Abstract
Human activities can generate a wide variety of direct and indirect effects on animals, which can manifest as environmental and genetic stressors. Several phenotypic markers have been proposed as indicators of these stressful conditions but have displayed contrasting results, depending, among others, on the phenotypic trait measured. Knowing the worldwide decline of multiple bumblebee species, it is important to understand these stressors and link them with the drivers of decline. We assessed the impact of several stressors (i.e. natural toxin-, parasite-, thermic- and inbreeding- stress) on both wing shape and size and their variability as well as their directional and fluctuating asymmetries. The total data set includes 650 individuals of Bombus terrestris (Hymenoptera: Apidae). Overall wing size and shape were affected by all the tested stressors. Except for the sinigrin (e.g. glucosinolate) stress, each stress implies a decrease of wing size. Size variance was affected by several stressors, contrary to shape variance that was affected by none of them. Although wing size directional and fluctuating asymmetries were significantly affected by sinigrin, parasites and high temperatures, neither directional nor fluctuating shape asymmetry was significantly affected by any tested stressor. Parasites and high temperatures led to the strongest phenotype modifications. Overall size and shape were the most sensitive morphological traits, which contrasts with the common view that fluctuating asymmetry is the major phenotypic marker of stress.
Similar content being viewed by others
Introduction
A large number of habitats and wildlife are under threat, especially due to human activities and many techniques have been developed to measure the potential stressors1. Indeed, the detection of populations undergoing such stresses has become a central point in conservation biology since it allows applying accurate conservation plans2. Numerous phenotypic markers have been proposed to detect these stresses3,4. Among them, fluctuating asymmetry (FA) is used to quantify the developmental stability under various conditions5. Developmental stability refers to the developmental processes that ensure phenotypic consistency under fixed environmental and genetic conditions in spite of small random variation during development (i.e. developmental noise)6,7,8. From the postulate that both sides of a symmetrical organism are controlled by the same genes and are exposed to the same environmental conditions, it is considered that deviations from perfect symmetry reflect those random errors of the development, and the ability of the organism to buffer them5. Increase in FA is thus usually interpreted as a decrease in developmental stability. In some field and laboratory studies, fluctuating asymmetry has been shown to increase under a range of both genetic and environmental stresses (e.g. inbreeding, low habitat connection, toxic stress; e.g.9,10). However, other studies have failed to detect such relation with stress e.g.11. The use of FA as a general and sensitive stress indicator has thus been seriously questioned12,13, see14 for a quantitative historical survey of the literature. Directional asymmetry (DA) is another type of asymmetry, which implies a predominant direction and a population mean of the left-right asymmetry different from zero5. Surprisingly enough, DA is common among insect wings15. This type of asymmetry has been thought to be adaptive and genetically determined16 but see15. Besides asymmetry, shift in overall wing shape, size and increase in trait variances have been reported under stressful conditions and have then been proposed as stress indicators17,18,19, although they have been very poorly investigated.
By pollinating major crops (e.g. tomato, oilseed rape) and various wild plants (e.g. Fabaceae), bumblebees show high economic and ecological value20. It has been demonstrated that their populations are suffering from a severe decline for the last decades; with potentially serious consequences on the ecosystem services they provide21,22. Five main factors are traditionally pinpointed: (i) modification and fragmentation of high quality habitats, (ii) agricultural practices, (iii) pathogen parasite spillover, (iv) competition with alien species, and (v) climate change reviewed in23. All these factors and their potential synergy lead to significant direct or indirect environmental and genetic stresses that have potentially lethal and sub-lethal effects on bumblebee individuals.
Here, we investigate the effect of a high diversity of distinct stressors, which are known to affect pollinators, on the size and shape of bumblebee anterior wings (Bombus terrestris; Hymenoptera: Apidae) reared in laboratory conditions. In bee populations under stabilizing selection, phenotypic shifts can negatively affect life history traits related to fitness, for example, mating success in honeybees24 and foraging range25. We tested four different stressors: (i) nutritional stress using a willow (i.e. Salix sp.) diet supplemented with plant secondary compounds, either sinigrin (i.e. glucosinolate found in some Brassicaceae) or amygdalin (i.e. cyanogenic glycoside found in Fabaceae and Rosaceae) at 100% and 200% of their natural concentration; (ii) parasite stress using the parasitic neogregarine Apicystis bombi (Apicomplexa: Neogregarinorida); (iii) thermic stress comparing rearing temperatures of 21 °C and 33 °C; and (iv) an inbreeding stress using F1 inbred generations. For each treatment, a control was established using non-supplemented Salix diet, a rearing temperature of 26 °C. We hypothesised that those stresses should lead to a significant shift in overall wing size and shape as well as to a higher trait variability and fluctuating asymmetry. Since fluctuating asymmetry displayed very contrasting results in previous studies, shifts in overall size and shape might turn out to be more accurate phenotypic markers of stress.
Results
Wing size and shape analyses
Size - No significant difference in centroid size was found among diets supplemented in amygdalin and the control group (Fig. 1B; F2,147 = 1.36; p-value = 0.152). One-way ANOVA of the CS showed significant differences between the different sinigrin treatments (F2,147 = 3.75; p-value = 0.02). Bumblebees fed on a 200% sinigrin supplemented diet were significantly larger than those fed on the control diet (Fig. 1C; Tukey’s HSD test; F1,49 = 3.03; p-value = 0.02). No significant effect of 100% supplementation was found irrespective of the plant secondary compound (Tukey’s HSD test; all p-values > 0.05). Both the A. bombi infection (Fig. 1D; t-test; t = −4.49; df = 93.509; p-value < 0.001) and the inbreeding (Fig. 1F; t-test; t = 3.35; df = 78.487; p-value < 0.001) significantly reduced the bumblebee wing size. Regarding the thermic stress, one-way ANOVA showed significant differences between the different treatments (Fig. 1E; F2,147 = 127; p-value < 0.001). The males emerging from the micro-colonies reared at 33 °C were significantly smaller than those from the control micro-colonies (Tukey’s HSD test; F1,49 = 60.65; p-value < 0.001). By contrast, the cold exposure (i.e. thermic stress at 21 °C) had no significant impact (Tukey’s HSD test; F1,49 = 0.2; p-value > 0.05).
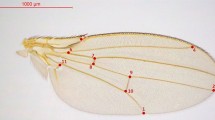
(A) Right forewing of Bombus terrestris with the 18 landmarks indicated to describe the shape. Length of radial cell is defined by the distance from landmark 1 to landmark 2. (B–F) Box plot of centroid size (CS) for the bumblebees bred in the five treatments: (B) Amygdalin treatment (Amy 0 = Amygdalin 0%, Amy100 = Amygdalin 100%, Amy200 = Amygdalin 200%). (C) Sinigrin treatment (Sin0 = Sinigrin 0%, Sin100 = Sinigrin 100%, Sin200 = Sinigrin 200%), (D) Parasite treatment, (E) Temperature treatment, (F). Inbreeding treatment. For each sub-dataset in each treatment, we measured 50 individuals.
Shape - The PCA displayed large overlap of stressed and control groups, except for the inbreeding stress (i.e. slight overlap between inbred and outbred bumblebees; Fig. 2). The perMANOVA on the PCA axes revealed significant differences between each treatment inside each sub-dataset (Table 1). The between-group PCA (Fig. S1) including all experiments and treatments mostly opposes the different experiments (opposing for example the samples used in the inbreeding analysis to those from the sinigrin treatments on the PC1). No clustering was detected within the samples undergoing stressful treatments.
Ordination of the bumblebees bred in five treatments along the first two axes of the principal component analysis. (A) Amygdalin treatment; (B) Sinigrin treatment, (C) Parasite treatment, (D) Temperature treatment, (E) Inbreeding treatment. The percentage in brackets represents the variance along the first and the second axis of the PCA.
Variance of shape and size - Variance analyses revealed significant impact of several tested stresses (Table 2). Diet supplementation with amygdalin, either 100% or 200%, led to higher size variance than in control group (F49,100 = 2.5 and F49,100 = 8.59 respectively; p-value = 0.008 and p-value = 0.01 respectively) whereas diet supplementation with sinigrin did not show higher size variance compared to the control group (F-test; all p-value > 0.05). Wing size displayed a higher variance for individuals originating from infected micro-colonies (F49,100 = 3.54; p-value < 0.001) and inbred colonies (F49,100 = 1.82; p-value < 0.001) compared to their respective control group. Similarly, the thermic stress impacted the wing size variance with a higher variance for males reared at 33 °C (heat wave) than those reared at 21 °C and 26 °C (F49,100 = 1.35 and F49,100 = 1.74 respectively; df = 2; p-value < 0.001) but no significant differences in wing size variance were found between bumblebees reared at 21 °C and 26 °C (F49,100 = 1.02; p-value = 0.367). No significant differences in shape variance were found for any tested stress (F-test; all p-value > 0.05).
Asymmetry analyses
In each group of each treatment, significant directional asymmetry (DA) was found in both wing size and shape (Table 1; Procrustes ANOVA, all p-value < 0.05), except for size DA of bumblebees bred in the control diet of the sinigrin treatment and those bred at a temperature of 33 °C (Procrustes ANOVA, both p-value > 0.05). When comparing groups, none tested stress impacted the shape DA (F-test, all p-value > 0.05) but significant differences in size DA were found for each stress (F-test, all p-value < 0.01). Regarding the nutritional stress, bumblebees fed on diet supplemented with sinigrin (both 100% and 200%) displayed a significantly higher size DA compared to the control group (F1,100 = 723.8 and F1,100 = 1872.4 respectively; df = 2; both p-value < 0.05) but no impact of amygdalin was detected (F-test; df = 2; p-value > 0.05). Whereas infested bumblebees displayed a higher size DA compared to non-infested ones (F1,100 = 5.43; df = 1; p-value < 0.001), those reared at 33 °C (i.e. thermic stress mimicking a heat wave) had a lower size DA compared to those exposed to cold and control groups (F1,100 = 33.05 and F1,100 = 252.96 respectively; df = 2; p-value < 0.001).
As for DA analysis, FA10 analysis did not show any difference in shape FA for the different tested stresses (Table 2; Procrustes ANOVA; p-value > 0.05). In contrast to shape FA, FA10 analysis revealed a significant increase of size FA in infested bumblebees (F49,100 = 4.73; df = 1; p-value < 0.001) and in bumblebees reared at 33 °C (F49,100 = 5.93; df = 2; p-value = 0.03) compared to respective control groups. Regarding nutritional stress, size FA was significantly lower in bumblebees fed with a diet supplemented in 200% sinigrin compared to the other treatments (100% supplemented and control group), although the p-value was quite close to the threshold (F49,100 = 1.24 and F49,100 = 1.34 respectively; df = 2; p-value = 0.049 and 0.033 respectively). Size FA was not significantly different between the respective control groups and the amygdalin and inbreeding treatments (F-test; df = 2 and 1 respectively; all p-value > 0.05).
The PCoA applied on the shape FA matrices did not suggest any clustering of the matrices of the stressed samples (not shown).
Discussion
There is evidence that secondary compounds e.g.26, parasites e.g.27, temperature e.g.17 and inbreeding e.g.28 may affect different morphological traits in their size and/or shape as confirmed in our results. While size and shape were always impacted by the tested stressors, size variance, size FA and DA were only impacted by some of them, and shape variance, FA and DA were never significantly impacted. Parasites and high rearing temperatures led to the strongest phenotype modifications: both impacted overall wing size and shape, size variance, size FA and DA, but also shape variance in the case of the parasitic stress. While in the literature, various stressors can induce unspecific and similar effects (e.g. on the immune system, metabolism or antioxidative response29), the effects can also be stressor-specific and differ for a same category of stressor (e.g. effects of secondary compounds on colony development30). In this paper, various stressful treatments did not generate similar shape changes (Fig. S1) which suggests that the nature of the treatment rather than stress per se determined the type of induced wing shape change. Similarly, for shape asymmetry, the lack of clustering of the FA matrices in the PCoA suggests that the nature of the shape asymmetry depends on the nature of the environmental treatment and that no general effect of stress can be detected.
Whereas food quantity and protein richness are known to have considerable effect on body size31, the impact of secondary compounds remains understudied. However there is evidence that several metabolic pathways can be affected by these secondary compounds32, which in turn can decrease the ability to buffer perturbations during the development although it has to be noticed that several secondary compounds can display beneficial effects on pollinators33. Our results suggested that such repercussions are probably compound-dependant as sinigrin and amygdalin displayed contrasted results, with a size increase for bumblebees fed on a diet supplemented in sinigrin and no significant effect for bumblebees fed on a diet supplemented in amygdalin. While several studies directly assessed the toxicity of amygdalin on bees e.g.34, the toxicity of sinigrin was only addressed in indirect studies on others insects35. Bees may have developed specific adaptation to those secondary compounds with the activation of detoxification mechanisms that could buffer perturbations of the developmental processes (i.e. only subtle modifications). Additionally the absence of significant effect of amygdalin on body size could result from an absence of biological targets in the process involved in wing development.
Although parasites can have a considerable impact on bees36 and interfere with their larval development37, the relationship among size, shape and parasitic prevalence has rarely been assessed27. Here we highlighted that infested bumblebees were significantly smaller than non-infested ones in the same rearing conditions. An interpretation of this effect is that the energy usually allocated towards the growth and development could be hijacked by the parasite and/or allocated to the immune response, thus leading to morphological changes and size decrease.
As predicted by the temperature-size (TS) rule, higher developmental temperature resulted in smaller body size. This TS rule is analogous to the Bergmann’s rule (i.e. larger body size at higher latitude) that applies to many ectotherms while mechanisms behind this trend remain debated38. However, recent studies provided opposite results, with bumblebees being smaller under higher latitudes and colder temperatures39,40. This converse Bergmann’s rule can result from a “season length effect” with starvation phenomenon (i.e. reduced availability of floral resources) that can occur at higher latitudes41. Such difference in larval feeding between in natura and laboratory conditions (i.e. fed ad libitum) could be responsible for those conflicting results. Moreover, since size does not increase monotonically with decreasing temperature, it would be more accurate to say that size is maximized at optimal physiological conditions.
Finally, while it is commonly assumed that inbreeding events affect several fitness components e.g.42, its impact on size is less consistently supported in the literature. In laboratory conditions, Gerloff and colleagues43 failed to detect any decrease in size after one generation of inbreeding using wild bumblebees. In our experiments, we used domesticated bumblebees obtained from industrial commercial breeding company that are likely to display lower levels of genetic diversity than wild ones44 since suffering from stronger inbreeding depression.
Regarding directional asymmetry and fluctuating asymmetry, no effect was detected on wing shape. By contrast, wing size asymmetry was significantly affected by the sinigrin, parasitic and thermic stresses; DA and FA being higher for the stressed individuals than for the control ones. With regard to FA, previous studies are inconsistent when assessing the impact of stress on developmental stability, whether studied organisms were insects or not45,46. For example, rearing temperature has repeatedly been found to affect FA in insects e.g.47,48, although some studies provided more equivocal or even negative results e.g.49. Extreme rearing temperature can affect the genetic cascade that is involved in the trait morphology during the larval development and could decrease the buffering developmental mechanisms of stabilizing proteins e.g.50. Additionally, inbreeding can lead to the expression of deleterious recessive mutations by increasing the homozygote frequency and thus could affect the developmental stability and increase FA4. Although the occurrence of diploid males in the inbred generation in our bioassays clearly demonstrated a genetic stress51, one generation of inbreeding might be insufficient to disrupt the bumblebee developmental stability, resulting in a similar FA between inbred and outbred colonies. Unfortunately, the lack of queens in the inbred generation prevented the production of additional inbred generations. Further bioassays are needed to test whether prolonged inbreeding may affect fluctuating asymmetry.
However, caution must be taken when considering the results based on FA as most of the groups displayed significant DA, which could lead to FA overestimation52. Genetic variation for DA could additionally inflate FA estimates e.g.53. Although DA has been widely reported in insects including bees e.g.15,54, it was not significant in most of the studies led on bee wings e.g.55. Actually, the use of FA as stress indicator seems to be traits-, stressors- and/or species-dependent, leading to non-congruent results among studies e.g.46,56. The setting of the study also influence this relationship. Indeed, different measures of FA can be used and display more or less accuracy to measure trait variability56. A review of environmental stressors56 even suggested that the nature of the stressor (e.g. temperature, nutrition, parasitism…) was not the main driver explaining the contrasting results among studies. By contrast, laboratory studies on fitness-related traits showed significantly larger negative effect of stressors on fluctuating asymmetry56. Even if our framework corresponds to those conditions, our results were still particularly contrasted.
Overall shape and size have been used in many fields such as Systematics and Palaeontology, mainly to detect changes among taxa or populations e.g.57,58. An additional application as stress estimator has been suggested since quantifying phenotype is less costly, can be non-lethal and is quite easy to obtain compared to other biomarkers (e.g. proteome59). Moreover, phenotypic changes occur usually before there is much mortality, which supports their use as a good stress marker for monitoring plan. Although fluctuating asymmetry has been used in various studies, the multiple responses in face of stressful conditions suggests that alternative phenotypic indicators of stress should be investigated. Our results have shown that size and/or shape are significantly affected by environmental and genetic stresses and are potentially accurate stress indicators18. Klingenberg and colleagues60 showed that variation within individuals was much lower than variation between individuals, corroborating the hypothesis that overall shape and size could be more sensitive stress indicators than FA. However, further studies are still needed to assess the accuracy of these stress indicators in additional taxa and their responses in natura.
Methods
Experimental design
Bombus terrestris (L., 1758) (see61 for an overview about this species) colonies were obtained from Biobest N.V. company (Westerlo, Belgium) and maintained in the laboratory of Zoology in Mons (Belgium).
Four different experimental bioassays were designed according to four tested stresses:
Plant toxin stress – We selected two secondary compounds detected in pollen collected by bumblebees: sinigrin and amygdalin. Sinigrin is a glucosinolate found in some Brassicaceae (2.226 µg/g in bee pollen samples62) and is toxic for most insects63. Amygdalin is a cyanogenic glycoside common in Fabaceae and Rosaceae (e.g. 1.889 mg/g in Prunus amygdalus pollen). When metabolized, it releases hydrogen cyanide (HCN) that is highly toxic for many animals and could impact bees through a long-term consumption34. This toxin induces “malaise” behaviour in honeybee, which consists in different behaviours like limited feeding and locomotion, and modifications of grooming behaviour64. Both molecules were detected in floral resources (i.e. pollen and nectar). We tested three diets per secondary compound: one control diet of willow pollen (Salix sp.)65,66 with water-ethanol mix; two diets of willow pollen (Salix sp.) added with the secondary compound (100% and 200% of their natural concentration in the pollen). We added some volume of BIOGLUC® syrup to each mixture to make consistent “candies” easier to manipulate. Salix pollen diet was prepared with commercial crushed honeybee pollen loads. For each treatment, ten queenless micro-colonies (i.e. colony containing only five workers66) were reared until emergence of at least five haploid males per micro-colony (n = 50 per treatment).
Parasitic stress – We used 20 queenless micro-colonies half of which have been infected with the neogregarine Apicystis bombi (Liu, Macfarlane & Pengelly, 1974) found in the body cavity of bumblebees67. This parasite appears to be highly virulent to bumblebee spring queens, inducing a rapid death through a pollen infested ingestion (i.e. B. pratorum68). Bumblebees were given a 20 μL droplet of syrup from a solution homogenised at 833 oocysts/μL. The 10 non-infected micro-colonies were used as a control. All micro-colonies were reared until emergence of at least five haploid males per micro-colony (n = 50 per treatment). Before the experiments the pollen diet was screened for the presence of Apicystis bombi following the protocol of69. No Apicystis bombi was detected in the pollen. Thermic stress – 30 queenless micro-colonies were reared at three different temperatures (21, 26 and 33 °C) in a climate controlled room. Bumblebee colonies are known to regulate their temperature to a set point between 27 and 33 °C70. Twenty one degrees corresponds to a cold stress: such low temperatures have indeed been shown to disrupt colony thermoregulation and have a negative impact on colony fecundity and brood incubation temperature71. Thirty three degrees is known as the upper limit where bumblebees can thermo-regulate their colonies by ventilation70 and is thus here considered a heat stress, mimicking the ground temperature during a heat wave. For each temperature, 10 micro-colonies were used. As for the previous experimental designs, all micro-colonies were reared until emergence of at least five haploid males per micro-colony (n = 50 per treatment).
Inbreeding stress – From the development of five colonies newly emerged queens and males were placed in a “flight cage” to force brother-sister mating. These mated queens were used to produce inbred generation colonies. During two months, the mated queens overwintered following the rearing method of 72. Fifteen out of 29 mated queens initiated colonies. For this inbreeding stress, we used workers (n = 50) to perform the subsequent morphometric analyses instead of haploid males because the inbred generation produced only few haploid males. Workers from the parent (outbred) generation were used as control (n = 50).
Our total dataset contains 650 individuals. The right and left forewings of each specimen were removed, placed on a slide and photographed using an Olympus SZH10 microscope with an AF-S NIKKOR 18–105 millimetres (Shinjuku, Japan) and GWH10X-CD oculars coupled with a Nikon D200 camera (Shinjuku, Japan).
Wing size and shape analysis
Two-dimensional coordinates of 18 landmarks were obtained (Fig. 1A) from right and mirror-reflected left wings pictures using tps-DIG v2.3073. The landmark configurations were scaled, translated and rotated against the consensus configuration using the GLS Procrustes superimposition method, to remove all non-shape components74. The superimposition was performed using the package geomorph75. Wing size was estimated by the centroid size (CS, i.e. the square root of the sum of squared distance between all landmarks and their centroid; e.g.76). Each wing was digitized twice by the same experimenter (MG), in order to account for measurement error.
Preliminary analyses revealed that wing CS is related to wing size and was thus used as proxy. After having checked residuals normality (Shapiro test) and homoscedasticity (Bartlett test), type one ANOVAs were used to test the effect of the nutritional and thermic stresses on CS. Multiple pairwise comparisons (i.e. post-hoc tests, Tukey HSD) were conducted when significant difference among treatments was detected. T-tests were performed to assess the effect of parasitic and inbreeding stresses on CS.
For shape, principal component analyses (PCA) were conducted for each experiment to investigate the shape variation among the different treatments, using the geomorph function “plotTangentSpace”. Permutational MANOVAs and multiple pairwise comparisons were then performed on these principal components to assess the effect of each stressor on wing shape (Euclidean distances, 999 permutations, vegan function “adonis”) after having tested for multivariate homogeneity.
A global comparison of wing shape across experiments was done using a between-group PCA, taking each experiment as a priori grouping. This allowed us to investigate whether the various selected stressors had similar effects on wing shape.
Asymmetry analyses
The procedure to measure FA (i.e. deviations from perfect symmetry by substracting left minus right sides values) is as follows. For shape, a two-way Procrustes ANOVA using individual, side, and their interaction as effects was applied to the superimposed coordinates. This Procrustes ANOVA allows, in a single procedure, to test for the significance of (i) the among-individual variation (individual effect), (ii) the directional asymmetry (DA) (side effect), and (iii) the fluctuating asymmetry (FA) relative to measurement error (interaction individual × side). The rationale for this procedure can be found in Palmer and Strobeck5,77 as well as in78. An error free estimate of shape FA was then derived (FA10 index77). Because FA10 is a variance estimate, F-test was used to compare differences in FA among the different treatments for each stressor. A similar two-way ANOVA was applied to centroid size to estimate size FA and an error corrected index (FA10) was computed. Inter-individual variances in size and shape were estimated as the mean squared (MS) of the individual effect, and the side MS was used as an estimate of directional asymmetry.
To investigate whether the patterns of shape asymmetry were similar across experiments and treatments (i.e. whether wings displayed the same ways to be asymmetric in response to different stressors), we performed a Principal Coordinates Analysis (PCoA) on the shape FA covariance matrices (i.e. interaction individual × side MSCP matrices; e.g.49).
Statistical analyses were performed using the software R version 3.4.079 (2017, http://www.R-project.org/).
Data Accessibility
All morphometric data will be available on the Dryad database as TPS file once the paper will be accepted.
References
Tarlow, E. M. & Blumstein, D. T. Evaluating methods to quantify anthropogenic stressors on wild animals. Appl. Anim. Behav. Sci. 102, 429–451 (2007).
Frankham, R. Genetics and extinction. Biol. Conserv. 126, 131–140 (2005).
Lerner, I. M. Genetc homeostasis. New York: Oliver and Boyd (1954).
Leamy, L. J. & Klingenberg, C. P. The genetics and evolution of fluctuating asymmetry. Annu. Rev Ecol. Evol. S 36, 1–21 (2005).
Palmer, A. R. & Strobeck, C. Fluctuating asymmetry: measurement, analysis, pattern. Annu. Rev. Ecol. Syst. 17, 391–421 (1986).
Waddington, C. H. The strategy of the genes. New-York: Macmillan (1957).
Debat, V. & David, P. Mapping phenotypes: canalization, plasticity and developmental stability. Trends Ecol. Evol. 16, 555–561 (2001).
Debat, V. & Peronnet, F. Asymmetric flies: the control of developmental noise in Drosophila. Fly 7, 70–77 (2013).
Rasmuson, M. Fluctuating asymmetry - indicator of what? Hereditas 136, 177–193 (2002).
Chang, X., Zhai, B. P., Wang, M. & Wang, B. Relationship between exposure to an insecticide and fluctuating asymmetry in a damselfly (Odonata, Coenagrionidae). Hydrobiologia 586, 213–220 (2007).
Kruuk, L. E. B., Slate, J., Pemberton, J. M. & Clutton-Brock, T. H. Fluctuating asymmetry in a secondary sexual trait: no associations with individual fitness, environmental stress or inbreeding, and no heritability. J. Evolution. Biol. 16, 101–113 (2003).
Palmer, A. R. Waltzing with asymmetry. Bioscience 46, 518–532 (1996).
Houle, D. High Enthusiasm and low R-squared. Evolution 52, 1872–1876 (1998).
Debat, V. Symmetry is beauty – or is it?: Grandeur et decadence de l’asymétrie fluctuante. Médecines/sciences 32, 774–780 (2016).
Pelabon, C. & Hansen, T. F. On the adaptive accuracy of directional asymmetry in insect wing size. Evolution 62, 2855–2867 (2008).
Windig, J. J. & Nylin, S. Adaptive wing asymmetry in males of speckled wood butterfly (Pararge aegeria)? P. Roy. Soc. Lond. B. Bio. 266, 1413–1418 (1999).
Hoffmann, A. A., Collins, E. & Woods, R. E. Wing shape and wing size changes as indicators of environmental stress in Helicoverpa punctigera (Lepidoptera: Noctuidae) moths: comparing shifts in means, variances and asymmetries. Environ. Entomol. 31, 965–971 (2002).
Hoffmann, A. A. et al. Wing shape versus asymmetry as an indicator of changing environmental conditions in insects. Aust. J. Entomol. 44, 233–243 (2005).
Pinto, N. S., Silva, D. P., Rodrigues, J. G. & De Marco, P. Jr. The size but not the symmetry of the wings of Eulaema nigrita Lepeletier (Apidae: Euglossini) is affected by human-disturbed landscapes in the Brazilian Cerrado savanna. Neotrop. Entomol. 44, 439–447 (2015).
Velthuis, H. H. W. & van Doorn, A. A century of advances in bumblebee domestication and the economic and environmental aspects of its commercialization for pollination. Apidologie 37, 421–451 (2006).
Potts, S. G. et al. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 25, 345–353 (2010).
Nieto A., et al. European Red List of bees, Publication Office of the European Union (2014).
Goulson, D., Lye, G. C. & Darvill, B. Decline and conservation of bumblebees. Annu. Rev. Entomol. 53, 191–208 (2008).
Jaffé, R. & Moritz, R. F. A. Mating flights select of symmetry in honeybee drones (Apis mellifera). Naturwissenschaften 97, 337–343 (2010).
Greenleaf, S. S., Williams, N. M., Winfree, R. & Kremen, C. Bee foraging ranges and their relationship to body size. Oecologia 153, 589–596 (2007).
Padro, J., Carreira, V., Corio, C., Hasson, E. & Soto, M. Host alkaloids differentially affect developmental stability and wing vein canalization in cactophilic Drosophila buzzatii. J. Evolution. Biol. 27, 2781–2797 (2014).
Polak, M. Parasites increase fluctuating asymmetry of male Drosophila nigrospiracula: implications for sexual selection. Genetica 89, 255–265 (1993).
Fredrickson, R. & Hedrick, P. Body size in endangered Mexican wolves: effects of inbreeding and cross-lineage matings. Anim. Conserv. 5, 39–43 (2002).
Münzel, T. & Daiber, A. Environmental Stressors and Their Impact on Health and Disease with Focus on Oxidative Stress. Antioxid. Redox. Signal. 28, 735–740 (2018).
Detzel, A. & Wink, M. Attraction, deterrence or intoxication of bees (Apis mellifera) by plant allelochemicals. Chemoecology 4, 8–18 (1993).
Roulston, T. H. & Cane, J. H. The effect of pollen protein concentration on body size in the sweat bee Lasioglossum zephyrum (Hymenoptera: Apiformes). Evol. Ecol. 16, 49–65 (2002).
Whitman, D. W. & Agrawal, A. A. What is phenotypic plasticity and why is it important? In: Phenotypic Plasticity of Insects, pp 1-63, Science Publishers, Enfield, NH (2009).
Stevenson, P. C., Nicolson, S. W. & Wright, G. Plant Secondary Metabolites in Nectar: Impacts on Pollinators and Ecological Functions. Funct. Ecol. 31, 65–75 (2016).
Kevan, P. G. & Ebert, T. Can Almond Nectar & Pollen Poison Honey Bees? Am. Bee J. 145, 507–509 (2005).
Morgan, E. D. Biosynthesis in Insects. The Royal Society of Chemistry, Cambridge (2004).
Otti, O. & Schmid-Hempel, P. A field experiment on the effect of Nosema bombi in colonies of the bumblebee Bombus terrestris. Ecol. Entomol. 35, 577–582 (2008).
Shimanuki, H., Know, D. A., Furgala, B., Caron, D. M. & Williams, J. L. Diseases and pests of honey bees. In: Graham J.M. (ed) The hive and the honey bee. Dadant & Sons Inc., Hamilton, pp 1083–151 (1992).
Angilletta, M. J. & Dunham, A. E. The Temperature-Size Rule in Ectotherms: Simple Evolutionary Explanations May Not Be General. Am. Nat. 162, 332–342 (2003).
Ramirez-Delgado, V. H., Sanabria-Urban, S. & Serrano-Meneses, M. A. Cueva del Castillo, R. The converse to Bergmann’s rule in bumblebees, a phylogenetic approach. Ecol. Evol. 6, 6160–6169 (2016).
Gérard, M. et al. Patterns of size variation in bees at a continental scale: does Bergmann’s rule apply? Oikos 127, 1095–1103 (2018).
White, T. C. R. The role of food, weather and climate in limiting the abundance of animals. Biol. Rev. 83, 227–248 (2008).
Keller, L. F. & Waller, D. M. Inbreeding effects in wild populations. Trends Ecol. Evol. 17, 1735–1741 (2002).
Gerloff, C. U., Ottmer, B. K. & Schmid-Hempel, P. Effects of inbreeding on immune response and body size in a social insect. Bombus terrestris. Funct. Ecol. 17, 582–589 (2003).
Medugorac, I. et al. Conservation priorities of genetic diversity in domesticated metapopulations: a study in taurine cattle breeds. Ecol. Evol. 1, 408–420 (2011).
Vollestad, L. A., Hindar, K. & Moller, A. P. A meta-analysis of fluctuating asymmetry in relation to heterozygosity. Heredity 83, 206–218 (1999).
Lens, L., Van Dongen, S., Kark, S. & Matthysen, E. Fluctuating asymmetry as an indicator of fitness: Can we bridge the gap between studies? Biol. Rev. Camb. Philos. 77, 27–38 (2002).
Imasheva, A. G., Loeschcke, V., Zhivotovsky, L. A. & Lazebny, O. E. Effects of extreme temperature on phenotypic variation and developmental stability in Drosophila melanogaster and Drosophila buzzatii. Biol. J. Linnean Soc. 61, 117–126 (1997).
Debat, V. et al. Multidimensional analysis of Drosophila wing variation in Evolution Canyon. J. Genet. 87, 407–419 (2008).
Debat, V., Debelle, A. & Dworkin, I. Plasticity, canalization and developmental stability of the Drosophila wing: join effects of mutations and of developmental temperature. Evolution 63, 2864–2876 (2009).
Runcie, D. A. et al. Genetics of gene expression responses to temperature stress in a sea urchin gene network. Mol. Ecol. 21, 4547–4562 (2012).
Zayed, A. Bee genetics and conservation. Apidologie 40, 237–262 (2009).
Graham, J. H., Emlen, J. M., Freeman, D. C., Leamy, L. J. & Kieser, J. A. Directional asymmetry and the measurement of developmental instability. Biol. J. Linnean Soc. 64, 1–16 (1998).
Stige, L. C., David, B. & Alibert, P. On hidden heterogeneity in directional asymmetry – can systematic bias be avoided? Evol. Biol. 19, 492–499 (2006).
Smith, D. R., Crespi, B. J. & Bookstein, F. L. Fluctuating asymmetry in the honey bee, Apis mellifera: effects of ploidy and hybridization. J. Evolution Biol. 10, 551–574 (1997).
Clarke, G. M. The genetic basis of developmental stability. III. Haplo-diploidy: Are males more unstable than females? Evolution 51, 2012–2028 (1997).
Beasley, D. A. E., Bonisoli-Alquati, A. & Mousseau, T. A. The use of fluctuating asymmetry as a measure of environmentally induced developmental instability: A meta-analysis. Ecol. Indic. 30, 218–226 (2013).
Dehon, M., Michez, D., Nel, A., Engel, M. S. & De Meulemeester, T. Wing shape of four new bee fossils (Hymenoptera: Anthophila) provides insights to bee evolution. PLoS ONE 9, e108865 (2014).
Gérard, M. et al. Discrimination of haploid and diploid males of Bombus terrestris (Hymenoptera; Apidae) based on wing shape. Apidologie 46, 644–653 (2015).
Leung, B., Knopper, L. & Mineau, P. A critical assessment of the utility of fluctuating asymmetry as a bioindicator of anthropogenic stress. In: Polak, M. (Ed.), Developmental Instability: Causes and Consequences. Oxford University Press, New York (2003).
Klingenberg, C. P., Badyaev, A. V., Sowry, S. M. & Beckwith, N. J. Inferring Developmental Modularity from Morphological Integration: Analysis of Individual Variation and Asymmetry in Bumblebee Wings. Am. Nat. 157, 11–23 (2001).
Rasmont, P., Coppée, A., Michez, D. & De Meulemeester, T. An overview of the Bombus terrestris (L. 1758) subspecies (Hymenoptera: Apidae). Ann. Soc. Entomol. Fr. 55, 243–250 (2008).
Ares, A. M., Nozal, M. J. & Bernal, J. Development and validation of a liquid chromatography-tandem mass spectrometry method to determine intact glucosinolates in bee pollen. J. Chromatogr. B. 1000, 49–56 (2015).
Ahuja, I., Rohloff, J. & Bones, A. M. Defence mechanisms of Brassicaceae: implications for plant-insect interactions and potential integrated pest management. A review. Agron. Sustain. Dev. 30, 311–348 (2010).
Hurst, V., Stevenson, P. C. & Wright, G. A. Toxins induce “malaise” behaviour in the honeybee (Apis mellifera). J. Comp. Physiol. A 200, 881–890 (2014).
Génissel, A., Aupinel, P., Bressan, C., Taseï, J. N. & Chevrier, C. Influence of pollen origin on performance of Bombus terrestris micro-colonies. Entomol. Exp. Appl. 104, 329–336 (2002).
Vanderplanck, M. et al. How Does Pollen Chemistry Impact Development and Feeding Behaviour of Polylectic Bees? PLoS ONE 9, e86209, https://doi.org/10.1371/journal.pone.0086209 (2014).
Lipa, J. J. & Triggiani, O. Apicystis gen nov and Apicystis bombi (Liu, Macfarlane and Pengelly) comb. nov. (Protozoa: Neogregarinida), a cosmopolitan parasite of Bombus and Apis (Hymenoptera: Apidae). Apidologie 27, 29–34 (1996).
Rutrecht, S. T. & Brown, J. F. M. The life-history impact and implications of multiple parasites for bumble bee queens. Int. J. Parasitol. 38, 799–808 (2008).
Meeus, I., de Graaf, D. C., Jans, K. & Smagghe, G. Multiplex PCR detection of slowly-evolving trypanosomatids and neogregarines in bumblebees using broad-range primers. J. Appl. Microbiol. 109, 107–115 (2010).
Vogt, F. D. Thermoregulation in bumblebee colonies I. Thermoregulatory versus Brood-Maintenance Behaviours during Active Changes in Ambient Temperature. Physiol. Zool. 59, 55–59 (1986).
Vogt, F. D. Thermoregulation in bumblebee colonies II. Behavioral and Demographic Variation throughout the Colony Cycle. Physiol. Zool. 59, 60–68 (1986).
Lhomme, P. et al. A method for year-round rearing of cuckoo bumblebees (Hymenoptera: Apoidea: Bombus subgenus Psithyrus). Ann. Soc. entomol. Fr. 49, 117–125 (2013).
Rohlf, F. J. tpsDig, version 2.17. Department of Ecology & Evolution, State University of New York, Stony Brook (2013).
Bookstein, F. L. Morphometric tools for Landmark Data: Geometry and Biology. Cambridge, Cambridge University Press (1991).
Adams, D. C. & Otárola-Castillo, E. Geomorph: An R package for the collection and analysis of geometric morphometric shape data. Methods Ecol. Evol. 4, 393–399 (2013).
Zelditch, M. L., Swiderski, D. L., Sheets, H. D. & Fink, W. L. Geometric Morphometrics for biologists: a primer. Elsevier Academic Press, San Diego (2004).
Palmer, A. R. & Strobeck, C. Fluctuating asymmetry analyses revisited. Pp. 279–319 in Polak, M. ed. Developmental instability: causes and consequences. Oxford University Press, Oxford, U.K (2003).
Klingenberg, C. P. & McIntyre, G. S. Geometric morphometrics of developmental instability: analyzing patterns of fluctuating asymmetry with Procrustes methods. Evolution 52, 1363–1375 (1998).
R Development Core Team R: A language and environment for statistical computing, version 3.0.2, ISBN 3-900051-07-0. R Foundation for Statistical Computing. Vienna, Austria (2013).
Acknowledgements
This work was partly supported by the “Fonds de la Recherche Scientifique - FNRS” and the Fonds “Wetenschappelijk Onderzoek - Vlaanderen (FWO)” under EOS Project n°30947854 and the FRFC project n°2461310. MG was supported by a grant from the Belgian Fonds pour la Recherche dans l’Industrie et l’Agriculture (FRIA) and MV was FNRS grant fellowship “Chargé de recherches”.
Author information
Authors and Affiliations
Contributions
M.G., M. Van, D.M., I.M., N.P., G.S. conceived the ideas and designed the methodology; M.G., M. Vas and O.S. collected the data; M.G. and V.D. analysed the data; M.G. and L.F. led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gerard, M., Michez, D., Debat, V. et al. Stressful conditions reveal decrease in size, modification of shape but relatively stable asymmetry in bumblebee wings. Sci Rep 8, 15169 (2018). https://doi.org/10.1038/s41598-018-33429-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-33429-4
Keywords
This article is cited by
-
Developmental instability and body condition in rodents: industrialized agriculture disturbances in anthromes from central Argentina
Mammalian Biology (2024)
-
Land-use changes in a neotropical biodiversity hotspot and its effects on Euglossini bees
Journal of Insect Conservation (2023)
-
Can the shape of the wing help in the identification of African stingless bee species? (Hymenoptera: Apidae: Meliponini)
International Journal of Tropical Insect Science (2023)
-
Effect of urbanization and its environmental stressors on the intraspecific variation of flight functional traits in two bumblebee species
Oecologia (2022)
-
Neonicotinoid insecticides hinder the pupation and metamorphosis into adults in a crabronid wasp
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.