Abstract
The dynamics of how metazoan phyla appeared and evolved – known as the Cambrian Explosion – remains elusive. We present a quantitative analysis of the temporal distribution (based on occurrence data of fossil species sampled in each time interval) of lophotrochozoan skeletal species (n = 430) from the terminal Ediacaran to Cambrian Stage 5 (~545 – ~505 Million years ago (Ma)) of the Siberian Platform, Russia. We use morphological traits to distinguish between stem and crown groups. Possible skeletal stem group lophophorates, brachiopods, and molluscs (n = 354) appear in the terminal Ediacaran (~542 Ma) and diversify during the early Cambrian Terreneuvian and again in Stage 2, but were devastated during the early Cambrian Stage 4 Sinsk extinction event (~513 Ma) never to recover previous diversity. Inferred crown group brachiopod and mollusc species (n = 76) do not appear until the Fortunian, ~537 Ma, radiate in the early Cambrian Stage 3 (~522 Ma), and with minimal loss of diversity at the Sinsk Event, continued to diversify into the Ordovician. The Sinsk Event also removed other probable stem groups, such as archaeocyath sponges. Notably, this diversification starts before, and extends across the Ediacaran/Cambrian boundary and the Basal Cambrian Carbon Isotope Excursion (BACE) interval (~541 to ~540 Ma), ascribed to a possible global perturbation of the carbon cycle. We therefore propose two phases of the Cambrian Explosion separated by the Sinsk extinction event, the first dominated by stem groups of phyla from the late Ediacaran, ~542 Ma, to early Cambrian stage 4, ~513 Ma, and the second marked by radiating bilaterian crown group species of phyla from ~513 Ma and extending to the Ordovician Radiation.
Similar content being viewed by others
Introduction
The Cambrian Explosion is a phenomenon that encompasses the dramatic appearance of diverse metazoans with biomineralized skeletons, an increase in metazoan complexity and behaviour, a substrate revolution that re-organised the sedimentary record, and the development of biodiverse marine ecosystems with complex food webs1,2,3,4,5. The relative importance of external drivers, such as rise of oxygen or seawater chemistry changes6,7,8,9, biological drivers, such as the influence of metazoan irrigation10, and feedbacks between the two11, remains unclear. Likewise, the relationship between Ediacaran and Cambrian biotas remains unresolved, with some arguing that the Cambrian Explosion has a ‘deep root’ in the terminal Ediacaran12, or that the first phase of the ‘Cambrian Explosion’ was either the Nama assemblage (~550–541 Ma)13, or appeared even earlier at the Avalon-White Sea boundary at ~561 Ma14. In addition, while it has been conjectured that extinction or turnover events of metazoans occurred at ~551 Ma13,15 and at the Ediacaran/Cambrian boundary at ~541 - 540 Ma (e.g.13,16), there is no consensus as to the precise form either of these dynamics, or indeed their timing, or causes (compare13,14,17).
The combined body and trace fossil record suggests the Cambrian Radiation of bilaterians may have followed a progressive two-stage diversification: the terminal Ediacaran (~560 Ma) to early Cambrian Stage 2 to 3 (mid-Tommotian to Atdabanian) interval dominated by stem groups, and after Cambrian Stage 2 to 3 when definitive crown group representatives of phyla appeared18. Most phylum-level body-plan evolution seems to have taken place well after the Cambrian Explosion, throughout the Cambrian and beyond; stem lineages are considered to have largely disappeared by the Ordovician18.
Placing extinct fossil taxa in phylogenetic order through the application of stem- and crown group concepts allows the order of character acquisition to be considered in both time and environmental context18. Even when highly problematic, all extinct taxa must have stem- or crown group relationships to extant taxa. A crown group is a monophyletic group consisting of the last common ancestor of all living forms and all of its descendants. A stem group is a paraphyletic group that lacks the defining morphological characters of the crown group, where all members are extinct. This therefore consists of the primitive relatives of the crown group, along the phylogenetic line up to, but not including, the last common ancestor of the crown group and their nearest living relatives19.
The considerable number of characters that can define crown groups were often acquired incrementally over geological time20. Random, background extinctions will inevitably erode the base of a clade through time, whether or not basal members are particularly prone to extinction19. Hence, the older a fossil, the more likely it is to fall outside the phylum-level of classification. But mass extinctions may operate quite differently, as they can remove taxa selectively based on particular ecological or other traits21 and lead to long-lasting changes in taxonomic composition and ecosystem functioning22.
Here we construct a high resolution temporal distribution of skeletal species (n = 1188) from the upper Ediacaran to the basal Cambrian Series 3 of the Siberian Platform in order to understand the evolutionary dynamics of the Cambrian Explosion (see Supplementary references). The Siberian Platform formed a separate province during the Ediacaran-Cambrian23,24,25,26, where the stratigraphy and age dating is relatively well known (Fig. 1) and the biota diverse. New coupled high-resolution δ13C and biostratigraphic data as well as improved U-Pb zircon dates suggests that terminal Ediacaran – early Cambrian sections on the northern and south-eastern Siberian Platform are more complete than previously thought, and also indicate that the Cambrian Explosion as shown by the record of skeletal biota may have been a more protracted event12,27. The first diverse skeletal assemblages of Cambrian type (including various halkieriids, chancelloriids, and hyoliths in addition to anabaritids and protoconodonts), occur between levels dated from 543.9 ± 0.24 to 529.7 ± 0.3 Ma which precede the strong basal Cambrian negative carbon isotope excursion (BACE), and in some areas even the basal Terreneuvian Trichophycus pedum ichnofossil assemblage12,28,29,30. Additionally, Ediacaran shelly taxa (cloudinids) co-occur with some of the earliest Cambrian shelly taxa (anabaritids) on the south-eastern Siberian Platform, indicating a continuity of the skeletal fossil record around the Precambrian-Cambrian boundary12. The additional presence of late Ediacaran soft-bodied rangeomorphs, including their biomineralized holdfasts, as well as chambered palaeopascichnids and Nenoxites (=Shaanxilithes) trace fossils found in immediately underlying strata of the same sections12,27 indicates that this record is, in turn, rooted in so-called “post-Kotlinian wormworld” (e.g.13).
Early Cambrian time scale for the Siberian Platform, Russia, with key radiometric dates (numbered; Siberian radiometric dates are in bold), international chronostratigraphy (ICS), and stages and zones accepted for the Siberian Platform. Radiometric dates from 127,93; 294; 395, 496; 597; 698,99,100; 730; 8101. Right column shows numbered temporal units, each c. 2.5 Myr in duration. ED = Ediacaran. 3 = Cambrian Series 3, pars. Modified from5.
In particular we consider the distribution of stem and crown group Lophotrochozoa, which is a monophyletic clade of protostome animals within the Spiralia, consisting of Mollusca, Lophophorata, Nemertea and Annelida31,32,33. The Lophotrochozoa constitutes a third of all modern marine animals34, and was chosen as it is species-rich and represented mostly by skeletal taxa in Ediacaran-Cambrian strata. Deuterostome and cnidarian fossils are too scarce for quantitative analysis, and putative poriferans do not allow detailed character subdivision, due to either an absence of diagnostic spicules (e.g. Archaeocyatha) or the frequently disarticulated preservation of spiculate classes. More importantly, neither the temporal fossil record nor comparative characters of the Lophotrochozoa are reliant upon exceptional preservation (Lagerstätten), as has been noted in other significant groups of the radiation such as euarthropods. This taphonomic bias is exemplified by the fact that crown group euarthropods appear before (521 Ma) stem lineage euarthropods (518 Ma), due in part to differential skeletonisation35. Our study thus enables an understanding of how important phyla including the Mollusca, Brachiopoda and Annelida, may have been assembled, in turn informing likely selective pressures and ecological consequences.
Results
Proposed stem-group Lophotrochozoa
The soft-bodied Ediacaran taxon Kimberella (~560 to ~550 Ma) has been proposed to represent a stem group mollusc36,37,38, although this placement remains problematic17. We exclude this from our analysis given this controversy and the lack of skeletonized hard parts.
We assign hyoliths (both hyolithimorphs and orthothecimorphs), tommotiids (including tannuolinids, Sunnaginia, and Lapworthella), and Oymurania to stem group lophophorates, and sachitids (including halkieriids and siphogonuchitids), wiwaxiids, and, probably, maikhanellids and helcionelloids to stem group molluscs following the phylogenetic and morphological inferences detailed below.
Hyoliths (Fig. 2j), despite their unusual, large calcareous conical shells incorporating a U-shaped intestine and an extendable tentacle-bearing lophophore, have molluscan-type microstructures, a thick compound operculum and, sometimes in hyolithimorphs, a pair of additional curved rigid lateral bar-like supports39,40.
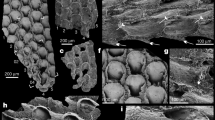
Early and early middle Cambrian skeletal stem- (a–f,i–j) and crown group (g,h) lophotrochozoans from the Siberian Platform. (a) Aldanella attleborensis (Shaler & Foerste), stem mollusc, helcionelloid; shell (29, Fig. 20A1); (b) Camenella garbowskae Missarzhevsky, stem lophophorate, tommotiid; sclerite (102, Fig. 37A); (c) Ceratoconus striatus Chen & Zhang, stem mollusc, helcionelloid; shell (29, Fig. 26A1); (d) Halkieria sp., stem lophotrochozoan; halkieriid; sclerite (29, Fig. 46C2); (e) Tannuolina pavlovi Kouchinsky et al., stem lophophorate, tommotiid; sclerite (103, Fig. 2A2); (f) Oymurania gravestocki Ushatinskaya, stem brachiopod; valve (104, Fig. 8A); (g) Pelmanotreta neguertchenensis (Pelman), crown brachiopod, paterinate; valve (105, Fig. 2i); (h) Pojetaia dentifera Kouchinsky et al., crown mollusc, bivalve; valve (106, Fig. 3A); (i) Purella antiqua (Abaimova), stem lophotrochozoan, maikhanellid; valve (29, Fig. 31B2); (j) Khetatheca cotuiensis (Sysoev), stem lophophorate, hyolith; valve (29, Fig. 50G). All photographs courtesy of Artem Kouchinsky.
While both tommotiids (Fig. 2b,e) and halkieriids s.l. (Fig. 2d) possess multi-element shells (scleritomes), tommotiid sclerites form a narrow conical shell and penetrated by setal canals which can preserve phosphatized setae, and exhibit dense, and fine lamination. In some cases a bivalved larval protegulum with a colleplax-plate typical of the oldest linguliformean brachiopods is present41,42,43,44,45. Oymurania (Fig. 2f) has setigerous canals and two shell layers, one of which shows acrotretoid brachiopod columnar microstructure, and the other resembles the prismatic framework of paterinid brachiopods46. The former problematic fossil Tumulduria is now reinterpreted as a detached central portion of the ventral interarea of a paterinid brachiopod47.
Intact calcareous sachitid scleritomes are considered to belong to a bilateral motile organism that possessed a radula and sclerites with a branching, aesthete type of canal system found in some molluscs48,49,50. Complete sachitid scleritomes from the Early Ordovician are recognized as stem-group aculiferan molluscs51. Chancelloriid sclerites possess the same morphology and microstructure despite the presence of a markedly different scleritome of a sedentary radial-symmetrical animal52,53,54. Thus, a more basal position of sachitids among molluscs, or even lophotrochozoans, cannot be not excluded. Wiwaxiids, although being organic, show the same overall scleritome organization55.
The cup-shaped maikhanellids (Fig. 2i) consist of merged sclerites identical to co-occurring sachitids56. Their cross lamellar microstructure is similar to that of some gastropods and the cap-shaped protoconch is typical of monoplacophorans57. Bivalved calcareous stenothecoids with their paired, serially arranged muscle scars on the inner surfaces of both valves represent a further group of mollusc-like fossils but with a set of features uncommon in crown group molluscs54.
The majority of Cambrian mollusc-like shells with essentially molluscan microstructures, protoconchs, and some features of torsia, are assigned to either the class Helcionelloida58, or subclass Archaeobranchia59. These mainly cup-shaped and low spiral, endogastrically coiled fossils are considered to be extinct lineages of the phylum Mollusca58,59. However, a helcionelloid affinity suggests that their untorted anatomy is due to aperture posterior emarginations, and the presence of a snorkel in some forms also suggests that helcionelloids (Fig. 2a,c) occupy a basal position within the phylum. The archaeobranchian hypothesis also emphasises a torted basic plan and ancestral gastropod affinities59. A stem group rather than crown group position for helcionelloids is further supported by the presence of paired bristle-like clusters extending from the aperture of the Pelagiella shell which have a striking resemblance to the parapodial chaetae of some polychaetes60. Additionally, helcionelloids are characterized by a different muscle system, densely porous shells that are more common in brachiopods than molluscs, and calcitic semi-nacre microstructures which are more typical of lophophorates61,62,63. These observations suggest that the Helcionelloida were stem-group molluscs that retained a number of shared basal features with lophotrochozoan ancestors. Pelagiella represents the most advanced branch of helcionelloids possessing a spirally coiled shell and asymmetric muscle scars suggesting at least partial torsion59.
Proposed crown-group Lophotrochozoa
Early Cambrian crown-group molluscs (Fig. 2h), are recognized among bivalves as well as gastropods of the Khairkhaniidae and Onychochilidae families, belonging to the Divasibranchia and Dextrobranchia orders, respectively59,64. Crown group Lophophorata are represented in the Cambrian by 13 orders of brachiopods (Fig. 2g), only one of which (Lingulida) survived beyond the Palaeozoic45,65,66.
Quantitative temporal distribution
Total skeletal species diversity on the Siberian Platform increases from the terminal Ediacaran to the middle of Cambrian Stage 2, then declines and rises again to reach a second peak at the beginning of Stage 4, followed by an abrupt and rapid decline at the end of Stage 4, followed by recovery around the Series 2/3 boundary (Fig. 3A). The Trilobita appears in Stage 3 and, as the most speciose group, mirrors this general trend. This is in contrast to the second most speciose group, the Archaeocyatha, which first appears in Stage 2 after which there is increase in diversity until the base of Stage 4 but then the group goes abruptly extinct shortly thereafter (Fig. 3A).
Diversity of skeletal species through the Ediacaran – early Cambrian of the Siberian Platform. (A) Total diversity of all skeletal species, Trilobita, and Archaeocyatha. (B) Total diversity of skeletal lophotrochozoan species, and stem group and crown group representatives. Ediacaran and Cambrian chronostratigraphic subdivisions are scaled according to Fig. 1.
Total skeletal lophotrochozoan species diversity likewise increases from the terminal Ediacaran to the middle of Cambrian Stage 2, but then declines until the middle of Stage 3, rises again to reach a second peak at beginning of Stage 4, followed by an abrupt and rapid decline until the middle of Stage 4, then followed by a further rise (Fig. 3B).
Of these, stem group lophophorates, brachiopods, and molluscs comprise a total of 354 species, and crown-groups a total of 76 species through the Ediacaran to Cambrian Stage 5 interval. Stem lophoporates, brachiopods and molluscs (halkieriids, chancelloriids and orthothecimorph hyoliths) appeared in the terminal Ediacaran (~542.5 Ma) and show two phases of diversification: the first through the Terreneuvian, and the second during the end of Stage 3 to beginning of Stage 4 (Fig. 3B). The first crown species are known from the late Fortunian (~537 Ma – gastropods; ~535 Ma – brachiopods and bivalves), and started to radiate later during the early Cambrian Epoch 2 (~522.5 Ma). Stem group species were devastated during the early Cambrian Stage 4 at ~513 Ma but crown group mollusc and brachiopod species, despite some changes in species composition, show no marked loss of diversity, and continued to diversify at a similar apparent rate (Fig. 3B).
Discussion
Possible taphonomic and sampling biases
Taphonomic studies have shown that the fossil record can test the proposition that marine community structure has changed over time67,68. Ediacaran to Cambrian skeletal lophotrochozoans are represented by taxa of comparable millimetric sizes, forming part of the small shelly fauna as shells and disarticulated sclerites. These fossils are generally either replaced by phosphate or present in the form of inner and outer moulds. Only lingulate brachiopods and tommotiids are preserved as original shells, and only rhynchonelliform brachiopods retain their original low-Mg calcite mineralogy. In the lower Cambrian of the Siberian Platform, such fossils are restricted to argillaceous limestones (mostly wackestones and packstones), and some grainstones, all of which accumulated onshore above either normal wave or storm wave base69. All fossils are extracted by the same method of dissolution in buffered acetic acid to isolate phosphatic and phosphatized shells, or moulds and steinkerns (e.g.29). Worker bias is unlikely given that the assemblages reflect multiple different studies and no single worker or study dominates. We infer that taphonomic biases are minimized, and sampling biases present are shared by all small skeletal fossils.
Trends through time
Total lophotrochozoan biodiversity increases until the middle of Stage 2, but then there is a notable decline that extends to approximately the middle of Stage 3 (Fig. 3B). This interval coincides in part with an expansion of anoxic sea floor around ~525 Ma inferred from U isotopes70. Stem- and crown group lophotrochozoan species show distinctly different temporal distributions, with stem group lophophorate, brachiopod and mollusc taxa originating and radiating first. The preferential extinction of stem group species in early Cambrian Stage 4, at ~513 Ma coincides with the well-known Sinsk Event, an episode of widespread shallow marine anoxia on the Siberian Platform and other locations globally, which also coincides with the major extinction of the Archaeocyatha71. It is probable that Archaeocyatha represent a poriferan stem group, and indeed a similar temporal separation of stem and crown group diversification is observed among other metazoans at phyla level, including the Porifera (where crown group demosponges are known by Cambrian Stage 3), Cnidaria and Echinodermata54,72,73,74.
The first probable metazoan body fossils (rangeomorphs) appeared at ~570 Ma75. Rangeomorphs are complex, macroscopic eukaryotes, probably stem group metazoan taxa, although an affinity higher than Porifera has been proposed76. Rangeomorph-dominated assemblages were devastated by the Kotlin Crisis, which marks a turnover event15. After this we propose two phases of the Cambrian Explosion separated by the Sinsk Event extinction. The first was dominated by non-bilaterians (Porifera, Cnidaria and Ctenophora) joined by indeterminate bilaterian stem groups at ~ 560 Ma18 and lasted until ~513 Ma. The general increase in diversity may have been interrupted by the global expansion of anoxic sea floor around ~525 Ma. Notably, this diversification started before, and continues across, the Ediacaran/Cambrian boundary and the Basal Cambrian Carbon Isotope Excursion (BACE) interval (~541 to ~540 Ma). The BACE has been ascribed to a possible global perturbation of the carbon cycle12.
The second phase was marked by radiating non-bilaterian and bilaterian (here determined as brachiopod and mollusc) crown group species, and started from ~513 Ma. This second radiation phase may have been interrupted or even terminated by the late Cambrian SPICE event, which marked a further minor extinction (Fig. 4). Crown groups brachiopod species continued to diversify during the remainder of the Cambrian and into the Ordovician. Bivalves and gastropods also formed a significant part of total global lophotrochozoan diversity and were joined by the appearance of bryozoans and cephalopods around the Cambrian/Ordovician boundary77,78. From that time onwards their diversity remained higher than stem group lophotrochozoans, which continued to decline dramatically during the Cambrian51,59,71,79,80. The last stem group taxa (a few hyolith genera) went extinct in the Permian81.
Schematic of hypothesised non-Bilaterian (total group Porifera, Cnidaria and Ctenophora) and Bilaterian diversification during the Ediacaran-Cambrian metazoan radaition, showing the fossil record of probable earliest metazoans (shown by a rangeomorph reconstruction), the Kotlin crisis, followed by two phases of Cambrian Explosion, separated by the Sinsk Event extinction (with a possible expanded interval of anoxia during Phase 1) and extending to the Ordovician Radiation through the SPICE extinction. Non-bilaterian stem group example is a stem group archaeocyath sponge; crown group is a crown group demosponge. Bilaterian stem group is shown by a tommotiid; crown group by a trilobite.
The Sinsk Event might therefore be considered a mass extinction, which appears to have preferentially removed skeletal stem group lophotrochozoans at a point when diversity was high. This rapid removal is in contrast to background extinctions that are expected to erode the base of a clade gradually through time. We note that crown group brachiopod and mollusc do not show a marked increase in diversity after the removal of stem group lophophorate, brachiopod and mollusc taxa, but continue their former diversity trajectory. This suggests that their radiation was not dependent upon the removal of incumbent stem group taxa, but rather that crown group taxa were in some way more resilient to shallow marine anoxia or other coeval environmental perturbations. Like other mass extinctions, the Sinsk Event led to significant and long-lasting changes in taxonomic composition and ecosystems22,79,82.
A similar sequential faunal replacement pattern of Phanerozoic metazoans has been established in the form of evolutionary marine faunas83, which are in part bounded by mass extinctions. During the Ediacaran to Cambrian interval, further distinguished were the Tommotian, Cambrian s.s. and Palaeozoic faunas84. All these faunas were discriminated by empirical and statistical analysis of family diversity patterns only without reference to phylogenetic relationships. Their existence was, however, challenged22 because their speciation/extinction trends could merely reflect replacement between major taxonomic groups that had coupled dynamics. But our analysis shows that evolutionary faunas may in fact be a manifestation of their composition, with the ‘Tommotian’ fauna being composed of mostly stem group lophotrophorates, molluscs and brachiopods, while the Cambrian s.s. and Palaeozoic faunas are dominated by crown group representatives of molluscs, brachiopods and many other phyla.
This pattern resembles the extinction of taxa at the Permo-Triassic boundary, when groups that originated in the early Palaeozoic either went extinct (tabulate and rugose corals, trilobites, cystoporates) or significantly declined (brachiopods, trepostomates, cryptostomates, conodonts) never to recover previous levels of diversity85. This is in contrast to the pattern shown by groups which appeared and diversified in the late Palaeozoic, such as gymnolaemates and new bivalve, gastropod and ammonoid orders85,86,87.
If ecological niches are relevant, the difference in maintaining the two phases of the Cambrian Explosion might be related to differences in ecospace that was actually “empty” for skeletal animals. During the earlier phase of stem taxa radiation (~543–513 Ma), speciation was most likely promoted by the lack of competition for existing niches. This is similar to the high rates of sympatric speciation, such as noted among modern benthic caenogastropods in lakes, where high phenotypic plasticity enables evolving ecophenotypes to diversify into different substrates (e.g.88). A similar pattern of early diversification as a result of adaptations to different substrates is shown by both helcionelloid mollusc and archaeocyath sponge species during the first phase of the Cambrian Explosion inferred here. The helcionelloids underwent rapid morphogenesis89, and archaeocyaths display extremely high inter-habitat diversity (that is, beta-diversity) in reef communities on the Siberian Platform90, which may also reflect high speciation rates. Niche partitioning is not inferred, as the alpha-diversity (species number per community) remains consistently low91. A similar effect as a result of low competition, and also correlated with a rise in beta-diversity, has been observed to be the main driver of general diversity increase in the early Cambrian92. This dynamic creates the unusual situation when the boundaries of even major lower Cambrian subdivisions have not been established due to an absence of cosmopolitan species. By contrast, even though stem group diversity was significantly reduced during the later crown group brachiopod and mollusc diversification (~513–508 Ma), older niches were not completely eliminated. Thus, in the aftermath of the Sinsk extinction, crown groups were able to diversify via competition for existing niches in order to incorporate into existing communities.
Conclusions
This quantitative analysis of lophotrochozoan skeletal stem- and crown group temporal distribution suggests that the Cambrian Explosion sensu lato may be redrawn as two successive phases of morphological and functional innovation that started in the terminal Ediacaran and were separated by an extinction event. This in turn allows exploration of this phenomenon as an expansion of ecological repertoires that are tractable from the fossil record.
Methods and Data
We divide the terminal Ediacaran to Cambrian Series 2 Siberian record from ~545 to ~505 Ma based on radiometric dates into 16 temporal units based on either sub-division, or combination of one to three Siberian biostratigraphic zones to create broadly equivalent units of ~2.5 Myr each. Units start at the Ediacaran Cloudina-Namacalathus-Sinotubulites assemblage zone through transitional Ediacaran-lowermost Cambrian zones (informally named in ascending order Anabarites trisulcatus, Protohertzina anabarica, and Purella antiqua zones) through Terreneuvian and Cambrian Series 2 zones up to the basal Ovatoryctocara granulata Zone of the Cambrian Stage 5 (Series 3) (Fig. 1). We use the timescale for this interval from available radiometric dates from fossiliferous strata of Siberia, South China, and Avalonia93,94,95,96,97,98,99,100,101 (see Supplementary data).
We quantify the distribution of described skeletal species (n = 1188) from the upper Ediacaran to the basal Cambrian Series 3 on the Siberian Platform (see Supplementary references). This is derived from occurrence data of fossil taxa sampled in each time interval. In particular we quantify the temporal distribution of lophotrochozoan skeletal species (n = 430) (see Supplementary data). Chancelloriids, although they may belong to stem lophotrochozoans, are excluded from analysis due to their frequently disarticulated nature.
Data Availability
The authors declare that the data supporting the findings of this study are available within the paper and its supplementary information files.
References
Brasier, M. D. The Cambrian radiation event. In The Origin of Major Invertebrate Groups (House, M. R. ed.) 103–159 (Academic Press, London; New York, 1979).
Crimes, T. P. Changes in the trace fossil biota across the Proterozoic-Phanerozoic boundary. J. Geol. Soc. Lond. 149, 637–646 (1992).
Bottjer, D. J., Hagadorn, J. W. & Dornbos, S. Q. The Cambrian substrate revolution. GSA Today 10, 1–7 (2000).
Dunne, J. A., Williams, R. J., Martinez, N. D., Wood, R. A. & Erwin, D. H. Compilation and network analyses of Cambrian food webs. PLoS Biol. 6(4), e102, https://doi.org/10.1371/journal.pbio.0060102 (2008).
Zhuravlev, A. Y., Naimark, E. B. & Wood, R. A. Controls on the diversity and structure of earliest metazoan communities: early Cambrian reefs from Siberia. Earth-Sci. Rev. 147, 18–29 (2015).
Towe, K. M. Oxygen-collagen priority and the early metazoan fossil record. Proc. Natl. Acad. Sci. USA 65, 781–788 (1970).
Cook, P. J. & Shergold, J. H. Phosphorus, phosphorites and skeletal evolution at the Precambrian–Cambrian boundary. Nature 308, 231–236 (1984).
Brennan, S. T., Lowenstein, T. K. & Horita, J. Seawater chemistry and the advent of biocalcification. Geology 32, 473–476 (2004).
Li, C. et al. Coupled oceanic oxygenation and metazoan diversification during the early-middle Cambrian? Geology. https://doi.org/10.1130/G39208.1 (2017).
Butterfield, N. J. Animals and the invention of the Phanerozoic Earth system. Trends Ecol. Evol. 26, 81–87 (2011).
Lenton, T. M., Boyle, R. A., Poulton, S. W., Shields-Zhou, G. A. & Butterfield, N. J. Co-evolution of eukaryotes and ocean oxygenation in the Neoproterozoic era. Nature Geosci. 7, 257–265 (2014).
Zhu, M., et al A deep root for the Cambrian Explosion: Implications of new bio- and chemostratigraphy from the Siberian Platform. Geology. https://doi.org/10.1130/G38865.1 (2017).
Darroch, S. A. F., Smith, E. F., Laflamme, M. & Erwin, D. H. Ediacaran extinction and Cambrian Explosion. TREE. https://doi.org/10.1016/j.tree.2018.06.003 (2018).
Tarhan, L. G., Droser, M. L., Cole, D. B. & Gehling, J. G. Ecological expansion and extinction in the late Ediacaran: Weighing the evidence for environmental and biotic drivers. Integr. Comp. Biol. https://doi.org/10.1093/icb/icy020 (2018).
Kolesnikov, A. V., Marusin, V. V., Nagovitsin, K. E., Maslov, A. V. & Grazhdankin, D. V. Ediacaran biota in the aftermath of the Kotlinian Crisis: Asha Group of the South Urals. Precambrian Res. 263, 59–78 (2015).
Amthor, J. E. et al. Extinction of Cloudina and Namacalathus at the Precambrian-Cambrian boundary in Oman. Geology 31, 431–434 (2003).
Muscente, A. D. et al. Exceptionally preserved fossil assemblages through geological time and space. Gondwana Res. https://doi.org/10.1016/j.gr.2017.04.020 (2018).
Budd, G. E. & Jensen, S. The origin of animals and ‘Savannah’ hypothesis for early bilaterian evolution. Biol. Rev. Camb. Philos. Soc. 92, 446–473 (2017).
Budd, G. E. The Cambrian fossil record and the origin of phyla. Integr. Comp. Biol. 43, 157–165 (2003).
Donoghue, P. C. G. Saving the stem group—a contradiction in terms? Paleobiol. 31, 553–558 (2006).
Knoll, A. H., Bambach, R. K., Payne, J. L., Pruss, S. & Fischer, W. W. Palaeophysiology of the end-Permian mass extinction. Earth Planet. Sci. Lett. 256, 295–313 (2007).
Alroy, J. Dynamics of origination and extinction in the marine fossil record. Proc. Natl. Acad. Sci. USA 105, 11536–11542 (2008).
Brock, G. A. et al. Palaeobiogeographic affinities of Australian Cambrian faunas. Assoc. Australas. Palaeontols Mem. 23, 1–61 (2000).
Williams, M., Siveter, D. J., Popov, L. E. & Vannier, J. M. C. Biogeography and affinities of bradoriid arthropods: Cosmopolitan microbenthos of the Cambrian seas. Palaeogeogr. Palaeoclimatol. Palaeoecol. 248, 202–232 (2007).
Meert, J. G. & Lieberman, B. S. The Neoproterozoic assembly of Gondwana and its relationship to the Ediacaran-Cambrian radiation. Gondwana Res. 14, 5–21 (2008).
Álvaro, J. J. et al. Global Cambrian trilobite palaeobiogeography assessed using parsimony analysis of endemicity. In Early Palaeozoic Biogeography and Palaeogeography (Harper, D. A. T. & Servais, T., eds) 273–296 (Geological Society of London Memoirs 38, 2013).
Rogov, V. I. et al. Duration of the first biozone in the Siberian hypostratotype of the Vendian. Russian Geol. Geophys. 56, 573–583 (2015).
Kouchinsky, A. et al. Chronology of early Cambrian biomineralization. Geol. Mag. 149, 221–251 (2012).
Kouchinsky, A. et al. Terreneuvian stratigraphy and faunas from the Anabar Uplift, Siberia. Acta Palaeontol. Polonica 62, 311–440 (2017).
Nagovitsin, K. E. et al. Revised Neoproterozoic and Terreneuvian stratigraphy of the Lena-Anabar Basin and north-western slope of the Olenek Uplift, Siberian Platform. Precambrian Res. 270, 226–245 (2015).
Giribet, G. New animal phylogeny: future challenges for animal phylogeny in the age of phylogenomics. Organisms Diversity & Evol. https://doi.org/10.1007/s13127-015-0236-4 (2015).
Laumer, C. E. et al. Spiralian phylogeny informs the evolution of microscopic lineages. Current Biol. 25, 1–7, https://doi.org/10.1016/j.cub.2015.06.068 (2015).
Kocot, K. M. et al. Phylogenomics of Lophotrochozoa with consideration of systematic error. Systematic Biol. 66, 256–282 (2017).
Appeltans, W. et al. The magnitude of global marine species diversity. Curr. Biol. 22, 2189–2202 (2012).
Daley, A. C., Antcliffe, J. B., Drage, H. B. & Pates, S. Early fossil record of Euarthropoda and the Cambrian Explosion. Proc. Natl. Acad. Sci. USA. https://doi.org/10.1073/pnas.1719962115 (2018).
Fedonkin, M. A. & Waggoner, B. M. The Late Precambrian fossil Kimberella is a mollusc-like bilaterian organism. Nature 388, 868–871 (1997).
Ivantsov, A. Y. A new reconstruction of Kimberella, a problematic Vendian metazoan. Paleontol. J. 43, 601–611 (2009).
Gehling, J. G., Runnegar, B. N. & Droser, M. L. Scratch traces of large Ediacaran bilaterian animals. J. Paleontol. 88, 284–298 (2014).
Moysiuk, J., Smith, M. R. & Caron, J.-B. Hyoliths are Palaeozoic lophophorates. Nature 541, 394–397 (2017).
Sun, H., Malinky, J. M., Zhu, M. & Huang, D. Palaeobiology of orthothecide hyoliths from the Manto Formation of Hebei Province, North China. Acta Palaeontol. Polonica 63, 87–101 (2018).
Balthasar, U., Skovsted, C. B., Holmer, L. E. & Brock, G. A. Homologous skeletal secretion in tommotiids and brachiopods. Geology 37, 1143–1146 (2009).
Holmer, L. E., Skovsted, C. B., Larsson, C., Brock, G. A. & Zhang, Z. First record of a bivalved larval shell in Early Cambrian tommotiids and its phylogenetic significance. Palaeontology 54, 235–239 (2011).
Skovsted, C. B., Brock, G. A., Topper, T. P., Paterson, J. R. & Holmer, L. E. Scleritome construction, biofacies, biostratigraphy and systematics of the tommotiid Eccentrotheca helenia sp. nov. from the early Cambrian of South Australia. Palaeontology 54, 253–286 (2011).
Murdock, D. E. J., Donoghue, P. C. J., Bengtson, S. & Marone, F. Ontogeny and microstructure of the enigmatic Cambrian tommotiid Sunnaginia Missarzhevsky, 1969. Palaeontology 55, 661–676 (2012).
Larsson, C. M. et al. Paterimitra pyramidalis from South Australia: scleritome, shell structure and evolution of a lower Cambrian stem group brachiopod. Palaeontology 54, 414–446 (2014).
Kouchinsky, A. & Bengtson, S. X-ray tomographic microscopy tightens affinity of the early Cambrian Oymurania to the brachiopod stem group. Acta Palaeontol. Polonica 62, 39–43 (2017).
Skovsted, C. B., Kouchinsky, A., Bengtson, S. & Holmer, L. E. The problematic early Cambrian fossil Tumulduria incomperta represents the detached ventral interarea of a paterinid brachiopod. Acta Palaeontol. Polonica 59, 359–365 (2014).
Conway Morris, S. & Peel, J. S. Articulated halkieriids from the Lower Cambrian of North Greenland and their role in early protostome evolution. Phil. Trans. R. Soc. Lond. B 347, 305–358 (1995).
Conway Morris, S. & Caron, J.-B. Halwaxiids and the early evolution of the lophotrochozoans. Science 315, 1255–1258 (2007).
Porter, S. M. Skeletal microstructure indicates chancelloriids and halkieriids are closely related. Palaeontology 51, 865–879 (2008).
Vinther, J., Parry, L., Briggs, D. E. G. & Van Roy, P. Ancestral morphology of crown-group molluscs revealed by a new Ordovician stem aculiferan. Nature. https://doi.org/10.1038/nature21055 (2017).
Butterfield, N. J. & Nicholas, C. J. Burgess Shale-type preservation of both non-mineralizing and ‘shelly’ Cambrian organisms from the Mackenzie Mountains, northwestern Canada. J. Paleontol. 70, 893–899 (1996).
Bengtson, S. & Collins, D. Chancelloriids of the Cambrian Burgess Shale. Palaeontol. Electronica 18.1.6A, 1–67. palaeo-electronica.org/content/2015/1031-chancelloriids (2015).
Zhuravlev, A. Y. The early history of the Metazoa—a palaeontologist’s viewpoint. Biology Bull. Revs 5, 415–461 (2015).
Zhang, Z., Smith, M. R. & Shu, D. New reconstruction of the Wiwaxia scleritome, with data from Chenjiang juveniles. Sci. Rep. 5, 14810, https://doi.org/10.1038/srep14810 (2015).
Bengtson, S. The cap-shaped Cambrian fossil Maikhanella and the relationship between coeloscleritophorans and molluscs. Lethaia 25, 401–420 (1992).
Ponder, W. F., Parkhaev, P., Yu. & Beechey, D. L. A remarkable similarity in scaly shell structure in Early Cambrian univalved limpets (Monoplacophora; Maikhanellidae) and a Recent fissurellid limpet (Gastropoda: Vetigastropoda) with a review of Maikhanellidae. Mollusc. Res. 27, 153–163 (2007).
Peel, J. S. Functional morphology, evolution, and systematics of Early Palaeozoic univalved molluscs. Grønl. Geol. Unders. 161, 1–116 (1991).
Parkhaev, P. Y. On the position of Cambrian archaeobranchians in the system of the class Gastropoda. Paleontol. J. 51, 453–463 (2017).
Thomas, R. D. K., Vinther, J. & Kerry, M. Structure and evolutionary implications of finely preserved chaetae associated with Pelagiella, a stem-group gastropod from the Kinzers Formation (Early Cambrian) at Lancaster, Pennsylvania. In International Palaeontological Congress 3, London, U.K. Programme & Abstracts 375 (2010).
Parkhaev, P. Y. On the genus Auriculina Vassiljeva, 1998 and shell pores of the Cambrian helcionelloid mollusks. Paleontol. J. 40, 20–33 (2006).
Parkhaev, P. Y. Structure of shell muscles in the Cambrian gastropod genus Bemella (Gastropoda: Archaeobranchia: Helcionellidae). Paleontol. J. 48, 17–25 (2014).
Vendrasco, M. J. & Checa, A. G. Shell microstructure and its inheritance in the calcitic helcionellid. Mackinnonia. Estonian J. Earth Sci. 64, 99–104 (2015).
Vendrasco, M. J., Checa, A. G. & Kouchinsky, A. V. Shell microstructure of the early bivalve Pojetaia and the independent origin of nacre within the mollusc. Palaeontology 54, 825–850 (2011).
Carlson, S. J. The evolution of Brachiopoda. Annu. Rev. Earth Planet. Sci. 44, 409–438 (2016).
Murdock, D. J. E., Bengtson, S., Marone, F., Greenwood, J. M. & Donoghue, P. C. J. Evaluating scenarios for the evolutionary assembly of the brachiopod body plan. Evo. Dev. 16(1), 13–24, https://doi.org/10.1111/ede.12059 (2014).
Kidwell, S. M. Preservation of species abundance in marine death assemblages. Science 294, 1091–1094 (2001).
Olszewski, T. D. & Erwin, D. H. Dynamic response of Permian brachiopod communities to long-term environmental change. Nature 428, 738–741 (2004).
Kruse, P. D., Zhuravlev, A., Yu. & James, N. P. Primordial metazoan-calcimicrobial reefs: Tommotian (Early Cambrian) of the Siberian Platform. Palaios 10, 291–321 (1995).
Wei, G. et al. Marine redox fluctuation as a potential trigger for the Cambrian explosion. Geology. https://doi.org/10.1130/G40150.1 (2018).
Zhuravlev, A. Y. & Wood, R. A. Anoxia as the cause of the mid-Early Cambrian (Botomian) extinction event. Geology 24, 311–314 (1996).
Park, T.-Y. et al. A stem-group cnidarians described from the mid-Cambrian of China and its significance for cnidarians evolution. Nat. Commun. 2, 442, https://doi.org/10.1038/ncomms1457 (2011).
Smith, A. B., Zamora, S. & Álvaro, J. J. The oldest echinoderm faunas from Gondwana show that echinoderm body plan diversification was rapid. Nat. Commun. 4, 1385, https://doi.org/10.1038/ncomms2391 (2013).
Botting, J. P. & Muir, L. A. Early sponge evolution: A review and phylogenetic framework. Palaeoworld 27, 1–29 (2018).
Pu, J. P. et al. Dodging snowball: Geochronology of the Gaskiers glaciation and the first appearance of the Ediacaran biota. Geology 44, 955–958 (2016).
Hoyal Cuthill, J. F. & Han, J. Cambrian petalonamid Stromatoveris phylogenetically links Ediacaran biota to later animals. Palaeontology. https://doi.org/10.1111/pala.12393 (2018).
Droser, M. D., Bottjer, D. J. & Li, X. The Ordovician radiation. Am. Sci. 84, 122–131 (1996).
Servais, T., Owen, A. W., Harper, D. A. T., Kröger, B. & Munnecke, A. The Great Ordovician Biodiversification Event (GOBE): The palaeoecological dimension. Palaeogeogr. Palaeoclimatol. Palaeoecol. 294, 99–119 (2010).
Zhuravlev, A. Y. Biota diversity and structure during the Neoproterozoic-Ordovician transition. In The Ecology of the Cambrian Radiation. (Zhuravlev, A. Yu. & Riding, R., eds) 173–199 (Columbia University Press, New York, 2001).
Pruss, S. B., Finnegan, S., Fischer, W. W. & Knoll, A. H. Carbonates in skeleton–poor seas: New insights from Cambrian and Ordovician strata of Laurentia. Palaios 25, 73–84 (2011).
Malinky, J. M. Permian Hyolithida of Australia: the last of the hyoliths? J. Paleontol. 83, 147–152 (2011).
Muscente, A.D. et al. Quantifying ecological impacts of mass extinctions with network analysis of fossil communities. Proc. Natl. Acad. Sci. USA, https://doi.org/10.1073/pnas.1719976115 (2018).
Sepkoski, J. J. Jr. A kinetic model of Phanerozoic taxonomic diversity. 2: Early Paleozoic families and multiple equilibria. Paleobiology 5, 222–252 (1979).
Sepkoski, J. J., Jr. Proterozoic–Early Cambrian diversification of metazoans and metaphytes. In The Proterozoic Biosphere: A Multidisciplinary Study. (Schopf, J. W. & Klein, C., eds) 553–561 (Cambridge University Press, Cambridge, 1992).
Erwin, D. H. Extinction: How Life Nearly Died 250 Million Years Ago. (Princeton University Press, Princeton, 2006).
Clapham, M. E. & Bottjer, D. J. Prolonged Permian-Triassic ecological crisis recorded by molluscan dominance in Late Permian offshore assemblages. Proc. Natl. Acad. Sci. USA 104, 12971–12975 (2007).
Payne, J. L. & Clapham, M. E. End-Permian mass extinction in the oceans: An ancient analog for the twenty-first century? Annu. Rev. Earth Planet. Sci. 40, 89–111 (2012).
Salzburger, W., Van Bocxlaer, B. & Cohen, A. S. Ecology and evolution of the African Great Lakes and their faunas. Annu. Rev. Ecol. Evol. S. 45, 519–545 (2014).
Parkhaev, P. Y. The Early Cambrian radiation of Mollusca. In Phylogeny and Evolution of the Mollusca. (Ponder, W. F. & Lindberg, D. F., eds) 33–69 (California University Press, Berkeley, 2008).
Zhuravlev, A. Y. & Naimark, E. B. Alpha, beta, or gamma: Numerical view on the Early Cambrian world. Palaeogeogr. Palaeoclimatol. Palaeoecol. 220, 207–225 (2005).
Hautmann, M. Diversification and diversity partitioning. Paleobiology 40, 162–176 (2014).
Na, L. & Kiessling, W. Diversity partitioning during the Cambrian radiation. Proc. Natl. Acad. Sci. USA 112, 4702–4706 (2015).
Bowring, S. A. et al. Calibrating rates of Early Cambrian evolution. Science 261, 1293–1298 (1993).
Compston, W., Zhang, Z., Cooper, J. A., Ma, G. & Jenkins, R. J. F. Further SHRIMP geochronology on the early Cambrian of South China. Am. J. Sci. 308, 399–420 (2008).
Zhu, R. et al. SIMS U-Pb zircon age of a tuff layer in the Meishucun section, Yunnan, southwest China: Constraint on the age of the Precambrian-Cambrian boundary. Sci. China Ser. D—Earth Sci. 52, 1385–1392 (2009).
Landing, E. et al. Duration of the Early Cambrian: U-Pb ages of volcanic ashes from Avalon and Gondwana. Can. J. Earth Sci. 35, 329–338 (1998).
Harvey, T. H. P. et al. A refined chronology for the Cambrian succession of southern Britain. J. Geol. Soc. Lond. 168, 705–716 (2011).
Okada, Y. et al. New chronological constraints for Cryogenian to Cambrian rocks in Three Gorges, Weng’an ang Chengjiang areas, South China. Gondwana Res. 25, 1027–1044 (2014).
Chen, D., Zhou, X., Fu, Y., Wang, J. & Yan, D. New U-Pb zircon ages of the Ediacaran-Cambrian strata in South China. Terra Nova 27, 62–68 (2015).
Wei, S. et al. Re-Os geochronology of the Cambrian stage-2 and −3 boundary in Zhijin County, Guizhou Province, China. Acta Geochimica 37, 323–333 (2018).
Hofmann, M. H., Li, X. H., Chen, J., MacKenzie, L. A. & Hinman, N. W. Provenance and temporal constraints of the Early Cambrian Maotianshan Shale, Yunnan Province, China. Gondwana Res. 37, 348–361 (2016).
Kouchinsky, A., Bengtson, S., Clausen, S. & Vendrasco, M. J. An early Cambrian fauna of skeletal fossils from the Emyaksin Formation, northern Siberia. Acta Palaeontol. Polonica 60, 421–512 (2015a).
Kouchinsky, A., Bengtson, S. & Murdock, D. E. J. A new tannuolinid problematic from the lower Cambrian of the Sukharikha River in northern Siberia. Acta Palaeontol. Polonica 55, 321–331 (2010).
Kouchinsky, A., Holmer, L. E., Steiner, M. & Ushatinskaya, G. T. The new stem-group brachiopod Oymurania from the lower Cambrian of Siberia. Acta Palaeontol. Polonica 60, 963–980 (2010).
Skovsted, C. B., Ushatinskaya, G. T., Holmer, L. E., Popov, L. E. & Kouchinsky, A. Taxonomy, morphology, shell structure and ontogeny of Pelmanotreta nom. nov. from the lower Cambrian of Siberia. GFF 137, 1–8 (2015).
Kouchinsky, A. et al. A middle Cambrian fauna of skeletal fossils from the Kuonamka Formation, northern Siberia. Alcheringa 35, 123–189 (2011).
Acknowledgements
Artem Kouchinsky is thanked for kindly providing all photographic images, and Jen Hoyal Cuthill, Doug Erwin, and an anonymous reviewer for thoughtful reviews.
Author information
Authors and Affiliations
Contributions
A.Yu. Z. collated the data and A.Yu. Z. and R.W. designed the research, wrote the main manuscript text, and prepared the figures.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhuravlev, A.Y., Wood, R.A. The two phases of the Cambrian Explosion. Sci Rep 8, 16656 (2018). https://doi.org/10.1038/s41598-018-34962-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-34962-y
Keywords
This article is cited by
-
Protomelission is an early dasyclad alga and not a Cambrian bryozoan
Nature (2023)
-
Brachiopod and mollusc biomineralisation is a conserved process that was lost in the phoronid–bryozoan stem lineage
EvoDevo (2022)
-
Dynamic and synchronous changes in metazoan body size during the Cambrian Explosion
Scientific Reports (2020)
-
The Scaly-foot Snail genome and implications for the origins of biomineralised armour
Nature Communications (2020)
-
Possible links between extreme oxygen perturbations and the Cambrian radiation of animals
Nature Geoscience (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.