Abstract
To evaluate the corneal spherical aberrations in cataract patients with and without high myopia, we conducted a retrospective case series of 502 cataract eyes with high myopia and 1500 age-related cataract eyes and measure their corneal biometric data and axial length using Pentacam and IOLMaster. Both the anterior and total corneal primary spherical aberrations were lower in the high myopia group than that in the control group (0.317 ± 0.215 vs 0.338 ± 0.148 μm, P = 0.043; and 0.281 ± 0.207 vs 0.314 ± 0.153 μm, P < 0.001). The incidence of eyes with negative total corneal primary spherical aberration increased as axial length increased in the high myopia group, and the overall incidence was higher in the high myopia group than that in the control group (2.59% vs 1.47%). These were mainly contributed to the younger age of cataract patients with high myopia (55.76 ± 13.10 vs 60.18 ± 15.72 years, P < 0.001), along with the positive correlations between age and anterior and total corneal primary spherical aberrations. In clinical practice, an aspheric IOL with a low negative or zero primary spherical aberration is recommended for cataract patients with high myopia. Negative total ocular primary spherical aberrations resulting from aspheric IOL implantation should be avoided in extremely high myopic eyes.
Similar content being viewed by others
Introduction
Intraocular lenses (IOLs) were designed to add spherical refractive power to the ocular optical system from which the neutral lenses had been removed. Further refinements were made to mimic specific ocular properties, such as the development of aspheric IOLs to compensate for positive corneal spherical aberrations1. The reduction of ocular spherical aberrations can lead to a better retinal image and optimized visual performance, even when the best corrected visual acuity remains the same2,3.
The designs of aspheric IOLs are based on the average corneal spherical aberrations reported in large-scale clinical studies to achieve ideal outcomes for subjects with ocular aspherical aberrations. However, few studies have investigated the distribution of corneal spherical aberrations in cataract patients with high myopia, so the suitability of aspheric IOLs for these patients has not been demonstrated.
The global prevalence of high myopia was estimated to be 163 million individuals (2.7% of the world population and 11.6% of all myopia patients) in 2000 and was predicted to be 938 million (9.8% of the world population and 19.7% of all myopia patients) by 20504. We have also identified an increasing trend in the proportion of subjects with high myopia cataract compared with age-related cataract in our clinical practice in recent years.
In general, cataract patients with high myopia are well educated and have high expectations of their postoperative subjective and objective visual performance (clearer, more comfortable, and longer lasting vision). Both patients and surgeons aspire not only to improvements in visual acuity and contrast sensitivity, but also to the relief and elimination of visual complaints such as starbursts and glare5.
Yu et al. compared the spherical aberrations after the implantation of aspheric and spherical IOLs in highly myopic subjects and found that aspheric IOLs were helpful6. However, the exact distribution of corneal spherical aberrations in highly myopic patients is still unknown. Considering the increasing prevalence of high myopia around the world and the high expectations of society overall, there is an obvious need for ophthalmologists to determine this distribution.
Consequently, in this study, we (1) determined the distributions of primary spherical aberrations in cataract patients with high myopia; (2) compared the corneal spherical aberrations in control cataract patients and cataract patients with high myopia; and (3) identified the main factors associated with spherical aberrations in highly myopic patients. We evaluated these factors and confirmed the effectiveness of aspheric IOL implantation during cataract surgery in patients with high myopia. Some suggestions are also made for the proper choice of aspheric IOLs.
Methods
In this retrospective study, we recruited patients scheduled for cataract surgery from July 10 to December 21, 2017, at the Eye and ENT Hospital of Fudan University, Shanghai, China. The inclusion criteria were age-related or high myopia cataract and the ability to understand and sign the informed consent form. Corneal biometric data and axial length were determined in all the patients. Patients with a history of previous ocular trauma or surgery, diagnosed dry eye disease, corneal comorbidities, such as uveitis or glaucoma, fundus abnormalities, or diabetic retinopathy, or who had worn contact lenses within the previous 2 weeks, were excluded. The patients were divided into two groups according to their axial length (cutoff = 26 mm): cataract patients with high myopia (high myopia group) and cataract patients without high myopia (control group). This study was approved by the Human Research Ethics Committee of the Eye and ENT Hospital of Fudan University and adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from each patient.
All data were collected in auto-mode, centered at corneal apex, with a rotating Scheimpflug camera (Pentacam; Oculus, Wetzlar, Germany) and partial coherence interferometry (IOLMaster; Carl Zeiss Meditec, Jena, Germany) by each of two skillful examiners to eliminate any latent examiner bias as far as possible. The examination was considered valid only if the quality expressed by the software was “OK”. Each patient’s last acceptable reading was used for the subsequent analysis. The measurements made at a pupil scan of 6 mm diameter included steep keratometric power, anterior and posterior corneal astigmatism, central corneal thickness (CCT), eccentricity (Ecc), Q value and the primary spherical aberration (Z 4 0) of the total cornea, anterior corneal surface, and posterior corneal surface. Conic coefficients were collected at a pupil scan of 8 mm diameter: Index of Surface Variance (ISV), Index of Vertical Asymmetry (IVA), Keratoconus Index(KI), Center Keratoconus Index (CKI), Index of Height Asymmetry (IHA), and Index of Height Decentration (IHD). The acquired corneal aberration data sets were expanded with normalized Zernike polynomials, with the magnitudes of their coefficients represented as the root mean square (in micrometers) and used to indicate wavefront aberrations. With-the-rule (WTR) astigmatism was defined as a cylindrical error for a steep corneal meridian of 90° ± 30° and against-the-rule (ATR) astigmatism was defined as a cylindrical error for a steep corneal meridian of 0° ± 30°. All other astigmatic readings outside these parameters were designated ‘oblique astigmatism’.
Statistical analysis
To avoid any potential contralateral effect or sympathetic effect, we recruited and included in the statistical analysis only one eye of patients who were scheduled for cataract surgery. To analyze the incidence of negative spherical aberration of the total cornea or the anterior corneal surface, the high myopia patients were stratified into three categories according to their axial length: ≥26 and <28; ≥28 and <30; and ≥30 mm.
All continuous data are presented as means ± standard deviations (SD). Statistical analysis of the quantitative data was performed for all variables. The Kolmogorov–Smirnov test was used to assess the normality of the distributions of continuous data. An independent-samples t test was performed for all consecutive items and Pearson’s χ2 test and Fisher’s exact test were used to compare categorical items in the high myopia and control groups. The exact statistical contributions of explanatory variables, such as age, axial length, CCT, corneal astigmatism, and steep keratometric power, to the spherical aberrations were investigated with a multiple regression analysis with a backward selection technique. All data were analyzed with SPSS 23.0 (SPSS, IBM Corp., Armonk, NY, USA). A P value < 0.05 was considered to indicate statistical significance.
Results
A total of 2002 eyes of 2002 patients were enrolled in this study: 502 eyes in the high myopia group and 1500 eyes in the control group. The demographic data, corneal biometric data, and axial lengths of these patients are listed in Table 1, with comparisons between the two groups. The distributions of primary spherical aberrations of the total cornea and the anterior and posterior corneal surfaces are presented in Figs 1–3. The results of the multiple linear regression analyses of the high myopia group, the control group, and the whole study population are presented in the Supplementary Material.
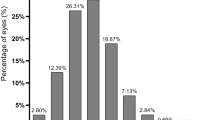
The distributions of anterior corneal primary spherical aberration in two groups. The ranges of anterior corneal primary spherical aberration were −1.009 to 0.918 μm in the control group and −0.400 to 2.421 μm in the high myopia group. Patients in the high myopia group had a lower value than those in the control group (0.317 ± 0.215 vs. 0.338 ± 0.148 μm, P = 0.043).
The distributions of posterior corneal primary spherical aberration in two groups. The ranges of posterior corneal primary spherical aberration were −0.328 to 0.097 μm in the control group and −0.645 to −0.017 μm in the high myopia group. Patients in the high myopia group had a slightly higher absolute value than those in the control group (−0.130 ± 0.044 vs. −0.123 ± 0.040 μm, P = 0.002).
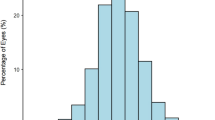
The distributions of total corneal primary spherical aberration in two groups. The ranges of anterior corneal primary spherical aberration were −0.777 to 0.981 μm in the control group and −0.389 to 1.969 μm in the high myopia group. Patients in the high myopia group had a lower value than those in the control group (0.281 ± 0.207 vs. 0.314 ± 0.153 μm, P < 0.001).
The average age of patients in the high myopia group was lower than that in the control group (55.76 ± 13.10 vs 60.18 ± 15.72 years, P < 0.001). In the multiple linear regression analysis of all enrolled patients, age correlated positively with primary spherical aberration of the total cornea and the anterior and posterior corneal surfaces (all three, P < 0.001).
Although the criterion used to divide the groups was axial length, axial length itself showed no or only a weak correlation with the spherical aberrations, especially the primary spherical aberration.
Anterior corneal astigmatism contributed to the primary spherical aberration in the control group (negatively for the total cornea and the anterior corneal surface and positively for the posterior corneal surface, all P < 0.001), but not in the high myopia group. The posterior corneal astigmatism in the high myopia group was similar to that in the control group (P = 0.129), and it was almost not identified as a significant factor in the multiple linear regression analyses of spherical aberrations (only in the analyses of anterior and posterior corneal primary spherical aberration with P = 0.054 and P = 0.002, respectively). A slightly lower proportion of ATR astigmatism was detected in the high myopia group than that in the control group (28.88% vs 35.80%, P = 0.016). The steep meridian keratometric powers of the both anterior and posterior corneal surfaces correlated negatively with the spherical aberrations. No statistically significant difference was identified in CCT between the two groups (539.99 ± 33.43 in the control group vs 539.77 ± 32.13 mm in the high myopia group, P = 0.897). Both Ecc and Q value of anterior and posterior corneal surface differed between two groups, while among all conic coefficients only KI differed (1.02 ± 0.03 in the control group vs 1.01 ± 0.04 in the high myopia group, P = 0.015).
Both the anterior and total corneal primary spherical aberrations were greater in the control group than that in the high myopia group (0.338 ± 0.148 vs 0.317 ± 0.215 μm, P = 0.043; and 0.314 ± 0.153 vs 0.281 ± 0.207 μm, P < 0.001). The incidence of eyes with negative total corneal primary spherical aberration increased as the axial length increased in the high myopia group (0.00%, 1.74%, and 5.79% in the three axial length categories, respectively), and the total incidence was higher in the high myopia group than that in the control group (2.59% vs 1.47%). Similar outcomes were detected for anterior corneal primary spherical aberrations: the incidence in the three axial length categories (≥26 and <28; ≥28 and <30; and ≥30 mm) were 0.51%, 1.74%, and 3.68%, respectively, and the total incidence was higher in the high myopia group (1.99%) than that in the control group (1.60%).
Discussion
Spherical aberrations have compelling and marked effects on optical quality, but among them, primary spherical aberrations can be compensated and partly adjusted with aspheric IOLs, improving visual performance6. Therefore, spherical aberrations have attracted our attention and that of many other ophthalmologists.
Recent researches into corneal spherical aberrations are presented in Table 2. Our findings for the primary spherical aberration in the control group are consistent with the findings of other researches in cataract patients.
The design of aspheric IOLs was based on large-scale studies of corneal spherical aberrations in the normal population, and they are only known to be appropriate for eyes with age-related cataract2. At present, the widely used aspheric IOLs have a primary spherical aberration of −0.27 μm or −0.20 μm, and conventional zero spherical aberration IOLs are selected for patients with low or negative corneal spherical aberrations1. However, in China, these choices are sometimes made regardless of the patient’s corneal spherical aberrations. Spherical aberrations of the anterior corneal surface and total corneal primary spherical aberrations were both smaller in the high myopia group than that in the control group (0.317 ± 0.215 vs 0.338 ± 0.148 μm, P = 0.043; and 0.281 ± 0.207 vs 0.314 ± 0.153 μm, P < 0.001). Therefore, it seems inappropriate to recommend the same aspheric IOLs for patients with age-related cataract and for those with high myopia.
At the outset of aspheric IOL development, the Tecnis Z9000 (Abbott Medical Optics, Inc.), with a primary spherical aberration of −0.27 μm, was deliberately designed to negate all the positive primary spherical aberrations of the cornea, and it effectively reduces ocular spherical aberrations after implantation7. Though the correction of spherical aberrations makes improvement in image quality, residual spherical aberrations are not devoid of any merit. Appropriate reservation of spherical aberrations can polish up the depth of focus and distance-corrected near and intermediate visual acuity to some extent8,9. Now the personalized correction of primary spherical aberrations is performed in cataract surgery, and a postoperative mean residual spherical aberration of approximately 0.1 μm results in better contrast sensitivity and therefore better visual quality7,10,11. In the present study, the primary spherical aberration of the total corneal surface in the high myopia group had a mean value of 0.281 μm. Using aspheric IOLs with a small primary spherical aberration compensation, such as −0.20 μm, we can achieve a residual primary spherical aberration of no less than 0.081 μm, very close to 0.1 μm, which provides the best contrast sensitivity and ideal visual outcomes for these patients.
One noteworthy finding was the incidence of negative corneal primary spherical aberrations. A slight increase in the number of eyes with negative total corneal primary spherical aberration was detected as the axial length increased in the high myopia group (0.00%, 1.74%, and 5.79% in three axial length categories, respectively), and the incidence in the whole group was higher than that in the controls (1.47% in the control group vs 2.59% in the high myopia group).
The increasing tendency for negative corneal primary spherical aberrations to occur in patients in the ≥26 and <28 mm group, ≥28 and <30 mm group and ≥30 mm group can be explained by the decreasing corneal primary spherical aberrations with increasing axial length in the high myopia group, which itself may be attributable to the lower age of the subjects in the high myopia group.
It is well recognized that high myopia is a risk factor for cataract and that highly myopic patients tend to suffer cataract at a younger age than the normal population12. Our results are consistent with those of previous studies, showing a lower average age in the high myopia group than that in the control group (55.76 ± 13.10 vs 60.18 ± 15.72 years, P < 0.001). Age is also considered to correlate positively with spherical aberrations13. This relationship is also supported by previous results (Table 2). Therefore, it is not unusual for corneal primary spherical aberrations to be less common in the younger group (high myopia group), which was strongly confirmed with the multiple linear regression analyses in this study (the standardized coefficients for age were all >0.2, with P < 0.001, for the total, anterior, and posterior corneal spherical aberrations).
With adjustment for age in the regression analyses, axial length itself, the criterion upon which the groups were divided, did not correlate statistically with anterior or total corneal primary spherical aberration. This differs from the low negative correlation between corneal primary spherical aberration and axial length demonstrated by Al-Sayyari et al.1. However, several theories can at least partly explain the smaller primary spherical aberrations in the high myopia group in this study.
Animal experiments and clinical studies have shown that subjects with myopia tend to have a more negative spherical aberration with accommodation and positive spherical aberration slowed the axial growth of the eye14,15,16,17,18. Thus, the implantation of aspheric IOLs in cataract patients with high myopia must be undertaken with caution. A preoperative examination of corneal aberrations should be performed with care, and prudent decisions should be made on the aspheric IOL selected. It is unclear whether postoperative negative total ocular primary spherical aberrations arising from overcompensation will increase the axial length and exacerbate pathological myopia. To avoid this potential risk, particular attention must be paid to these patients and individualized spherical aberration adjustments are warranted. If this is not possible, zero-spherical-aberration IOLs might be the best second choice.
However, it is really difficult for us to pick up and hardly practical for ophthalmologists to employ a cutoff age under which patients are more suitable for the aspheric IOLs with a low negative or zero primary spherical aberration if no personalized primary spherical reduction is achieved in the cataract surgery. “High myopia” is widely recognized and can be easily detected. Ophthalmologists would always pay serious attention to the design of these patients’ IOLs due to their complex ocular condition. These process would be easier when keep the following in mind: An aspheric IOL with a low negative or zero primary spherical aberration is recommended for cataract patients with high myopia. Negative total ocular primary spherical aberrations resulting from aspheric IOL implantation should be avoided in extremely high myopic eyes. These two were the keys of our investigations.
There were some limitations in this study. First, the use of Scheimpflug analysis alone may have limited the acuity of the corneal biometrics in the high myopia group. Although previous studies have reported the repeatability and reproducibility of measurements of the anterior segment made with the Scheimpflug instrument in normal eyes, the poor preoperative fixation stability in cataract patients with long axial lengths could lead to fluctuations in the data19,20. Therefore, more stable corneal topography is required. Second, we did not measure ocular surface dryness. Despite the exclusion of all eyes with a diagnosis of dry eye, different tear film conditions still affect the measurement of aberrations21. Last but not least, we did not analyze the spherical aberrations after aspheric IOL implantation. Had we done so, we could have evaluated the validity of our recommendation. However, too many patients were recruited to be followed up over a short period. Testing and confirmation of our recommendation will be the focus of our future work.
Conclusions
Cataract patients with high myopia had younger age and smaller corneal primary spherical aberrations and an increased proportion of negative corneal primary spherical aberrations than age-related cataract patients did. The incidence of negative corneal primary spherical aberrations increased slightly as the axial length increased, although the exact number of these patients was small. These were mainly contributed to the positive correlations between age and anterior and total corneal primary spherical aberrations. In clinical practice, an aspheric IOL with a low negative primary spherical aberration is recommended for cataract patients with high myopia. Cataract patients with extremely high myopia should be considered seriously. Negative total ocular spherical aberrations resulting from aspheric IOL implantation in extremely high myopic eyes should be avoided.
References
Alsayyari, T. M., Fawzy, S. M. & Alsaleh, A. A. Corneal spherical aberration and its impact on choosing an intraocular lens for cataract surgery. Saudi Journal of Ophthalmology Official Journal of the Saudi Ophthalmological Society 28, 274 (2014).
Schuster, A. K., Tesarz, J. & Vossmerbaeumer, U. Ocular wavefront analysis of aspheric compared with spherical monofocal intraocular lenses in cataract surgery: Systematic review with metaanalysis. J Cataract Refract Surg 41, 1088–1097, https://doi.org/10.1016/j.jcrs.2015.04.005 (2015).
Holladay, J. T., Piers, P. A., Koranyi, G., Van, d., M. M. & Norrby, N. E. A new intraocular lens design to reduce spherical aberration of pseudophakic eyes. Journal of Refractive Surgery 18, 683–691 (2002).
Holden, B. A. et al. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology 123, 1036–1042, https://doi.org/10.1016/j.ophtha.2016.01.006 (2016).
Cillino, G. et al. Working-age cataract patients: visual results, reading performance, and quality of life with three diffractive multifocal intraocular lenses. Ophthalmology 121, 34–44, https://doi.org/10.1016/j.ophtha.2013.06.034 (2014).
Yu, A. Y. et al. Spherical aberration after implantation of an aspheric versus a spherical intraocular lens in high myopia. Clin Exp Ophthalmol 37, 558–565, https://doi.org/10.1111/j.1442-9071.2009.02096.x (2009).
Khan, S. & Rocha, G. Cataract surgery and optimal spherical aberration: as simple as you think? Can J Ophthalmol 43, 693–701, https://doi.org/10.3129/i08-152 (2008).
Yi, F., Iskander, D. R. & Collins, M. Depth of focus and visual acuity with primary and secondary spherical aberration. Vision Res 51, 1648–1658, https://doi.org/10.1016/j.visres.2011.05.006 (2011).
Rocha, K. M., Soriano, E. S., Chamon, W., Chalita, M. R. & Nose, W. Spherical aberration and depth of focus in eyes implanted with aspheric and spherical intraocular lenses: a prospective randomized study. Ophthalmology 114, 2050–2054, https://doi.org/10.1016/j.ophtha.2007.01.024 (2007).
Beiko, G. H. Personalized correction of spherical aberration in cataract surgery. J Cataract Refract Surg 33, 1455–1460, https://doi.org/10.1016/j.jcrs.2007.04.019 (2007).
Ferrer-Blasco, T. Effect of partial and full correction of corneal spherical aberration on visual acuity and contrast sensitivity. J Cataract Refract Surg 35, 949–951, https://doi.org/10.1016/j.jcrs.2008.12.041 (2009).
Harding, J. J., Harding, R. S. & Egerton, M. Risk factors for cataract in Oxfordshire: diabetes, peripheral neuropathy, myopia, glaucoma and diarrhoea. Acta Ophthalmologica 67, 510–517 (1989).
Hashemi, H. et al. Higher order aberrations in a normal adult population. Journal of Current Ophthalmology 27, 115 (2016).
Swarbrick, H. A., Alharbi, A., Watt, K., Lum, E. & Kang, P. Myopia control during orthokeratology lens wear in children using a novel study design. Ophthalmology 122, 620–630, https://doi.org/10.1016/j.ophtha.2014.09.028 (2015).
Cheng, X., Xu, J., Chehab, K., Exford, J. & Brennan, N. Soft Contact Lenses with Positive Spherical Aberration for Myopia Control. Optom Vis Sci 93, 353–366, https://doi.org/10.1097/OPX.0000000000000773 (2016).
Hiraoka, T., Kakita, T., Okamoto, F. & Oshika, T. Influence of ocular wavefront aberrations on axial length elongation in myopic children treated with overnight orthokeratology. Ophthalmology 122, 93–100, https://doi.org/10.1016/j.ophtha.2014.07.042 (2015).
Smith, E. L. 3rd. Optical treatment strategies to slow myopia progression: effects of the visual extent of the optical treatment zone. Exp Eye Res 114, 77–88, doi:10.1016/j.exer.2012.11.019 (2013).
Liu, Y. & Wildsoet, C. The effect of two-zone concentric bifocal spectacle lenses on refractive error development and eye growth in young chicks. Invest Ophthalmol Vis Sci 52, 1078–1086, https://doi.org/10.1167/iovs.10-5716 (2011).
Zhu, X., He, W., Du, Y. & Lu, Y. Effect of fixation stability during biometry measurements on refractive prediction accuracy in highly myopic eyes. J Cataract Refract Surg 43, 1157–1162, https://doi.org/10.1016/j.jcrs.2017.06.039 (2017).
Altiparmak, Z. et al. Repeatability and Reproducibility of Anterior Segment Measurements in Normal Eyes Using Dual Scheimpflug Analyzer. Turk J Ophthalmol 45, 243–248, https://doi.org/10.4274/tjo.16768 (2015).
Montes-Mico, R., Cervino, A., Ferrer-Blasco, T., Garcia-Lazaro, S. & Orti-Navarro, S. Optical quality after instillation of eyedrops in dry-eye syndrome. J Cataract Refract Surg 36, 935–940, https://doi.org/10.1016/j.jcrs.2009.12.044 (2010).
Acknowledgements
This study was funded by the National Natural Science United Foundation of China (grant no. U1503124) and the National Natural Science Foundation of China (grant no. 81770908).
Author information
Authors and Affiliations
Contributions
All authors contributed to the research design. Min Zhang and Dongjin Qian made contributions to data acquisition and all authors made contributions to data analyses and interpretation. Manuscript was initially prepared by Min Zhang and was modified by all authors. Min Zhang prepared all tables and figures. All authors reviewed and proved the final version of this manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, M., Qian, D., Jing, Q. et al. Analysis of Corneal Spherical Aberrations in Cataract Patients with High Myopia. Sci Rep 9, 1420 (2019). https://doi.org/10.1038/s41598-018-36539-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-36539-1
This article is cited by
-
Leaving trusted paths: Estimating corneal keratometric index in cataract surgery eyes with zero-power implants
Graefe's Archive for Clinical and Experimental Ophthalmology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.