_{{O}_{2}}\)) and log10(1/tc) as response variables, either or both of log10-transformed speed (U, m s−1) and log10-transformed body mass (M, kg) as fixed effects, and phylogenetic relatedness as a random effect (it was not possible to include species identity as a random effect in the multivariate models because there was insufficient data to fit covariance matrices for both phylogenetic relatedness and species identity). Completely parameterised (unstructured) (co)variance matrices were specified for the random effect associated with the phylogeny, as well as the residuals. The significance of phylogenetic correlations between log10(\({\dot{V}}_{{O}_{2}}\)) and log10(1/tc), conditioned on the fixed effects, were inferred by determining if the phylogenetic covariance between log10(\({\dot{V}}_{{O}_{2}}\)) and log10(1/tc) differed significantly from zero by using a likelihood ratio test to compare models with and without the appropriate covariances fixed at zero. Approximate standard errors for phylogenetic correlations were calculated using the R ‘pin’ function84.
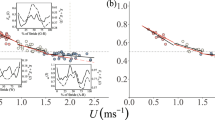
Before running the multivariate phylogenetic mixed model, we first visually verified that the relationships between log10(U) and both of log10(\({\dot{V}}_{{O}_{2}}\)) and log10(1/tc) were approximately linear for all species (Fig. 2), and used univariate phylogenetic mixed models to test for significant interactions between the fixed effects of log10(U) and log10(M) for both log10(\({\dot{V}}_{{O}_{2}}\)) and log10(1/tc). The significance of fixed effects was tested using Wald-type F-tests with conditional sums of squares and denominator degrees of freedom calculated according to Kenward and Roger85. Phylogenetic heritability (h2), a measure of phylogenetic correlation equivalent to Pagel’s86 λ77, was estimated as the proportion of variance attributable to the random effect of phylogeny; the proportion of variance attributable to species identity independent of phylogeny was also calculated. The significance of phylogenetic heritability and the random effect of species identity were assessed using likelihood ratio tests to compare models with and without the random effects. Approximate standard errors for the estimate of phylogenetic heritability were calculated using the R ‘pin’ function84.
Relationships between (A) inverse of foot contact time and (B) rate of oxygen consumption, against locomotion speed. Each line in each panel represents an individual data set for a distinct species (N = 21), which are coloured by body mass from small (blue) to large (orange). Note that data are shown on log-transformed axes. The data set includes a total of 414 observations, and all data are provided in the online information.
To test for correlations between NCOT and each of the putative eco-physiological correlates of NCOT, because there was only a single value per species we used the phylogenetic generalised least squares (PGLS) method82,83 and the ‘ape’ v3.287 and ‘caper’ v0.5.288 packages of R v3.0.279, with Pagel’s λ86 estimated using maximum likelihood. Body mass was included as a covariate in all analyses, and all data were log10-transformed for analysis. The PGLS method is equivalent to univariate phylogenetic mixed models when only one response value per species is analysed, as is the case for NCOT data.
The phylogeny used for all analyses was obtained from the open tree of life89 using R v3.2.2 and the R package ‘rotl’90, with branch lengths estimated using the arbitrary method of Grafen83 in ‘ape’ v3.287.
Parameter and variance component estimates are shown ± SE, unless otherwise noted, and α was set at 0.05 for all tests. Multiple comparison corrections were not performed91.
Results
Assessed using univariate phylogenetic mixed models, there was no interaction between log10(M) and log10(U) as predictors of log10(\({\dot{V}}_{{O}_{2}}\)) (Table 2) or log10(1/tc) (Table 3) during treadmill locomotion, indicating that there is no need to include an interaction terms in the multivariate phylogenetic mixed model. With only the additive effects of log10(M) and log10(U) included, there was no significant phylogenetic heritability for log10(\({\dot{V}}_{{O}_{2}}\)). However, there was significant among-species variation unrelated to phylogeny (Table 4), indicating that there are consistent mass- and speed-independent differences among-species in log10(\({\dot{V}}_{{O}_{2}}\)) that are not related to patterns of phylogenetic relatedness (i.e. related species are not more similar than unrelated species). Conversely, for log10(1/tc) there was significant phylogenetic heritability but no significant among-species variation unrelated to phylogeny (Table 5); this indicates that there are significant speed- and mass-independent differences in log10(1/tc) among species, but that related species are more similar than unrelated ones.
Assessing multivariate phylogenetic mixed models, the phylogenetic correlation between log10(\({\dot{V}}_{{O}_{2}}\)) and log10(1/tc) was positive and significantly different from zero when accounting for log10(M) only (r = 0.87 ± 0.08, χ21 = 17.1, P < 0.001, Fig. 3A) and when accounting for log10(U) only (r = 0.64 ± 0.13, χ21 = 9.64, P = 0.002, Fig. 3B). Thus, on a logarithmic scale, \({\dot{V}}_{{O}_{2}}\) relates to 1/tc when accounting for body mass, or when accounting for speed. The correlation was not significantly different from zero when both log10(M) and log10(U) were accounted for (r = −0.02 ± 0.27, χ21 = 0.003, P = 0.95, Fig. 3C). Thus \({\dot{V}}_{{O}_{2}}\) no longer relates to 1/tc when both body mass and speed are accounted for.
Relationships between log10(\({\dot{V}}_{{O}_{2}}\)) and log(1/contact time [tc]), accounting for (A) log10(mass), (B) log10(speed), and (C) both log10(mass) and log10(speed). \({\dot{V}}_{{O}_{2}}\) has units of ml kg−1 min−1 and tc has units of s. Each point is the best linear unbiased predictor (BLUP) for the random effect of phylogeny for each species (N = 21), shown ±SE, which provides shrunken estimates of the differences between terms and the overall means. The BLUP values are used for visualisation, and quantify the phylogenetic component of each species’ deviation from the overall means; a positive relationship between the BLUPs for log10(\({\dot{V}}_{{O}_{2}}\)) and log10(1/tc) indicates that, once the fixed effects are accounted for, species that evolve a high log10(\({\dot{V}}_{{O}_{2}}\)) also evolve a high log10(1/tc), and vice versa. The choice of x and y axes for these visualisations is arbitrary. The phylogenetic correlations in panels (A) and (B) are significant (r = 0.87 and 0.64, respectively, P ≤ 0.002), the correlation in panel (C) is not (r = −0.02, P = 0.95).
None of the eco-physiological traits were significantly related to NCOT when body mass was accounted for (Table 6).
Discussion
Most explanations for animal scaling of mechanical efficiency during locomotion are premised on the concept that energy costs of locomotion derive primarily from the muscular force to support the body and, particularly for larger animals, to accelerate the body as it oscillates through the step cycle6,40. Smaller animals have higher stride frequencies due to their shorter limbs, which necessitate higher rates of force generation due to shorter foot contact times9. This requires the recruitment of faster, less efficient muscle fibres, which demand higher rates of cross-bridge cycling and Ca2+ pumping, and thus use more ATP per gram of active muscle16,92,93. Consequently, at a higher stride frequency more energy is expended to deliver the necessary ground reaction force to support the body. Superficially, this theory appears to explain the present results, because foot contact time – a proxy for rate of force production – describes variation in the energy cost of locomotion between species of different sizes locomoting at the same speed (Fig. 3B), as well as of the same size moving at different speeds (Fig. 3A). However, the among-species relationship disappears when both speed and body size are accounted for simultaneously (Fig. 3C). This finding indicates that the apparent relationships among speeds (Fig. 3A) and sizes (Fig. 3B) arise because of the independent effects of speed and size on both foot contact time (Fig. 2A) and \({\dot{V}}_{{O}_{2}}\) (Fig. 2B), rather than because of an among-species link between (speed- and size-independent) foot contact time and \({\dot{V}}_{{O}_{2}}\). Stated another way, for animals of similar size locomoting at similar speeds, there is no among-species relationship between foot contact time and \({\dot{V}}_{{O}_{2}}\). In turn, this suggests, surprisingly, that rate of ground force production is not a substantive influence on NCOT between species. There must therefore be overriding, alternate factors at play determining the often considerable variation in energy cost of transport between species of similar size.
Not only does NCOT not relate inter-specifically to foot contact time, it also does not relate with any of the eco-physiological variables investigated, from home range size to running speed to body fat mass. While such data may be noisy, this consistent lack of correlative evidence raises the question as to whether NCOT – a measure obtained from prescribed and highly controlled protocols – is meaningful beyond the laboratory i.e. meaningful within an ecological context.
In the remainder of the Discussion we first consider what factors other than rate of force generation might explain variation in \({\dot{V}}_{{O}_{2}}\) between species during terrestrial locomotion, and then interpret and consider the implications for the lack of correlation between NCOT and all available eco-physiological variables.
Possible mechanisms underlying NCOT
The phylogenetically informed estimate of the scaling exponent for the relationship between log10(NCOT) and log10(body mass) is −0.284. Given that NCOT in that study, as in the current one, is presented in mass-specific terms, the whole-animal scaling exponent for NCOT is 1 −0.28 = 0.72, which is similar to the phylogenetically informed estimate of the scaling exponent of BMR of 0.69 ± 0.0147. This similarity in turn raises the question as to whether the scaling laws governing NCOT are the same as those governing BMR. However, similarity of scaling exponents does not mean that two traits are functionally related94, and our analyses found no association between NCOT and BMR once the effect of body mass on BMR was accounted for. Thus the relationship between NCOT and mass is not explained by the relationship between BMR and mass.
Morphological differences between species are likely to include the amount of active muscle that is applying force during periods of foot-ground contact1 (see also95). For example, birds have longer legs than do mammals and thus longer muscle fibres and a greater muscle volume, which can explain the considerably higher energy expenditure of birds for a given rate of force production15,96. Effective mechanical advantage based on the ratio of the anatomical moment arm of the muscles to the load arm of the ground force vector97 can also vary considerably between species, including when muscle fibre length has been accounted for98. Indeed, Pontzer et al.98 demonstrate that step length, a strong proxy for foot contact time9, is only a limited predictor of NCOT across (eight) species of birds and mammals, arguing that this is because of diversity within those species of muscle fibre length and effective mechanical advantage. Similar effective limb lengths in animal species can be represented by diverse arrangements of the limb skeletal structure resulting in differing degrees of upright or crouched stance; the more upright the limb (the more erect the animal’s posture) the lower the cross-sectional area of muscle required to generate sufficient force to counter gravity37,99. There may also be differences between species in the relative shortening velocity of active muscle. For example, due to the force-velocity relationship of skeletal muscle, in cases where the fibres are operating at lower shortening velocities a smaller cross-sectional area of muscle would need to be activated to provide the same force as muscles operating at higher shortening velocities15,100. Recent work by Cavagna and Legramandi101 provides a new approach to considering the biomechanics underpinning the scaling of locomotion efficiencies with size. They demonstrated that there is variability in the hysteresis energy loss experienced by animals during the lower part of the vertical oscillation of the centre of mass. While muscle-tendon units are undergoing their stretch-shortening cycle, in heavier animals a greater role is played by more elastic tendons at the expense of less elastic muscles. Pontzer102 also argues for a graded difference across body mass in the mechanics underlying locomotion energy costs, but through a different pathway. He presents a model which indicates that force production becomes a smaller part of the cost, and mechanical work a greater part of the cost, as animals become larger.
In conclusion, while briefer ground-foot contact times may require faster, less energetically efficient muscle fibres, this mechanism appears insufficient to explain speed- and size-independent differences in the energy cost of locomotion between species. Indeed, an interpretation of recent work by Gutmann and Bertram (2017)103 is that if species of a similar size have very different stride frequencies at a given locomotion speed, as is apparent from data presented by54, then Kram and Taylor’s (1990) relationship between metabolic rate and foot contact time should not hold. Instead, the volume of muscle activated per step44, variations in effective mechanical advantage for instance due to posture102, muscle contraction efficiency in terms of mechanical power104, and/or bigger animals having a lower energy loss by hysteresis during each step101, may explain an important proportion of the variation in residual movement costs. Sufficient information across species on duty factor, limb length, musculature and moment arms along with kinematic information will be necessary to investigate the covariance of these factors with the energy cost of locomotion across species. Alternatively, the considerable diversity in the species represented in inter-specific scaling relationships of NCOT perhaps hints that, for any given species, there could be a plethora of reasons explaining its relatively high or low costs of transport and, furthermore, these could be idiosyncratic. Consequently, mechanisms influencing the energy cost of locomotion beyond mass and, for example, effective limb length may not be generalizable.
Ecological correlates with NCOT
For many terrestrial species, the amount of energy they expend to locomote substantially decreases the energy remaining that they can channel into reproduction105,106,107. Thus animals are expected to optimise the efficiency of their energy expenditure during movement, within certain constraints108. In turn, we might predict correlations between species ecology and energy costs of transport. Yet our analysis on mammals found no relationships between NCOT and a host of ecological variables once body mass, which accounts for the fact that bigger animals have a lower NCOT and can range further102, was accounted for. Effectively, our analyses show that for different species of the same size, home range size, daily movement distance, geographic range, group size, terrestriality, diet breadth, trophic level and habitat breadth do not relate to NCOT. Furthermore, despite the fact that animals which can run faster or more energetically cheaply perhaps have the opportunity to roam more extensively and those with greater energy output might be predicted to carry greater energy stores to buffer against short falls109, neither maximum running speed nor percentage body fat levels were related to NCOT. Finally, NCOT was also not related to FMR, MMR, AMR or aerobic scope.
Thus NCOT appears to be disconnected from animal foraging behaviour, broader measures of energetics and varying aspects of ecology. This either calls into question the importance of locomotion energy expenditure in influencing an animal’s ecology, or the relevance of NCOT for understanding the locomotion energetics of terrestrial animals beyond the realms of biomechanics or comparisons among many animals of varying size. If either or both possibilities are true, in turn it is important to understand why NCOT has little ecological relevance. Pontzer110 argues that most extant terrestrial animals may have already evolved to be efficient foragers. Given the adaptive losses that these species would experience as a consequence of further enhancements to their locomotion efficiencies108, for example longer legs might reduce an animal’s capacity to accelerate97, there is little such selective pressure. Harris and Steudel111 report that details of prey pursuit and capture are the factors that describe hind limb length, in contrast finding no predictive power in home range size or daily movement distance. Perhaps other physical factors that influence locomotion energy efficiencies, such as leg muscle mass, for similar reasons are also under minimal selection for enhancement in terms of energy efficiencies, particularly in species for which locomotion costs are a relatively small proportion of their total energy expenditure112. The possibility that NCOT is simply a poor representation of an animal’s energy costs of locomotion is particularly supported in the present study by the lack of relationship between NCOT and field metabolic rate or activity metabolic rate. NCOT, calculated as the slope of the linear fit between rate of energy expenditure and locomotion speed, tends towards the total energy costs of locomotion per unit distance at high running speeds33. However, the majority of movements by animals are conducted at low speeds relative to their maximum obtainable113,114,115,116. Under these circumstances, per unit distance the cost of transport is higher than indicated by NCOT due to fixed costs associated with locomotion33, most notably the ‘postural cost of transport’ PCOT72. Furthermore, animals often incur additional energy costs while walking associated with, for example, intermittent locomotion117, turning118, and negotiating various terrains e.g. Fig. 4B in119. NCOT therefore underestimates an animal’s true energy costs to move, and the magnitude of this error presumably varies between species depending upon factors such as their typical running speeds and overall movement behaviours.
To progress our understanding of the energetic costs and constraints for terrestrial animals traversing their habitats, if and how these costs help shape their ecology, and how those costs relate to their locomotion characteristics, perhaps now it is time to step off the laboratory treadmill and measure transport costs in the wild33,120,121,122.
Data Availability
We have made the data collated for the present study available on Dryad.
References
Full, R., Zuccarello, D. & Tullis, A. Effect of variation in form on the cost of terrestrial locomotion. J Exp Biol 150, 233–246 (1990).
Taylor, C., Heglund, N. & Maloiy, G. Energetics and mechanics of terrestrial locomotion. I. Metabolic energy consumption as a function of speed and body size in birds and mammals. J Exp Biol 97, 1–21 (1982).
Taylor, C., Schmidt-Nielsen, K. & Raab, J. Scaling of energetic cost of running to body size in mammals. Am. J. Physiol. 219, 1104–1107 (1970).
White, C. R., Alton, L. A., Crispin, T. S. & Halsey, L. G. Phylogenetic comparisons of pedestrian locomotion costs: confirmations and new insights. Ecology and Evolution, in press (2016).
Halsey, L. G. & White, C. R. A different angle: comparative analyses of whole-animal transport costs running uphill. J. Exp. Biol. 220, 161–166 (2017).
Reilly, S. M., McElroy, E. J. & Biknevicius, A. R. Posture, gait and the ecological relevance of locomotor costs and energy-saving mechanisms in tetrapods. Zoology 110, 271–289, https://doi.org/10.1016/j.zool.2007.01.003 (2007).
Heglund, N. C., Fedak, M., Taylor, C. & Cavagna, G. Energetics and mechanics of terrestrial locomotion. IV. Total mechanical energy changes as a function of speed and body size in birds and mammals. J. Exp. Biol. 97, 57–66 (1982).
Taylor, C. R., Heglund, N. C., McMahon, T. A. & Looney, T. Energetic cost of generating muscular force during running. J. Exp. Biol. 86, 9–18 (1980).
Kram, R. & Taylor, C. R. Energetics of running: a new perspective. Nature 346, 265–267 (1990).
Taylor, C. R. Relating mechanics and energetics during exercise. Advances in veterinary science and comparative medicine 38, 181–215 (1994).
Heglund, N. C. & Taylor, C. R. Speed, stride, frequency and energy cost per stride: how do they change with body size and gait? J. Exp. Biol. 138, 301–318 (1988).
Williams, T. M. Locomotion in the North American mink, a semi-aquatic mammal. II. The effect of an elongate body on running energetics and gait patterns. J. Exp. Biol. 105, 283–295 (1983).
Bhambhani, Y. & Singh, M. Metabolic and cinematographic analysis of walking and running in men and women. Med. Sci. Sports Ex. 17, 131–137 (1985).
Lees, J. J., Nudds, R., Folkow, L., Stokkan, K.-A. & Codd, J. R. Understanding sex differences in the cost of terrestrial locomotion. Proceedings of the Royal Society B: Biological Sciences 279, 826–832, https://doi.org/10.1098/rspb.2011.1334 (2011).
Roberts, T., Kram, R., Weyand, P. & Taylor, C. R. Energetics of bipedal running. I. Metabolic cost of generating force. J. Exp. Biol. 201, 2745–2751 (1998).
Tokui, M. & Hirakoba, K. Estimation of oxygen cost of internal power during cycling exercise with changing pedal rate. Journal of physiological anthropology 27, 133–138 (2008).
White, C. R., Alton, L. A., Crispin, T. S. & Halsey, L. G. Phylogenetic comparisons of pedestrian locomotion costs: confirmations and new insights. Ecology and evolution 6, 6712–6720 (2016).
Full, R. J. In Energy transformations in cells and animals (eds Wieser, W. & Gnaiger, E.) 175–182 (Georg Thieme Verlag, 1989).
Kram, R. Taylor’s treadmill menagerie. J. Exp. Biol. 215, 2349–2350, https://doi.org/10.1242/jeb.062778 (2012).
Parsons, P. E. & Taylor, C. R. Energetics of brachiation versus walking: a comparison of a suspended and an inverted pendulum mechanism. Physiol. Zool. 50, 182–188 (1977).
Bamford, O. S. & Maloiy, G. M. O. Energy metabolism and heart rate during treadmill exercise in the Marabou stork. J. Appl. Physiol.: Respirat. Environ. Excerise Physiol. 49, 491–496 (1980).
Scantlebury, D. M. et al. Flexible energetics of cheetah hunting strategies provide resistance against kleptoparasitism. Science 346, 79–81, https://doi.org/10.1126/science.1256424 (2014).
Speakman, J. R. & Selman, C. Physical activity and resting metabolic rate. Proc. Nutr. Soc. 62, 621–634, https://doi.org/10.1079/PNS2003282 (2003).
Garland, T. Jr. Scaling the ecological cost of transport to body mass in terrestrial mammals. Am. Nat. 121, 571–587 (1983).
Rezende, E. L., Gomes, F. R., Chappell, M. A. & Garland, T., Jr. Running behavior and its energy cost in mice selectively bred for high voluntary locomotor activity. Physiol. Biochem. Zool. 82, 662–679, 10.1086/605917 (2009).
Gefen, E. The relative importance of respiratory water loss in scorpions Is correlated with species habitat type and activity pattern. Physiol. Biochem. Zool. 84, 68–76, 10.1086/657688 (2011).
Halsey, L. G., Matthews, P. G. D., Rezende, E. L., Chauvaud, L. & Robson, A. A. The interactions between temperature and activity levels in driving metabolic rate: theory, with empirical validation from contrasting ectotherms. Oecologia 177, 1117–1129, https://doi.org/10.1007/s00442-014-3190-5 (2015).
Gils, J. A. V. & Tijsen., W. Short‐Term Foraging Costs and Long‐Term Fueling Rates in Central‐Place Foraging Swans Revealed by Giving‐Up Exploitation Times. Am. Nat. 169, 609–620, https://doi.org/10.1086/513114 (2007).
Houston, A. I. & McNamara, J. M. Foraging currencies, metabolism and behavioural routines. J. Anim. Ecol. 83, 30–40, https://doi.org/10.1111/1365-2656.12096 (2014).
Stearns, S. C. The evolution of life histories. Vol. 249 (Oxford University Press Oxford, 1992).
Sibly, R. M. & Calow, P. Physiological ecology of animals. (Blackwell Scientific Publications, 1986).
Kooijman, S. A. & Lika, K. Comparative energetics of the 5 fish classes on the basis of dynamic energy budgets. Journal of Sea Research 94, 19–28 (2014).
Halsey, L. Terrestrial movement energetics: current knowledge and its application to the optimising animal. J. Exp. Biol. 219, 1424–1431 (2016).
Pinshow, B., Fedak, M. & Schmidt-Nielsen, K. Terrestrial locomotion in penguins: it costs more to waddle. Science 195, 592–594 (1977).
Dewasmes, G., Le Maho, Y., Cornet, A. & Groscolas, R. Resting metabolic rate and cost of locomotion in long-term fasting emperor penguins. J. Appl. Physiol.: Respirat. Environ. Excerise Physiol. 49, 888–896 (1980).
Griffin, T. M. & Kram, R. Penguin waddling is not wasteful. Nature 408, 929 (2000).
Sih, B. L. & Stuhmiller, J. H. The metabolic cost of force generation. Med. Sci. Sports Ex. 35, 623–629 (2003).
Kram, R. & Dawson, T. Energetics and biomechanics of locomotion by red kangaroos. Comparative Biochemistry and Physiology Part B 120, 41–49 (1998).
Minetti, A. E., Boldrini, L., Brusamolin, L., Zamparo, P. & McKee, T. A feedback-controlled treadmill (treadmill-on-demand) and the spontaneous speed of walking and running in humans. J. Appl. Physiol. 95, 838–843 (2003).
Pontzer, H. A new model predicting locomotor cost from limb length via force production. J Exp Biol 208, 1513–1524, https://doi.org/10.1242/jeb.01549 (2005).
Gottschall, J. S. & Kram, R. Energy cost and muscular activity required for propulsion during walking. J. Appl. Physiol. 94, 1766–1772 (2003).
Marsh, R., Ellerby, D., Carr, J., Henry, H. & Buchanan, C. Partitioning the energetics of walking and running: swinging the limbs is expensive. Science 303, 80–83 (2004).
Tickle, P. G., Richardson, M. F. & Codd, J. R. Load carrying during locomotion in the barnacle goose (Branta leucopsis): The effect of load placement and size. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 156, 309–317 (2010).
Pontzer, H. Predicting the energy cost of terrestrial locomotion: a test of the LiMb model in humans and quadrupeds. J. Exp. Biol. 210, 484–494 (2007).
Pontzer, H. Effective limb length and the scaling of locomotor cost in terrestrial animals. J. Exp. Biol. 210, 1752–1761 (2007).
Jones, K. E. et al. PanTHERIA: a species-level database of life history, ecology, and geography of extant and recently extinct mammals. Ecology 90, 2648–2648, https://doi.org/10.1890/08-1494.1 (2009).
Sieg, A. E. et al. Mammalian metabolic allometry: do intraspecific variation, phylogeny, and regression models matter? Am. Nat. 174, 720–733 (2009).
Speakman, J. R. & Król, E. Maximal heat dissipation capacity and hyperthermia risk: neglected key factors in the ecology of endotherms. J. Anim. Ecol. 79, 726–746 (2010).
Dlugosz, E. M. et al. Phylogenetic analysis of mammalian maximal oxygen consumption during exercise. J. Exp. Biol. 216, 4712–4721 (2013).
Garland, T. Jr. The relation between maximal running speed and body mass in terrestrial mammals. Journal of Zoology 199, 157–170 (1983).
Pitts, G. C. & Bullard, T. R. In Body composition in animals and man (eds Reid, J. T., Bensadoun, A. & Bull, L. S.) (National Academy of Science, 1968).
Navarrete, A., Van Schaik, C. P. & Isler, K. Energetics and the evolution of human brain size. Nature 480, 91–93, https://doi.org/10.1038/nature10629 (2011).
Fedak, M. A., Pinshow, B. & Schmidt-Nielsen, K. Energy cost of bipedal running. American Journal of Physiology–Legacy Content 227, 1038–1044 (1974).
Hoyt, D., Wickler, S. J. & Cogger, E. A. Time of contact and step length: the effect of limb length, running speed, load carrying and incline. J. Exp. Biol. 203, 221–227 (2000).
Thompson, S. D., MacMillen, R. E., Burke, E. M. & Taylor, C. R. The energetic cost of bipedal hopping in small mammals. Nature 287, 223–224 (1980).
Hoyt, D. F. & Kenagy, G. J. Energy costs of walking and running gaits and their aerobic limits in golden-mantled ground squirrels. Physiol. Zool. 61, 34–40 (1988).
Seeherman, H. J., Taylor, C. R., Maloiy, G. M. & Armstrong, R. B. Design of the mammalian respiratory system. II. Measuring maximum aerobic capacity. Resp. Physiol. 44, 11–23 (1981).
Cerretelli, P., Piiper, J., Mangili, F. & Ricci, B. Aerobic and anaerobic metabolism in exercising dogs. J. Appl. Physiol. 19, 25–28 (1964).
Hoyt, D. F. & Taylor, C. R. Gait and the energetics of locomotion in horses. Nature 292, 239–240 (1981).
Wickler, S. J., Hoyt, D. F., Cogger, E. A. & Myers, G. The energetics of the trot-gallop transition. J. Exp. Biol. 206, 1557–1564 (2003).
Watson, R. R. et al. Gait-specific energetics contributes to economical walking and running in emus and ostriches. Proceedings of the Royal Society B 278, 2040–2046 (2011).
Bransford, D. R. & Howley, E. T. Oxygen cost of running in trained and untrained men and women. Med. Sci. Sports 9, 41–44 (1977).
Webster, K. & Dawson, T. Locomotion energetics and gait characteristics of a rat-kangaroo, Bettongia penicillata, have some kangaroo-like features. Journal of Comparative Physiology B 173, 549–557 (2003).
Wilkinson, H., Thavarajah, N. & Codd, J. The metabolic cost of walking on an incline in the Peacock (Pavo cristatus). PeerJ 3, e987 (2015).
Fahlman, A. et al. Effect of fasting on the VO2-fH relationship in king penguins, Aptenodytes patagonicus. Am. J. Physiol. (Regulatory Integrative Comp. Physiol.) 287, R870–R877 (2004).
Lees, J., Folkow, L., Stokkan, K.-A. & Codd, J. The metabolic cost of incline locomotion in the Svalbard rock ptarmigan (Lagopus muta hyperborea): the effects of incline grade and seasonal fluctuations in body mass. The Journal of Experimental Biology 216, 1355–1363, https://doi.org/10.1242/jeb.078709 (2013).
Nudds, R. L. & Codd, J. R. The metabolic cost of walking on gradients with a waddling gait. The Journal of Experimental Biology 215, 2579–2585, https://doi.org/10.1242/jeb.071522 (2012).
White, C. R., Martin, G. & Butler, P. Pedestrian locomotion energetics and gait characteristics of a diving bird, the great cormorant, Phalacrocorax carbo. Journal of Comparative Physiology B 178, 745–754, https://doi.org/10.1007/s00360-008-0265-9 (2008).
Rose, K. A., Nudds, R. L. & Codd, J. R. Intraspecific scaling of the minimum metabolic cost of transport in leghorn chickens (Gallus gallus domesticus): links with limb kinematics, morphometrics and posture. The Journal of Experimental Biology 218, 1028–1034, https://doi.org/10.1242/jeb.111393 (2015).
Fish, F. E., Frappell, P. B., Baudinette, R. V. & MacFarlane, P. Energetics of terrestrial locomotion of the platypus Ornithorhynchus anatinus. J. Exp. Biol. 204, 797–803 (2001).
Sparrow, L. The effect of limb length on the cost of locomotion and gait in the Longshanks mouse, University of Calgary, (2015).
Halsey, L. G. The relationship between energy expenditure and speed during pedestrian locomotion in birds: a morphological basis for the elevated y-intercept? Comparative Biochemistry and Physiology A 165, 296–298, https://doi.org/10.1016/j.cbpa.2013.03.027 (2013).
Ricklefs, R. E., Konarzewski, M. & Daan, S. The relationship between basal metabolic rate and daily energy expenditure in birds and mammals. Am. Nat. 147, 1047–1071 (1996).
Portugal, S. J. et al. Associations between Resting, Activity, and Daily Metabolic Rate in Free-Living Endotherms: No Universal Rule in Birds and Mammals. Phys. Biochem. Zool. 89, 251–261 (2016).
Lynch, M. Methods for the analysis of comparative data in evolutionary biology. Evolution 45, 1065–1080, https://doi.org/10.2307/2409716 (1991).
Housworth, E. A., Martins, E. P. & Lynch, M. The phylogenetic mixed model. Am. Nat. 163, 84–96, https://doi.org/10.1086/380570 (2004).
Hadfield, J. D. & Nakagawa, S. General quantitative genetic methods for comparative biology: phylogenies, taxonomies and multi-trait models for continuous and categorical characters. J. Evol. Biol. 23, 494–508 (2010).
Gilmour, A. R., Gogel, B. J., Cullis, B. R. & Thompson, R. ASReml user guide. Release 3.0. (NSW Department of Industry and Investment, 2009).
R Development Core Team. (R Foundation for Statistical Computing, Vienna, Austria, 2013).
Hadfield, J. D. MCMC methods for multi-response generalized linear models: the MCMCglmm R Package. Journal of Statistical Software 33 (2010).
Felsenstein, J. Phylogenies and the comparative method. Am. Nat. 125, 1–15 (1985).
Rezende, E. L. & Diniz-Filho, J. A. F. Phylogenetic analyses: comparing species to infer adaptations and physiological mechanisms. Comprehensive Physiology 2, 639–674 (2012).
Grafen, A. The phylogenetic regression. Philosophical Transactions of the Royal Society of London B 326, 119–157 (1989).
White, I. R pin function. http://www.homepages.ed.ac.uk/iwhite//asreml/. (2013).
Kenward, M. G. & Roger, J. H. The precision of fixed effects estimated from restricted maximum likelihood. Biometrics 53, 983–997 (1997).
Pagel, M. Inferring the historical patterns of biological evolution. Nature 401, 877–884 (1999).
Paradis, E., Claude, J. & Strimmer, K. APE: Analyses of Phylogenetics and Evolution in R language. Bioinformatics 20, 289–290 (2004).
Caper: Comparative Analyses of Phylogenetics and Evolution in R. v. R package version 0.5.2. (2013).
Hinchliff, C. E. et al. Synthesis of phylogeny and taxonomy into a comprehensive tree of life. Proceedings of the National Academy of Sciences 112, 12764–12769, https://doi.org/10.1073/pnas.1423041112 (2015).
Michonneau, F., Brown, J. & Winter, D. rotl: Interface to the ‘Open Tree of Life’ API. R package version 0.5.0. https://CRAN.R-project.org/package=rotl. (2016).
Rothman, K. No adjustments are needed for multiple comparisons. Epidemiology 1, 43–46 (1990).
He, Z.-H., Bottinelli, R., Pellegrino, M. A., Ferenczi, M. A. & Reggiani, C. ATP Consumption and Efficiency of Human Single Muscle Fibers with Different Myosin Isoform Composition. Biophysical Journal 79, 945–961, https://doi.org/10.1016/S0006-3495(00)76349-1 (2000).
Brooks, H. P., Andrews, M. H., Gray, A. J. & Osborne, M. A. Comparison of models for the physiological estimation of internal mechanical power in Cycling. Journal of Science and Cycling 2, 58 (2013).
White, C. R. & Kearney, M. R. Metabolic scaling in animals: methods, empirical results, and theoretical explanations. Comprehensive Physiology 4, 231–256 (2014).
Kipp, S., Grabowski, A. M. & Kram, R. What determines the metabolic cost of human running across a wide range of velocities? The Journal of Experimental Biology 221, https://doi.org/10.1242/jeb.184218 (2018).
Roberts, T. J., Chen, M. S. & Taylor, C. R. Energetics of bipedal running. II. Limb design and running mechanics. J. Exp. Biol. 201, 2753–2762 (1998).
Biewener, A. Scaling body support in mammals: Limb posture and muscle mechanics. Science 245, 45–48 (1989).
Pontzer, H., Raichlen, D. A. & Sockol, M. D. The metabolic cost of walking in humans, chimpanzees, and early hominins. J. Hum. Evol. 56, 43–54, https://doi.org/10.1016/j.jhevol.2008.09.001 (2009).
Biewener, A. A. & Roberts, T. J. Muscle and tendon contributions to force, work, and elastic energy savings: a comparative perspective. Exercise and sport sciences reviews 28, 99–107 (2000).
Roberts, T. J., Marsh, R. L., Weyand, P. G. & Taylor, C. R. Muscular force in running turkeys: the economy of minimizing work. Science 275, 1113–1115 (1997).
Cavagna, G. A. & Legramandi, M. A. Running, hopping and trotting: tuning step frequency to the resonant frequency of the bouncing system favors larger animals. J. Exp. Biol. 218, 3276–3283, https://doi.org/10.1242/jeb.127142 (2015).
Pontzer, H. A unified theory for the energy cost of legged locomotion. Biol. Lett. 12, https://doi.org/10.1098/rsbl.2015.0935 (2016).
Gutmann, A. K. & Bertram, J. E. A. The apparently contradictory energetics of hopping and running: the counter-intuitive effect of constraints resolves the paradox. The Journal of Experimental Biology 220, 167–170, https://doi.org/10.1242/jeb.144832 (2017).
Curtin, N. A. et al. Remarkable muscles, remarkable locomotion in desert-dwelling wildebeest. Nature (2018).
Perrigo, G. Breeding and feeding strategies in deer mice and house mice when females are challenged to work for their food. Anim. Behav. 35, 1298–1316 (1987).
Perrigo, G. & Bronson, F. Sex differences in the energy allocation strategies of house mice. Behavioral Ecology and Sociobiology 17, 297–302 (1985).
Zhao, Z.-J., Król, E., Moille, S., Gamo, Y. & Speakman, J. R. Limits to sustained energy intake. XV. Effects of wheel running on the energy budget during lactation. The Journal of Experimental Biology 216, 2316–2327, https://doi.org/10.1242/jeb.078402 (2013).
Arnold, S. J. Morphology, performance and fitness. American Zoologist 23, 347–361 (1983).
Pontzer, H. et al. Metabolic acceleration and the evolution of human brain size and life history. Nature advance online publication, https://doi.org/10.1038/nature17654, http://www.nature.com/nature/journal/vaop/ncurrent/abs/nature17654.html#supplementary-information (2016).
Pontzer, H. Relating ranging ecology, limb length, and locomotor economy in terrestrial animals. J. Theor. Biol. 296, 6–12, https://doi.org/10.1016/j.jtbi.2011.11.018 (2012).
Harris, M. A. & Steudel, K. Ecological correlates of hind‐limb length in the Carnivora. Journal of Zoology 241, 381–408 (1997).
Baudinette, R. The energetics and cardiorespiratory correlates of mammalian terrestrial locomotion. J. Exp. Biol. 160, 209–231 (1991).
Pennycuick, C. On the running of the gnu (Connochaetes taurinus) and other animals. J. Exp. Biol. 63, 775–799 (1975).
Jayne, B. C. & Irschick, D. J. A field study of incline use and preferred speeds for the locomotion of lizards. Ecology 81, 2969–2983 (2000).
Perry, A. K., Blickhan, R., Biewener, A. A., Heglund, N. C. & Taylor, C. R. Preferred speeds in terrestrial vertebrates: are they equivalent? J. Exp. Biol. 137, 207–219 (1988).
O’Neill, M. C. Gait-specific metabolic costs and preferred speeds in ring-tailed lemurs (Lemur catta), with implications for the scaling of locomotor costs. Am J Phys Anthrop 149, 356–364, https://doi.org/10.1002/ajpa.22132 (2012).
Seethapathi, N. & Srinivasan, M. The metabolic cost of changing walking speeds is significant, implies lower optimal speeds for shorter distances, and increases daily energy estimates. Biol. Lett. 11, 20150486 (2015).
Wilson, R. et al. Turn costs change the value of animal search paths. Ecology Letters 16, 1145–1150, https://doi.org/10.1111/ele.12149 (2013).
Williams, T. M. et al. Instantaneous energetics of puma kills reveal advantage of felid sneak attacks. Science 346, 81–85 (2014).
Wilson, R., Quintana, F. & Hobson, V. Construction of energy landscapes can clarify the movement and distribution of foraging animals. Proc Roy Soc B 279, 975–980, https://doi.org/10.1098/rspb.2011.1544 (2011).
Lees, J., Gardiner, J., Usherwood, J. & Nudds, R. Locomotor preferences in terrestrial vertebrates: An online crowdsourcing approach to data collection. Scientific Reports 6 (2016).
Bidder, O. R. et al. Does the treadmill support valid energetics estimates of field locomotion? Integrative and comparative biology 57, 301–319 (2017).
Acknowledgements
We would like to recognise the valuable ideas and inspiration that supported the development of this article, which came from reading the PhD thesis of Kayleigh Rose, discussions with Jon Codd, and a conversation at the 2015 SICB conference with Robert Full. We are particularly grateful for the detailed suggestions generously provided by Herman Pontzer after reading a draft of this article, and email correspondence with John Bertram. CRW was supported by an Australian Research Council Future Fellowship (project FT130101493).
Author information
Authors and Affiliations
Contributions
L.G.H. and C.R.W. designed the study. L.G.H. and C.R.W. compiled the data. C.R.W. analysed the data and prepared the figures. L.G.H. and C.R.W. wrote the paper.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Halsey, L.G., White, C.R. Terrestrial locomotion energy costs vary considerably between species: no evidence that this is explained by rate of leg force production or ecology. Sci Rep 9, 656 (2019). https://doi.org/10.1038/s41598-018-36565-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-36565-z
This article is cited by
-
Leg loss decreases endurance and increases oxygen consumption during locomotion in harvestmen
Journal of Comparative Physiology A (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.