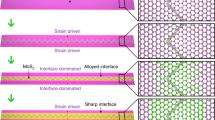
Abstract
Thin films of layered semiconductors emerge as highly promising materials for energy harvesting and storage, optoelectronics and catalysis. Their natural propensity to grow as oriented crystals and films is one of their distinct properties under recent focal interest. Specifically, the reaction of transition metal films with chalcogen vapor can result in films of vertically aligned (VA) layers, while metal-oxides react with chalcogens in vapor phase to produce horizontally aligned crystals and films. The growth mechanisms of vertically oriented films are not yet fully understood, as well as their dependence on the initial metal film thickness and growth conditions. Moreover, the resulting electronic properties and the role of defects and disorder had not yet been studied, despite their critical influence on catalytic and device performance. In this work, we study the details of oriented growth of MoS2 with complementary theoretical and experimental approaches. We present a general theoretical model of diffusion-reaction growth that can be applied to a large variety of layered materials synthesized by solid-vapor reaction. Moreover, we inspect the relation of electronic properties to the structure of vertically aligned MoS2 and shed light on the density and character of defects in this material. Our measurements on Si-MoS2 p-n hetero-junction devices point to the existence of polarizable defects that impact applications of vertical transition-metal dichalcogenide materials.
Similar content being viewed by others
Introduction
The growing interest in layered inorganic materials over the last decade was stimulated by the development of novel synthetic methods. Among such methods chemical vapor deposition (CVD) dominated the production methods of scalable, device-grade transition-metal dichalcogenides (TMDC)1,2,3. In turn, the success of the synthetic research has propelled the research on novel device applications4,5,6,7 and fundamental physics of layered materials8,9,10. Hitherto, CVD growth of TMDCs was mostly focused on the growth of ultrathin, few-layer crystals and heterostructures11,12,13,14,15. The recent reemergence of vertically aligned (VA) thin films of TMDCs is imperative to diverse device applications and of fundamental interest, owing to their unique growth mechanism16,17,18,19. Moreover, thin VA-MoS2 films were established as scalable, high quality electronic materials, enabling highly-responsive near infrared photodetectors20,21,22,23,24 and energy storage devices based on Li+ and Na+ intercalation25,26. However, the role of defects and crystalline disorder can be critically important in these materials. Solar energy harvesting with self-driven photodiodes27 of VA-MoS2 on P-Si yields relatively low quantum efficiency due to the enhanced recombination rates associated with defects in VA-MoS2. Catalytic activity of VA-MoS2 is associated with its high density of edge states and is also prone to be influenced by defects and crystalline disorder17. Therefore, understanding the formation and properties of defects and disorder in VA-MoS2 is critically important for a variety of applications and may pave the way for achieving the full potential of vertically aligned layered compounds.
Early studies of VA-MoS2 deposition were conducted by RF magnetron sputtering28,29. More recently, studies on the CVD growth mechanisms of VA-MoS2 found that sulfurization of thin Mo films changes character from planar to vertical crystal growth, controlled by the thickness of the Mo film30,31. Specifically, a critical thickness of 5 nm was found to be the threshold for vertical growth, while sulfurization of thinner metal films resulted in planar oriented MoS219. The vertical orientation of VA-TMDCs is attributed to a diffusion-driven growth mechanism19,32,33. In this mechanism, sulfur diffuses through the Van der Waals interlayer gap at a much higher rate than across the crystal layers – and thus the preferred orientation of the growth is established from kinetic considerations. However, the details of the vertical alignment mechanism and the turning point from planar to vertical growth are not yet fully understood. These details are significant for the advancement of several important device applications across multiple fields32,34,35,36,37. Moreover, the impact of a multi-domain (vertical and randomly oriented) structure on the electronic properties of MoS2 remains unclear. In this work, we study the formation mechanism of VA-MoS2 films on P-Si and their charge carrier characteristics as resolved from transport measurements of p-n junction devices.
Results and Discussion
Our devices are constructed from 70 nm thick MoS2 grown on P+-Si by CVD in the following process: 90 nm Si/SiO2 wafers were patterned by photolithography and etched to form through-oxide vias (Fig. 1a), on which 30 nm metal Mo was deposited and lifted-off. Mo films were then sulfurized under flow of 30 sccm nitrogen in background pressure of 5 mTorr, carrying sulfur vapor from a boat heated to 150 °C. The sample was kept at 800 °C during the growth. Clean Raman spectra were taken from the samples at a parallel geometry between the laser line and the c-axis of the crystal, with linearly polarized light (Fig. 1c), obtaining the signal of 2H-MoS2 and crystalline Si, without traces of molybdenum silicide. Then, a second lithographic process was applied for metallizing the grown MoS2 with Ti/Pd contacts (Fig. 1b).
(a) Graphical representation of a MoS2 device fabrication process: 90 nm SiO2/Si selective etch by photolithography and reactive-ion etching, Mo deposition and lift-off, sulfurization growth of VA-MoS2, and metallization of devices. (b) A cross-sectional TEM micrograph of the resulting device. (c) Raman spectrum of the MoS2 taken immediately after growth.
A detailed characterization of the MoS2 layer was performed with cross-section transmission electron microscopy of samples as shown in Fig. 2, with two types of thermal processing; in both cases a thin film of Mo on Si/SiO2 (as in Fig. 1) were sulfurized at 800 °C for two hours under continuous temperature ramping. Interestingly, samples of fast temperature ramping (20 °C/min) exhibit thicker domains of randomly oriented crystals as compared to slow temperature ramping samples (5 °C/min). Details of the process are given below and in the supporting information. Temperature cycles are displayed for clarity in Fig. S1. The proposed growth mechanism assumes that during the reaction, the growth front propagates into the Mo film from the vapor-solid interface inwards, finally reaching the Si substrate. Complementary, the case of thick Mo films representing the growth process at the semi-infinite samples was studied on thick Mo foils to elucidate the limit of long reaction time and depth (Figs S2 and S3), as discussed in the supplementary information. During the reaction on Mo on Si/SiO2, the growth front propagates into the Mo film, from the vapor-solid interface inwards, finally reaching the Si substrate. The image for the fast growth (Fig. 2a) reveals a gradual establishment of preferred crystallographic orientation. The MoS2 is crystalline along the reaction path, initially showing random orientation that after a threshold thickness becomes a vertically oriented layer. Thus, the crystal orientation ordering towards VA-MoS2 started only after an incubation thickness that we term as “surface reaction”. Following the surface reaction a strongly preferred orientation is evidenced by the sharpening of the orientation distribution around the perpendicular axis (Figs S4 and S5), in proximity to the substrate. In order to understand this growth process and the parameters controlling the thickness of the disordered layer formed at the early stages of the reaction, we quantified the crystal orientation of the films as a function of the reaction coordinate (Fig. S4). Results of the quantification procedure are displayed in Fig. S5. This transition in growth leaves a 30–40 nm thick randomly oriented layer at the fast growth conditions. In contrast, for the sample grown at slow growth conditions (Fig. 2b), the typical thickness of the surface reaction layer is much smaller than that of the fast growth (<10 nm), in agreement with previous studies16,19,33. Under low growth rate the disordered layer is thinner, thus indicating a lower surface reactivity and dominant diffusion-limited growth mechanism. The examples of Fig. 2 shows that the thickness of the randomly oriented film is strongly affected by the growth kinetics, yet the growth mechanisms and the role of the disordered layer were not thoroughly examined.
TEM micrograph of VA-MoS2 grown on Si substrate at 800 °C for 2 h with a temperature ramping rate of 20 °C/min (a) and 5 °C/min (b), respectively. Insets show the magnified view of vertically aligned MoS2 in the initial growth at the top (dashed rectangle) and at the growth interface (solid rectangle). The surface reaction results in an initial growth of a significantly thick randomly oriented MoS2 layer for fast growth, in contrast with slow growth where the layers are vertically aligned (dashed rectangle).
Here, we present a model describing the growth process resulting in the co-existence of two distinct morphologies: disordered layer of surface reaction and VA-MoS2. Using the model, we show the underlying principles to achieve control over the structural features and the associated electronic properties. Details of the model are described in the supporting information, and in Fig. S6. In this model the random MoS2 extends from the surface to a time-dependent depth x0, and the VA-MoS2 extends from x0 to x1. The model assumes that: (i) Mo is stationary and the reaction is driven by sulfur diffusion only; (ii) sulfur is fully reacted and no inclusions exist in the Mo film; (iii) the consumption of sulfur takes place at the moving boundary between Mo and MoS2, i.e., at the reaction front.
The set of Eq. (1) present the model for an equilibrium growth at the Mo-MoS2 interface38, while the initial and boundary conditions are fit to represent the conditions of the chemical process.
Here, C is the sulfur concentration and DMo and \({D}_{Mo{S}_{2}}\) are the diffusion coefficients of sulfur in Mo and in MoS2, respectively; η is the concentration of Mo consumed at the interface which is linearly related to C by the constant R by \(\eta (x,t)=RC(x,t)\). x0(t) is the position of the reaction front, representing the moving boundary of the Mo-MoS2 interface. The reaction obeys the following conditions: initially, the sample comprises Mo only [x0(t ≤ 0) = 0]. The self-limited (see SI for details) reaction time for the growth of random MoS2 is τ: \([{x}_{0}(t\ge \tau )={x}_{0}(\tau )]\). The random layer grown within time τ forms a diffusion barrier and the reaction rate, dx0/dt- decays after τ (see Fig. 3b), where the reaction becomes diffusion-limited. From this point, the growth front advances through vertical seeds and is dominated by the higher diffusion kinetics of sulfur along the Van- der Waals gap that defines the fast axis within MoS2 with a diffusion coefficient D⊥ for sulfur. Therefore, the reaction continues along a new Mo/VA-MoS2 interface that we label as x1(t > τ). Figure 3a schematically displays our model at which a surface reaction advances from the molybdenum surface into its depth, up to a distance x0(τ)- followed by a diffusion-limited growth along the fast axis of MoS2 that obeys Eq. (1), with a time-dependent boundary condition CB(t) = C(x0, t > τ):
(a) Graphical illustration of growth process starting from a surface reaction of depth x0 and reaction time τ, followed by a diffusion-limited growth of VA-MoS2. (b) The solution to the propagation velocity of x0(t) with a scaled amplitude showing its rapid decay and (c) the resulting VA-MoS2 growth with several values for x0.
Figure 3b shows the scaled reaction front velocity, dx0/dt, as obtained from the analytical solution (see SI for the full solution), showing that the surface reaction dominates the initial stages of the growth. Figure 3c provides the solution x1(t) - the thickness of VA-MoS2 with several values of the disoriented film thickness - x0(τ), demonstrating the role of this diffusion barrier layer and its impact on growth kinetics, being a barrier for sulfur diffusion. The diffusion coefficients and the parameter R can be calculated from ab-initio and molecular dynamics of Mo and sulfur32,34,36,37.
In some cases, the existence of a randomly oriented layer may be desired and can serve as a diffusion barrier in several processes, such as intercalation, that are widely spread across various applications. In case that a sharp interface is required, this layer can be removed by dry etching.
It is well known that defects in MoS2 films are the main source for charge carriers39,40,41,42, which raises the question of the quality of devices made with VA-MoS2. The disordered layer contains unique structural features and their influence on the electronic properties is so far unexplored. To this end, we prepared diodes from VA-MoS2 grown on p+-Si (similar to the sample presented in Fig. 2a), and we confirmed that they obey the standard model of diffusive transport (Fig. 4a), I = I0(exp[qV/ηkBT] − 1). On these well-behaved diodes we could measure the electrical characteristic of the carriers and the impact of charge traps. Figure 4b shows a sizable hysteresis under cyclic bias applied on the diodes, corresponding to charge trapping at crystalline defects, rendering them polar43,44,45. By analogy with polar ions observed in perovskites46, we assign this electrical behavior to trap charging and discharging. The quenching of the hysteresis with increased frequency is a result of the trap capacitance and the resulting trapping/detrapping rate. From the capacitance measurements in Fig. 4c, the estimation of the built-in potential is 1.2 eV and the carrier density is ~1019 cm−3. The measured built-in potential represents the p-n quasi-Fermi level difference and the band misalignment across the junction that can be roughly estimated to ~100 meV in this case of highly doped, sharp junction. We assume that the measured carrier density is comparable with the density of traps (Nt ≈ ND) and that the vast majority of carriers in VA-MoS2 originate from shallow traps47,48.
To conclude, we analyzed the growth of MoS2 by sulfurization of metallic Mo films from several perspectives. We applied a high-temperature, low pressure growth on thin film Mo resulting in a gradual transition from disordered crystalline MoS2 to a vertically oriented morphology. By applying a theoretical diffusion-reaction model to the growth, we provided a plausible growth mechanism that explains the experimental observations. Our model is general and applicable to a large variety of layered materials. If provided with diffusion parameters retrieved from experiments or ab-initio calculations, it will provide a predictive, quantitative tool to characterize the growth process. Regarding the electronic properties of samples containing a disordered layer, we found that the band misalignment between Si and VA-MoS2 can be estimated to ~100 meV and that structural defects result in trap states that can be observed in cyclic I-V measurements. Details of the trap energy and density are a subject for future work. Yet without loss of generality, we conclude that rapid reactions result in higher defect density and that the surface reaction taking place at the beginning of the process is the source for the majority of defects. These conclusions may have critical implications to the performance of VA-MoS2 in several fields including catalysis, lithium ion storage and solar energy harvesting and optoelectronics devices.
Methods
Sample preparation
Si wafer preparation
Photolithography was applied to a sample of 90 nm SiO2/Si P-type wafer to create an array of 324 circles of 40 µm diameter each. The oxide layer in the circles was then selectively etched by reactive-ion etching, followed by evaporation and lift-off of ~30 nm Molybdenum, after which the CVD process was applied. Metal contacts were finally deposited by e-beam lithography and evaporation.
CVD process
Si wafer
Samples were annealed under 160 sccm N2 flow at 200 °C for 1 h. Sulfur powder was placed upstream at low temperature zone of ~150 °C. The quartz furnace was heated (20 °C/min and 5 °C/min, for the fast/slow temperature ramping samples, respectively) to 800 °C under 30 sccm nitrogen flow and were held at this set-point for 2 h. Then the temperature was reduced to 600 °C, the sulfur was solidified and afterwards the temperature was reduced (7 °C/min until 400 °C and afterward 2 °C/min till reaches to room temperature).
Mo foil
The sample was annealed under 160 sccm N2 flow at 200 °C for 1 h. Sulfur powder was placed upstream at low temperature zone of ~150 °C until liquefied. The quartz furnace was heated to 500 °C (20 °C/min) under 100 sccm nitrogen flow and was held at this set point for 30 min. Subsequently the quartz furnace was heated to 600 °C (20 °C/min) under a nitrogen flow of 100 sccm and it was held at this set point for a period of 30 min. An additional heating step was added while the temperature rose to 750 °C (20 °C/min) under a 30 sccm nitrogen flow. This state was maintained for a period of 24 h. In the last thermal cycling step the temperature was reduced to 600 °C, the sulfur was solidified and the temperature was further reduced to 400 °C (5 °C/min) and then brought down to room temperature (2 °C/min). Further details and a graphical representation of the temperature cycle are presented in the supporting information.
TEM
TEM lamella of the CVD-grown VA-MoS2 devices were prepared using a FEI Helios 600 Focused Ion Beam (FIB) microscope. Pt was deposited during the FIB process for the purpose of material protection. TEM micrographs of the cross-sectional device samples were collected on a JEOL JEM-2100 high resolution transmission electron microscope operated at 200 kV.
Raman Spectroscopy
Raman spectra were taken by a Horiba Scientific Labram HR Evolution using a 532 nm laser. The exciting laser propagated parallel to the crystal c-axis with a linear polarization.
Electrical measurements
Samples were wire bonded to ceramic leadless chip carrier, and then were measured in a cryostat vacuum chamber (Montana instruments) with a Keysight B2912A and B1500A instruments.
References
Lee, Y. H. et al. Synthesis of large‐area MoS2 atomic layers with chemical vapor deposition. Adv. Mater. 24, 2320–2325 (2012).
Wang, X., Feng, H., Wu, Y. & Jiao, L. Controlled synthesis of highly crystalline MoS2 flakes by chemical vapor deposition. J. Am. Chem. Soc 135, 5304–5307 (2013).
Amani, M. et al. Electrical performance of monolayer MoS2 field-effect transistors prepared by chemical vapor deposition. Appl. Phys. Lett. 102, 193107 (2013).
Radisavljevic, B., Radenovic, A., Brivio, J., Giacometti, I. V. & Kis, A. Single-layer MoS2 transistors. Nature nanotechnology 6, 147–150 (2011).
Jariwala, D., Sangwan, V. K., Lauhon, L. J., Marks, T. J. & Hersam, M. C. Emerging device applications for semiconducting two-dimensional transition metal dichalcogenides. ACS nano 8, 1102–1120 (2014).
Radisavljevic, B., Whitwick, M. B. & Kis, A. Integrated circuits and logic operations based on single-layer MoS2. ACS nano 5, 9934–9938 (2011).
Wang, H. et al. Integrated circuits based on bilayer MoS2 transistors. Nano Lett. 12, 4674–4680 (2012).
Xu, X., Yao, W., Xiao, D. & Heinz, T. F. Spin and pseudospins in layered transition metal dichalcogenides. Nature Physics 10, 343–350 (2014).
Mak, K. F., McGill, K. L., Park, J. & McEuen, P. L. The valley Hall effect in MoS2 transistors. Science 344, 1489–1492 (2014).
Wu, S. et al. Vapor–solid growth of high optical quality MoS2 monolayers with near-unity valley polarization. Acs Nano 7, 2768–2772 (2013).
Tongay, S. et al. Tuning interlayer coupling in large-area heterostructures with CVD-grown MoS2 and WS2 monolayers. Nano Lett. 14, 3185–3190 (2014).
Gong, Y. et al. Vertical and in-plane heterostructures from WS2/MoS2 monolayers. Nature materials 13, 1135–1142 (2014).
Hong, X. et al. Ultrafast charge transfer in atomically thin MoS2/WS2 heterostructures. Nature nanotechnology 9, 682–686 (2014).
Roy, T. et al. Dual-gated MoS2/WSe2 van der Waals tunnel diodes and transistors. Acs Nano 9, 2071–2079 (2015).
Li, M.-Y. et al. Epitaxial growth of a monolayer WSe2-MoS2 lateral pn junction with an atomically sharp interface. Science 349, 524–528 (2015).
Yu, J. H. et al. Vertical heterostructure of two-dimensional MoS2 and WSe2 with vertically aligned layers. Nano Lett. 15, 1031–1035 (2015).
Wang, H. et al. Electrochemical tuning of vertically aligned MoS2 nanofilms and its application in improving hydrogen evolution reaction. Proceedings of the National Academy of Sciences 110, 19701–19706 (2013).
Jung, Y. et al. Metal seed layer thickness-induced transition from vertical to horizontal growth of MoS2 and WS2. Nano Lett. 14, 6842–6849 (2014).
Zhang, X. et al. Direct synthesis of large-scale hierarchical MoS2 films nanostructured with orthogonally oriented vertically and horizontally aligned layers. Nanoscale 8, 431–439 (2015).
Qiao, S. et al. Large lateral photovoltage observed in MoS2 thickness-modulated ITO/MoS2/p-Si heterojunctions. ACS Applied Materials & Interfaces (2017).
Mao, J. et al. Ultrafast, Broadband Photodetector Based on MoSe2/Silicon Heterojunction with Vertically Standing Layered Structure Using Graphene as Transparent Electrode. Advanced Science 3, 1600018 (2016).
Dhyani, V. & Das, S. High-Speed Scalable Silicon-MoS2 PN Heterojunction Photodetectors. Scientific Reports 7, 44243 (2017).
Zhang, Y. et al. In Situ Fabrication of Vertical Multilayered MoS2/Si Homotype Heterojunction for High‐Speed Visible–Near‐Infrared Photodetectors. Small 12, 1062–1071 (2016).
Cheng, R. et al. Electroluminescence and photocurrent generation from atomically sharp WSe2/MoS2 heterojunction p–n diodes. Nano Lett. 14, 5590–5597 (2014).
Gao, J. et al. Vertically oriented arrays of ReS2 nanosheets for electrochemical energy storage and electrocatalysis. Nano Lett. 16, 3780–3787 (2016).
Chao, D. et al. Pseudocapacitive Na-ion storage boosts high rate and areal capacity of self-branched 2D layered metal chalcogenide nanoarrays. ACS nano 10, 10211–10219 (2016).
Wang, L. et al. MoS2/Si Heterojunction with Vertically Standing Layered Structure for Ultrafast, High‐Detectivity, Self‐Driven Visible–Near Infrared Photodetectors. Adv. Funct. Mater. 25, 2910–2919 (2015).
Bichsel, R. & Levy, F. Morphological and compositional properties of MoSe2 films prepared by r.f. magnetron sputtering. Thin Solid Films 116, 367–372 (1984).
Bertrand, P. A. Orientation of rf-sputter-deposited MoS2 films. Journal of Materials Research 4, 180–184 (1989).
Shokhen, V. et al. On the impact of Vertical Alignment of MoS2 for Efficient Lithium Storage. Scientific Reports 7 (2017).
Zhan, Y., Liu, Z., Najmaei, S., Ajayan, P. M. & Lou, J. Large‐area vapor‐phase growth and characterization of MoS2 atomic layers on a SiO2 substrate. Small 8, 966–971 (2012).
Shang, S.-L. et al. Lateral versus vertical growth of two-dimensional layered transition-metal dichalcogenides: Thermodynamic insight into MoS2. Nano Lett. 16, 5742–5750 (2016).
Kong, D. et al. Synthesis of MoS2 and MoSe2 films with vertically aligned layers. Nano Lett. 13, 1341–1347 (2013).
Rajan, A. G., Warner, J. H., Blankschtein, D. & Strano, M. S. Generalized mechanistic model for the chemical vapor deposition of 2D transition metal dichalcogenide monolayers. ACS Nano 10(4), 4330–4344 (2016).
Zhu, D. et al. Capture the growth kinetics of CVD growth of two-dimensional MoS2. npj 2D Materials and Applications 1, 8 (2017).
Nie, Y. et al. A kinetic Monte Carlo simulation method of van der Waals epitaxy for atomistic nucleation-growth processes of transition metal dichalcogenides. Scientific Reports 7, 2977 (2017).
Nie, Y. et al. First principles kinetic Monte Carlo study on the growth patterns of WSe2 monolayer. 2D Materials 3, 025029 (2016).
Crank, J. The mathematics of diffusion. (Oxford university press, 1979).
Li, H. et al. Kinetic study of hydrogen evolution reaction over strained MoS2 with sulfur vacancies using scanning electrochemical microscopy. J. Am. Chem. Soc. 138, 5123–5129 (2016).
Sim, D. M. et al. Controlled doping of vacancy-containing few-layer MoS2 via highly stable thiol-based molecular chemisorption. ACS nano 9, 12115–12123 (2015).
Kim, S. et al. Effects of plasma treatment on surface properties of ultrathin layered MoS2. 2D Materials 3, 035002 (2016).
Zhou, W. et al. Intrinsic structural defects in monolayer molybdenum disulfide. Nano Lett. 13, 2615–2622 (2013).
Lee, E. G., Wouters, D. J., Willems, G. & Maes, H. E. Voltage shift and deformation in the hysteresis loop of Pb (Zr, Ti) O3 thin film by defects. Appl. Phys. Lett. 69, 1223–1225 (1996).
Prosandeyev, S. et al. Study of intrinsic point defects in oxides of the perovskite family: I. Theory. J. Phys.: Condens. Matter 8, 6705 (1996).
Park, C. & Chadi, D. Microscopic study of oxygen-vacancy defects in ferroelectric perovskites. Physical Review B 57, R13961 (1998).
Meloni, S. et al. Ionic polarization-induced current–voltage hysteresis in CH3NH3PbX3 perovskite solar cells. Nature communications 7, 10334 (2016).
Lu, C.-P., Li, G., Mao, J., Wang, L.-M. & Andrei, E. Y. Bandgap, mid-gap states, and gating effects in MoS2. Nano letters 14, 4628–4633 (2014).
Radisavljevic, B. & Kis, A. Mobility engineering and a metal–insulator transition in monolayer MoS2. Nature Materials 12, 815 (2013).
Acknowledgements
DN would like to thank the Israeli Science Foundation for funding of this work with grant #1055/15. The authors would like to thank Yafit Fleger for preparing TEM sample using focused ion beam, Ilana Perelshtein for assistance with TEM imaging.
Author information
Authors and Affiliations
Contributions
C.S. performed CVD growth; C.S., H.A. and M.O. fabricated the devices; C.S., S.G., M.K. and O.S. performed electrical measurements; D.N., S.G. and C.S. analyzed the results; M.B.S. and O.E.M. conducted TEM measurements, M.B.S. and L.H. analyzed TEM images; all authors read and agreed with the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stern, C., Grinvald, S., Kirshner, M. et al. Growth Mechanisms and Electronic Properties of Vertically Aligned MoS2. Sci Rep 8, 16480 (2018). https://doi.org/10.1038/s41598-018-34222-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-34222-z
Keywords
This article is cited by
-
Single step growth of vertical MoS2 thin films by chemical vapor deposition for hydrogen evolution reaction
Journal of Materials Science: Materials in Electronics (2023)
-
Two-dimensional halide perovskites: A review on their orientations
Science China Physics, Mechanics & Astronomy (2023)
-
Carbide-free one-zone sulfurization method grows thin MoS2 layers on polycrystalline CVD diamond
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.