Abstract
To date, studies that reported seasonal patterns of adult neurogenesis and neuronal recruitment have correlated them to seasonal behaviors as the cause or as a consequence of neuronal changes. The aim of our study was to test this correlation, and to investigate whether there is a seasonal pattern of new neuronal recruitment that is not correlated to behavior. To do this, we used adult female zebra finches (songbirds that are not seasonal breeders), kept them under constant social, behavioral, and spatial environments, and compared neuronal recruitment in their brains during two seasons, under natural and laboratory conditions. Under natural conditions, no significant differences were found in the pattern of new neuronal recruitment across seasons. However, under artificial indoor conditions that imitated the natural conditions, higher neuronal recruitment occurred in late summer (August) compared to early spring (February). Moreover, our data indicate that “mixing” temperature and day length significantly reduces new neuronal recruitment, demonstrating the importance of the natural combination of temperature and day length. Taken together, our findings show, for the first time, that neuroplasticity changes under natural vs. artificial conditions, and demonstrate the importance of both laboratory and field experiments when looking at complex biological systems.
Similar content being viewed by others
Introduction
Seasonal neuronal recruitment has been linked to seasonal behavior. A prominent example is the song control system in the avian brain, where seasonal changes in song behavior are accompanied by changes in the song nuclei in the brain, including seasonal neuronal recruitment, in almost every seasonally breeding songbird species that has been examined1. For example, in adult canaries, which are seasonal breeders, neuronal recruitment in the vocal nucleus HVC of males is temporally related to changes in song, with a peak at the end of summer/early fall2. It was demonstrated that both the increase of number of new neurons and singing behavior correlate with the regeneration of HVC-RA (robust nucleus of the arcopallium) circuit in breeding birds3. The identity of the new neurons had been confirmed by Burd and Nottebohm4, and Paton and Nottebohm5 showed that these neurons have neurophysiological profiles and clear neuronal anatomy, and that they are incorporated into functional neural circuits.
Brain plasticity is also correlated to migratory behavior in birds. We have found that in passerines, more new neurons are recruited in the migrant reed warbler (Acrocephalus scirpaceus) than in the resident Clamorous warbler (A. stentoreus), in two forebrain regions that are known to process spatial information - the Hippocampus (HC) and the Nidopallium Caudolaterale (NCL), during spring, summer and autumn6. In Columbiformes, on the other hand, we found an overall higher neuronal recruitment in these regions in brains of the resident laughing dove (Streptopelia senegalesis) than in those of the migratory turtle dove (S. turtur)7. However, regardless of the differences in levels of neuronal recruitment and their directions within each of the tested pairs of species, it is evident that in doves as well as in passerines, there is lower neuronal recruitment in spring compared to other seasons.
A similar pattern of marked seasonality in neuronal recruitment was reported in blackcapped chickadees8, where seasonal brain plasticity correlated with the seasonal food storing behavior and the need to acquire spatial information. The lowest recruitment of new neurons in the HC was evident in early spring (February-March) compared to other seasons (August or October). However, later studies in the same species indicated either another seasonal pattern9, or no seasonality at all10. Sherry and MacDougall-Shackleton11, who reviewed these studies, as well as other studies that investigated additional aspects of hippocampal seasonal changes (e.g. volume), provided evidence that these seasonal changes are not under photoperiod control. They suggested that the variation in the seasonal change in the HC of food-storing parids is the result of various experience-dependent effects, such as the intensity of the behavior, stress, or the amount of exercise in captivity. Another comprehensive recent review by Pravosudov et al.12 on two food-storing species – black capped and mountain chickadees, pointed to inconsistencies between results from different studies regarding the seasonal effect on neuronal recruitment in the HC. However, in both species they found that rates of new neuronal recruitment in the HC were significantly associated with winter climate harshness, with birds from harsher climates having higher neurogenesis rates.
Testing the interactions between behavior and neuronal recruitment is challenging, because it is easy to confound correlation and causation, and difficult to determine the direction of the causal relationships13. Do seasonal environmental changes (e.g. day length, temperature) cause changes in behavior, which, in turn, cause changes in brain nuclei that are associated with this behavior? Or do seasonal environmental changes cause changes in brain nuclei, which, in turn, cause changes in behavior? Brenowitz14 presents evidence for the latter suggestion, arguing that seasonal changes in the song nuclei in seasonal species are predominantly regulated by hormonal changes, and that the subsequent changes in song behavior play a secondary role in reinforcing neuronal changes. On the other hand, Ball et al.15 proposed that singing behavior itself could influence the seasonal neuronal plasticity in the song system, through mediation of testosterone. In addition, as already mentioned above, Sherry & MacDougall-Shackleton11 suggest that, in relation to the seasonal food-storing behavior, seasonal changes in the HC may be a consequence of the behavior itself.
In any case, whichever seasonal pattern had been found in the brain, in most of the above mentioned studies the explanation was related to a seasonal behavior – migration, food-storing, song production, or reproduction. The assumption was that neurogenesis (i.e. neuronal proliferation, which in birds occurs only in the ventricular zone; VZ) does not vary seasonally, but rather the survival of these new neurons that migrate from the VZ to the HC is the neurogenic process that varies seasonally. This was supported only in the seasonal black-capped chickadees, where neurogenesis was stable throughout the seasons whereas neuronal recruitment varied16. However, in many of our previous studies, we used another species - zebra finches (Taeniopygia guttata; ZF), which are considered to be non-seasonal, because they breed at any time, and do not exhibit seasonal changes in singing17. Their diet is very narrow throughout the year: they eat seeds from a limited number of grass species, and rarely anything else18,19. Zebra finches are also very restricted to specific areas all year round, do not travel long distances and do not migrate (reviewed in Zann17). Therefore in our outdoors experiments we assumed that if all behavioral, social, and spatial conditions are constant, then neurogenesis, as well as neuronal recruitment, should be stable throughout the year. Accordingly, we stated that “seasonal changes in temperature and photoperiod were unlikely to have affected the outcome of our studies”20. Others have avoided that issue altogether by keeping the birds under constant indoor conditions (e.g. Tokarev, et al.21). However, to the best of our knowledge, this assumption has not been tested yet.
Accordingly, in the present study we tested whether there is a seasonal neuronal recruitment pattern that is not correlated to behavior, and if so – whether this pattern exhibits less plasticity in early spring than in late summer. We used ZF, which, as mentioned above, are non-seasonal breeders that reproduce opportunistically throughout the year whenever conditions are favorable, both in the wild17 and in captivity22. To avoid effects of singing behavior, we used females, and kept the social, behavioral, and spatial environment constant. Another advantage of females in this respect is that gonadal steroid hormones and corticosterone have no effect on cell proliferation in the VZ23,24. We compared neuronal recruitment rates under two natural outdoor seasons and under artificial settings in the laboratory that imitated the natural conditions. In addition, we used unnatural combinations of photoperiod and temperature to study whether the compatibility between these two cues is significant for neuronal recruitment – namely, whether “mixing” of summer temperature with winter day length and vice versa will affect neuronal recruitment compared with exposure to compatible conditions. Lastly, we tested whether any seasonal effect on neuronal recruitment is region-dependent by analyzing three brain regions that process different sensory inputs, as follows: the HC, which is involved in the processing of spatial information25,26,27 and in stress response28; the Nidopallium Caudale (NC), which contains auditory relays29 and is thought to be involved in vocal communication and integration of auditory information30,31,32, and one of its sub-regions, Nidopallium Caudolaterale (NCL), is considered functionally equivalent to the prefrontal cortex in mammals33 and might have a role in working memory and executive function (e.g.34); and the Medial Striatum (MSt), formerly known as Lobus parolfactorius (LPO) which is linked to visual perception and associative learning35,36,37,38. We believe that testing the possibility of a seasonal neuronal recruitment pattern that is not correlated to behavior will add to the open discussion regarding the functional significance of adult neurogenesis and whether it is vestigial or a consequence of active maintenance14. This question is also highly relevant for the constantly growing research community working with ZF, in planning of future experiments that are designed to examine mechanisms that regulate neuronal recruitment.
Materials and Methods
Experimental design
Female ZF were reared in outdoor breeding colonies at the Meier Segals Garden for Zoological Research at Tel-Aviv University. The birds were banded with numbered and colored plastic rings for individual identification, and at the age of 4–18 months (after reaching adulthood), they were removed from their native colonies and randomly divided into six experimental groups, to achieve the best possible age-matched distribution, according to availability in the different seasons. Each group consisted of 5 females that were housed in a standard cage (65 × 35 × 45 cm).
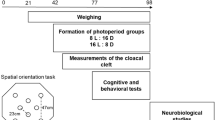
The six experimental groups are presented in Fig. 1. Two control groups were kept outdoors, one in August and the other in February, and exposed to natural ambient temperature and day length of that season: outdoor control August - cA (mean temperature of 29 °C; mean day length of 13.5hrs), and outdoor control February - cF (mean temperature of 16 °C; mean day length of 11hrs). During each of these months, a group of other females were transferred to indoors cages, where they were exposed to artificial conditions of day length and temperature of the opposing season. Thus, in August the birds that were transferred to indoor cages were exposed to artificial conditions of February temperature and February day length – tFdF (mimicking the conditions in cF), while in February the birds that were transferred to indoor cages were exposed to artificial conditions of August temperature and August day length – tAdA (mimicking the conditions in cA). The “seasonal reversal” in these two indoor groups was done in order to use birds from the same pool, which grew up and kept in identical conditions until the onset of the experiment, and create pairs of reversed groups (i.e. cA vs. tFdF in August; and cF vs. tAdA in February). This design enabled us to simultaneously expose birds, which were randomly chosen from the same pool, to temperature and day length conditions of opposite seasons. Sometime between August and February we transferred two more groups indoors, where they were kept under “mixed” temperature and day length, so that they were exposed to artificial and opposite combinations of temperature and day length: indoor August temperature and February day length – tAdF, and indoor February temperature and August day length – tFdA.
A schematic representation of the six groups that were included in the experimental design, as follows: outdoor ambient August (cA), outdoor ambient February (cF), indoor artificial February temperature and day length (tFdF), indoor artificial August temperature and day length (tAdA), indoor artificial August temperature and February day length (tAdF), and indoor artificial February temperature and August day length (tFdA). For more details see text.
All the six groups were acclimatized to their experimental conditions for 21–24 days, a time period that ensures thermogenic acclimation39, and that is also usually accepted for physiological acclimation in birds (e.g.40,41,42). Then, each female received, during two consecutive days, four intramuscular injections of 130 μl (i.e. 100 mg/kg) of bromodeoxyuridine (BrdU; SigmaUltra, diluted 10 mg/ml in sterile water; Sigma). BrdU is a cell birth-date marker, and our dose is the same as previously used to study adult neurogenesis in this species43,44,45. Then, the birds were kept under the same conditions for additional 24 days, a period that allows enough time for neurons born at the time of treatment to migrate to their final destination, undergo final anatomical differentiation46,47, and incorporate into functional neural circuits4.
Histology, immunohistochemistry and mapping of brain regions
Twenty four days after BrdU treatment the birds were weighed, killed, their brains were fixed, embedded, cut transversely at 6 µm intervals, and processed for immunohistochemical staining as we described in details previously (e.g.45). In short, we used double-labeling fluorescence staining: Hu (a neuronal specific marker) was stained green in the cytoplasm of all neurons, and BrdU (the cell birth-date marker) was stained red in the nuclei of new neurons. Therefore, cells with co-localization of green cytoplasm and a red nucleus were identified as new neurons (Fig. 2).
Three microphotographs of the same field, showing a newly formed neuron in the hippocampus of female zebra finch. (A) BrdU-labeled cells were identified by their red-stained nuclei; (B) All neurons were identified by their green-stained cytoplasm; and (C) cells with co-localization of green cytoplasm and a red nucleus were identified as new neurons. (See text for more details). (The picture was taken by S. Barkan and published with his consent).
As already detailed in the Introduction, we focused our attention on three brain regions (Fig. 3A): The Hippocampal complex (HC), Nidopallium Caudale (NC), and Medial Striatum (MSt). ZF recognize each other and communicate by vocalization and visual information. Hence, because the social and spatial environment were the same and remained constant in all groups, if there is no seasonal effect on neuronal recruitment, one can expect to find similar levels of neuronal recruitment in these regions across groups. However, if neuronal recruitment differs between groups, it will provide an indication of a seasonal effect. Furthermore, the groups in which we created a combination of temperature and day length of different seasons will enable to test possible interactions between these two factors.
Schematic views of the three investigated brain regions (A) Top view of the brain: rostral is to the left, caudal is to the right. We indicate the range within which frontal sections were taken from the Nidopallium caudale (NC), Hippocampus (HC), and Medial Striatum (MSt). Five sections were sampled along the rostro-caudal axis of each brain region. For NC (B) and HC (C) only three are shown: the most rostral, the middle, and the most caudal (from left to right). For MSt only two are shown: the most rostral and the most caudal (C). Abbreviations: Cerebellum (Cb), Lamina arcopallialis dorsalis (LAD), Lateral ventricle (V), Tectum opticum (TeO). Orientations: Dorsal (D), Lateral (L), Ventral (V), and Medial (M); adapted from Cattan et al.43.
All three brain regions have easily recognizable boundaries in transverse sections. To determine the boundaries of NC (Fig. 3B) and those of HC (Fig. 3C), as well as the locations of the five sections that were sampled along the rostral-caudal axis in each of these brain regions, we followed the criteria that are described in Cattan et al.43. For MSt, the lateral and the dorsal boundaries were defined by lamina medullaris dorsalis (LMD); the ventral boundary was defined by the surface of the brain and the medial boundary was defined by the midline (Fig. 3C). MSt stretches rostro-caudally over a distance of approximately 1200 µm; its most rostral section corresponded to A4.0 in the canary atlas48 (Fig. 3C), and its most caudal section corresponded to A1.8 there. Similar to HC and NC, within this distance we sampled five sections along the rostral-caudal axis, with an average interval of 240–360 µm between them.
We used a computerized brain-mapping system (Neurolucida; Stereo Investigator; Micro-BrightField Ltd.) to draw boundaries of the mapped areas, to count labeled and unlabeled neurons, and to quantify other neuronal parameters, as described below. Each of the five sampled sections of HC, MSt and NC were scanned using a 63X objective. In the HC and MSt, which are relatively small regions, we completely scanned all the mapped sections, using the meander scan sampling in our mapping system. In the NC, which is a relatively large brain region, we scanned about 35% of the entire sampled area, by using the fractionator sampling, which chose random sampling squares. From our previous work43 we know that this sampled portion yields results that are equivalent to mapping of the entire area. Accordingly, in each mapped section, we recorded the location of all BrdU labeled neurons and counted them. The total number of BrdU labeled neurons per group ranged between 119–603 in MSt, 20–435 in NC, and 15–86 in the HC.
In each brain and for each region, we also measured the nuclear diameters of ten BrdU labeled neurons, and those of a sample of all neurons (labeled and unlabeled), as described in Vistoropsky et al.45. The values of section thickness and the mean neuronal nuclear diameter of labeled neurons in a certain brain region served to estimate the number of new neurons per mm3 in each section and compensate for over-counting [number of cells/mm3 = number of cells/area * (T/(T + D)]; where T = section thickness (6 µm, as indicated above) and D = mean nuclear diameter], using the Abercrombie stereological correction equation49. Data from all the five mapped sections in each region of each brain were averaged so that each region in that brain was represented by a single estimated number of BrdU labeled neurons per mm3.
Statistical analysis
Numbers of BrdU labeled neurons per mm3 were transformed into square root values to achieve a normal distribution50. The transformed data were analyzed by repeated measures analyses of variance (ANOVA), in which the fixed factor was the six groups, and brain regions (HC, NC, MSt) served as the repeated measures. This was done because brain regions are considered to be dependent variables - they are all in the same brain of a particular bird, and were found to be significantly (p < 0.0001) correlated51. Post hoc Bonferroni test was carried for comparisons of the means. In order to discern the effect of location and seasonality we performed two-way ANOVA on the four groups of natural combinations: cF, cA, tFdF, tAdA. First on all three brain regions by repeated measures and then for each brain region separately. Individual one-way ANOVAs were done for pre-planned comparisons. Alpha was 0.05. JMP® Pro 13 (SAS Institute Inc., Cary, NC) was used for all analyses.
Ethics Statement
This study was approved by the Tel-Aviv University Institutional Animal Care and Use Committee (permit L-15-013) and was carried out in accordance with its regulations and guidelines regarding the care and use of animals for experimental procedures.
Results
An overall comparison between natural, artificial, and “mixed” conditions
Overall, levels of new neuronal recruitment (presented as mean numbers of BrdU labeled neurons per mm3 ± SE; Fig. 4), with values in the different regions (HC, NC, MSt) serving as repeated measures, differed significantly among the six experimental groups (F(5,23) = 15.3, p < 0.001). Post-hoc comparisons indicated that neuronal recruitments in the two “mixed” groups (i.e., those with the combination between the two seasons) were lower than in most of the other four groups. More specifically, neuronal recruitment in tFdA was significantly lower than all groups except tAdF, whereas that in tAdF was significantly lower only from the August groups (cA and tAdA). The two mixed groups did not differ from each other. Because the two “mixed” groups (tFdA, tAdF) were different from most of the other groups, and because they represent unnatural combinations of temperature and day length, we excluded these two groups in the following assessment of seasonal effects:
Mean number (±SE) of new neurons per mm3 in the six experimental groups in each of the three investigated brain regions. Abbreviations for experimental groups: cF - control February; cA - control August; tFdF - artificial conditions mimicking those in cF; tAdA - artificial conditions mimicking those in cA; tFdA – a “mixed” group, of February temperature and August day length; and tAdF – a “mixed” group, of August temperature and February day length. Abbreviations for brain regions: HC - hippocampal complex, NC - nidopallium caudale, MSt – medial striatum. *indicates a significant difference (p < 0.05). Dashed lines indicate the separation of the two “mixed” groups from the other groups (natural and artificial conditions).
Analysis of the overall effect of season (August/February) and location (indoor/outdoor) in a two-way repeated measures ANOVA showed that neuronal recruitment was higher during August (cA and tAdA) than during February conditions (cF and tFdF), with F(1,16) = 25.34, p = 0.0001 (Fig. 4), and higher under the artificial indoor conditions (tFdF and tAdA) than under the natural outdoor conditions (cF, cA; F(1,16) = 10.37, p = 0.0053; Fig. 4). Neuronal recruitment differed significantly among the brain regions (F(2,19.9) = 60.23, p < 0.0001), with the highest recruitment in the MSt and the lowest in the HC.
No significant interactions were found between season and location, between season and brain region, or between location and brain region. However, the three-factor interaction of season X location X brain region was significant (F(2,19.9) = 3.8, p = 0.04), indicating that the interaction between season and location varies across the brain regions. Therefore, two-way ANOVAs were conducted for each brain region separately. Again, neuronal recruitment was higher during August than during February conditions in all brain regions (F(1,15) = 20.49, p = 0.0004 for HC; F(1,16) = 12.91, p = 0.0024 for NC; and F(1,16) = 10.18, p = 0.0057 for MSt), and higher under artificial indoor conditions than under natural outdoor conditions in the HC and NC (F(1,15) = 12.71, p = 0.0028; F(1,16) = 12.82, p = 0.0025, respectively), but not in the MSt. In addition, a significant season X location interaction was found in the NC (F(1,16) = 11.31, p = 0.004).
Analysis of the effect of season for each location and brain region separately revealed that neuronal recruitment was significantly higher in all brain regions during August than during February under artificial indoor conditions (tAdA vs. tFdF) but not under natural outdoor conditions (cA vs. cF). However, the effect of location for each season and brain region separately was significant during August conditions (tAdA vs. cA) in the HC and NC brain regions but not in the MSt, and significant during February conditions (tFdF vs. cF) in the HC and not in the other two brain regions (indicated by * in Fig. 4).
These results indicate the following:
No seasonal effect on neuronal recruitment under natural outdoor conditions: A comparison between the groups that were kept under natural conditions revealed no seasonal effects on neuronal recruitment, in all the three tested brain regions (cF vs. cA).
A seasonal effect on neuronal recruitment under artificial indoor conditions: A comparison between the groups that were kept under artificial indoor conditions and imitated natural seasons (tAdA vs. tFdF) revealed a seasonal effect, with significantly higher levels of neuronal recruitment during August compared to February, in all the brain regions tested.
Natural outdoor conditions and similar artificial indoor ones have differential effects on neuronal recruitment: We found that levels of neuronal recruitment under artificial indoor conditions were significantly higher than those observed under natural and outdoor ones, during both August and February in the HC and during August in the NC. No such differences were observed during February in the NC as well as in the MSt during both seasons.
Discussion
We aimed to study, for the first time in birds, in a controlled and systematic manner, whether there is a seasonal neuronal recruitment pattern that is not correlated to behavior, and if so – whether this pattern exhibits fewer neurons that are incorporated into brain regions in early spring than in late summer. To date, only little evidence supports this possibility: for example, Tramontin & Brenowitz52 found that wild song sparrows show higher neuronal recruitment in the HVC in the fall than in the spring, even though they retain the same song throughout adulthood. From other animals there is also relatively little information about this question. For example, in rodents there are indications that temperature (hot climate) is correlated with higher HC neuronal recruitment53,54, while in sheep a seasonal effect has been demonstrated on hypothalamic neurogenesis, which is modulated by photoperiod55. In turtles there is a temperature-dependent effect on cell proliferation, with higher new neuronal densities in warmer temperatures than in colder ones56. In lizards, a photoperiod effect on neurogenesis was recorded, with highest rates in spring compared to autumn57,58, while in snakes an opposite pattern was found59. However, all these examples can still be interpreted in the contexts of seasonal behaviors, particularly in relation to reproduction, and most of them did not separate day length from temperature.
Lack of a seasonal effect on neuronal recruitment under natural outdoor conditions
Our data suggest that, under natural photoperiod and temperature conditions, without any association with behavioral patterns, there is no seasonal pattern in new neuronal recruitment, as no differences were found in neuronal recruitment in any of the three investigated brain regions when we compared two opposite seasons – August (cA) and February (cF). This implies that in the absence of behavioral, social or spatial changes, neuronal recruitment is not affected by the seasons of the year. We can assume that a similar output would occur also in Australia, in the natural habitat of zebra finches, because there they encompass diverse climatic zones, including tropical and subtropical types17, which are even milder than the Israeli climate, where our study had been conducted. Nevertheless, it can definitely be interesting to conduct a similar experiment in Australia and test this hypothesis.
We are aware of the fact that we need to be cautious when interpreting our results. For example, it could be that although we did not find any seasonal effect on densities of new neurons, a seasonal effect on overall neuronal recruitment could still exists, if the volumes of the investigated brain regions (which we did not measure in the current study) show seasonal variation. However, this is unlikely, because in a previous study, in which we kept zebra finches outdoors for 1, 2, and up to 5 months, we did not find any variation in the volumes of both the HC and NC60. In addition, in another study61, in which we compared neuronal recruitment between breeding and non-breeding zebra finches, we also did not find any changes in volumes of these brain regions. Another possible explanation for the lack of a seasonal effect in new neuronal recruitment that we have found in zebra finches could be related to the fact that they are non-seasonal birds (in relation to various behaviors, such as breeding, singing, diet, etc., as described in the Introduction). Hence, it is important to further test this question also in a seasonal songbird, for example the canary (Serinus canaria domestica), which can be easily studied. A comparison between seasonal and non-seasonal species will enable to conclude whether our finding, of a constant basic neuronal recruitment across seasons, is a general and broad phenomenon, or restricted only to non-seasonal birds. If our findings occur in other species, it will provide further support to the notion that had often been expressed in previous studies, including ours, as detailed in the Introduction, that seasonal neuronal recruitment is correlated with seasonal behavior or change, and enables organisms to adapt to their changing environment.
A seasonal effect on neuronal recruitment under artificial indoor conditions
Surprisingly, when we tested the same question of seasonal neuronal recruitment under artificial conditions, we observed a different outcome than the one obtained under natural conditions. This time we found a seasonal pattern, with higher levels of neuronal recruitment in August (tAdA) compared with those recorded in February (tFdF). The difference was significant in all the three investigated brain regions.
The direction of this difference under artificial indoor conditions, i.e. of higher neuronal recruitment in August than in February, resembles that from other studies, which correlated it to behavior: neuronal recruitment into HC was highest in late summer/early fall in food-storing chickadees8, in migrating warblers6, and in HVC of singing canaries62. Moreover, a similar seasonal difference was also found in non-migrating warblers6, and in the song nucleus HVC of wild sparrows, despite the fact that in this species, unlike canaries, males retain the same song throughout adulthood51. Nevertheless, it is fair to note, as already mentioned in the Introduction, that in regards to food-storing behavior, others have found other results (reviewed by Sherry and MacDougall-Shackleton11).
It is not clear why outdoor and indoor conditions yielded different results. It could be suggested that the subtle and non-significant trend of higher recruitment in August than in February that occurs under outdoor conditions (cA vs. cF) becomes more pronounced and hence significant under indoor conditions (tAdA vs. tFdF) in all the three investigated brain regions. In any case, the overall evident difference between the results that were obtained under natural vs. artificial conditions could be due to inherent differences between the two settings. This is because, by definition, the differences between the two months that we tested (August and February) were sharper and more evident under artificial conditions, at least in two aspects. Firstly, the temperature in the indoor setup was kept constant, unlike the natural situation, in which there are always temperature fluctuations between days during the tested time period, as well as fluctuations within the 24-hour period, with a natural temperature drop during the night. Secondly, under natural conditions there is a gradual light-dark transition between day and night, both at sunrise and sunset. This transition, which might be behaviorally and physiologically important, was completely absent in our artificial conditions. Hence, the differences in neuronal recruitment patterns between indoor and outdoor conditions should be taken into consideration when planning indoor experiments, and they further emphasize the importance of conducting experiments under natural conditions. The need to be cautious in interpreting results that are obtained from indoor experiments is also demonstrated by the enhanced levels of neuronal recruitment that were recorded in both NC and HC under August indoors conditions and in the HC also during February conditions, so that they were significantly higher than those observed under the respective outdoor conditions. Taken together, these differences between indoor and outdoor indicate that we should be aware of the risk or possibility that data obtained from experiments that are conducted under indoor and artificial conditions might not accurately represent the natural situation.
Artificial indoor conditions induce differential effects on neuronal recruitment in the investigated brain regions
Although neuronal recruitment in the HC and NC was affected by location (in the HC there was a significant difference between outdoor natural conditions and indoor artificial ones in both seasons and in NC only in August), neuronal recruitment in the MSt was not affected by location, in both seasons. Hence, MSt seems to be more resilient to the effect of artificial indoor conditions, maybe due to its highest rates of neuronal recruitment compared to HC and NC, which might moderate the effect of artificial conditions. Nevertheless, it is not clear why artificial August conditions had a more pronounced effect on neuronal recruitment (both in HC and NC), compared to those of artificial February (only in HC).
The relative resilience of neuronal recruitment in MSt under artificial indoor conditions is intriguing. MSt was formerly known as Lobus Paraolfactorius (LPO), but because this region contains mainly striatal cellular characteristics, it nomenclature had been revised into Medial Striatum35. Nevertheless, it is important to note that MSt lacks the one-to-one homology to the medial part of the mammalian striatum63. As suggested by lesioning experiments to the mammalian medial striatum, this area may have a crucial role in behavioral flexibility64,65,66. In birds, there is evidence for its role in associative learning, involvement in the rewarding effects of morphine and processing of object feature information37,38,67,68. Another study has indicated that inactivation of dorsomedial striatum of rats impaired behavioral flexibility that involved shifts between response and visual cues discrimination69. A similar link to visual perception was found in pigeons, when MSt lesioning caused cognitive rigidity that was expressed by impaired ability to discriminate based on color36. Sicard et al.70 suggested that MSt is photosensitive and is involved in the regulation of gonadotrophin secretion, however that study was conducted on quails that are known to be seasonal breeders. Thus, a possible explanation for the relative resilience pattern in neuronal recruitment in MSt may be due to a lack of seasonal significance in this area when there is no correlation to behavior. It could be that in non seasonal birds such as our zebra finches, which breed throughout the year, high visual vigilance for mate recognition is required regardless of the season.
“Mixed” seasonal conditions significantly reduce neuronal recruitment
An important outcome of our study is that “mixing” temperature and day length (i.e. combinations between the two seasons; groups tAdF and tFdA) yielded significantly lower neuronal recruitment rates in the three examined brain regions. Originally, the purpose of these two groups was to test whether one of these seasonal cues is more crucial for neuronal recruitment than the other. However, the similar low neuronal recruitment in both mixed groups suggests that a natural combination of both temperature and day length is important for neuronal recruitment. When these two factors are mixed, recruitment is significantly reduced, and in the case of tFdA it is even lower than the low neuronal recruitment that was observed under both outdoor and artificial February conditions (cF and tFdF, respectively). This outcome is along the same line as suggested by Stevenson et al.71, who present evidence that even slight seasonal disruptions affect the health and welfare of organisms. It is possible (although not proven) that by mixing the two major seasonal components - temperature and day length - we interrupted the internal clock in our birds, which in turn affected neuronal recruitment. It is known that stress decreases neurogenesis (reviewed in Schoenfeld and Gould72) and that disruption of circadian rhythm induces stress (reviewed in Koch et al.73). Therefore, it is plausible that the birds in the “mixed” groups underwent chronic stress due to the alteration of the seasonal components, and that, in turn, reduced neuronal recruitment in their brains.
While evidence indicates a correlation between circadian disruption and mood disorders (reviewed in Bedrosian and Nelson74), and also demonstrates the role of impaired neurogenesis in affective disorders (reviewed in Samuels and Hen75), we still miss evidence that link aberrations in these processes. In the light of a new study76 that provides the first indication of seasonal effects on human cognitive function, our “mixed” groups might propose such a possible link between circadian and/or seasonal disruption, neurogenesis and mood disorders. Even though the synchrony that we altered was between temperature and day length (seasonal disruption) and not time and day length as the cited above (circadian disruption), our data reinforce the significance of such synchrony between light and temperature, and might suggest potential translational implications. This question, of course, is still to be tested.
Overview and conclusions
Here we show that under natural photoperiod and temperature conditions, and in the absence of behavioral, social or spatial changes, neuronal recruitment remains constant across seasons (cA vs. cF). This lack of seasonality in new neuron recruitment could be due to the a-priori non-seasonal nature of our model – the zebra finch. Hence, to conclude whether this is a general and broad phenomenon, it has to be tested also in seasonal species. Surprisingly, under artificial conditions, the same set-up yielded a different outcome, of higher neuronal recruitment in August compared to February (tAdA vs. tFdF). The different results between natural and artificial conditions could be because of two reasons: Firstly, indoor temperature was constant, unlike the natural situation where fluctuations occur between days and within the 24-hour period. Secondly, the natural gradual light-dark transition was absent in our artificial conditions. We believe that our findings indicate that one has to be aware of the possibility that data obtained from indoor experiments under artificial conditions might not accurately represent the natural situation. Moreover, our study shows that artificial indoor conditions differentially affect neuronal recruitment in various brain regions, and we discuss possible explanations of this effect.
Lastly, our data indicate that “mixing” temperature and day length (tAdF and tFdA) results in a significant reduction of neuronal recruitment, demonstrating the importance of the natural combination of temperature and day length. Taken together, our results support the review by Calisi and Bentley77, which presents examples for experiments performed in a laboratory environment that are sometimes different than findings from similar experiments performed in the “real world”. While the authors suggest that endocrinological and behavioral experiments are particularly susceptible to influence from the environmental set-up, our study adds another dimension to this conclusion, by showing that neuroplasticity also changes under natural vs. artificial conditions. Another example for the importance of understanding how changes in housing conditions affect the question under investigation is the study by Dickens & Bentley78 who showed remarkable differences in breeding behavior and hypothalamic pituitary-adrenal activity between European starlings (Sturnus vulgaris) that were housed outdoors or indoors. We join in with the conclusion of these authors that experiments in both lab and field are needed when looking at complex biological systems.
References
Brenowitz, E. A. Plasticity of the song control system in adult birds in Neuroscience of birdsong (ed. H.P. Zeigler and P. Marler) 332–349 (Cambridge University Press, 2008).
Kirn, J., O’Loughlin, B., Kasparian, S. & Nottebohm, F. Cell death and neuronal recruitment in the high vocal center of adult male canaries are temporally related to changes in song. Proc. Natl. Acad. Sci. USA 91, 7844–7848 (1994).
Cohen, R. E., Macedo-Lima, M., Miller, K. E. & Brenowitz, E. A. Adult neurogenesis leads to the functional reconstruction of a telencephalic neural circuit. J. Neurosci. 36, 8947–8956, https://doi.org/10.1523/JNEUROSCI.0553-16.2016 (2016).
Burd, G. D. & Nottebohm, F. Ultrastructural characterization of synaptic terminals formed on newly generated neurons in a song control nucleus of the adult canary forebrain. J. Comp. Neurol. 240, 143–152 (1985).
Paton, J. A. & Nottebohm, F. Neurons generated in the adult brain are recruited into functional circuits. Science 225, 1046–1048 (1984).
Barkan, S., Yom-Tov, Y. & Barnea, A. A possible relation between new neuronal recruitment and migratory behavior in Acrocephalus warblers. Dev. Neurobiol. 74, 1194–1209, https://doi.org/10.1002/dneu.22198 (2014).
Barkan, S., Roll, U., Yom-Tov, Y., Wassenaar, L. & Barnea, A. Possible linkage between neuronal recruitment and relocation distance in migratory birds. Sci. Rep. 6, 21983, https://doi.org/10.1038/srep21983 (2016).
Barnea, A. & Nottebohm, F. Seasonal recruitment of hippocampal neurons in adult free-ranging black-capped chickadees. Proc. Natl. Acad. Sci. USA 91, 11217–11221 (1994).
Hoshooley, J. S., Phillmore, L. S., Sherry, D. F. & Macdougall-Shackleton, S. A. Annual cycle of the black-capped chickadee: seasonality of food-storing and the hippocampus. Brain Behav. Evol. 69, 161–168 (2007).
Hoshooley, J. S. & Sherry, D. F. Greater hippocampal neuronal recruitment in food-storing than in non food storing birds. Dev. Neurobiol. 67, 406–414 (2007).
Sherry, D. F. & MacDougall-Shackleton, S. A. Seasonal change in the avian hippocampus. Front. Neuroendocrinol. 37, 158–167, https://doi.org/10.1016/j.yfrne.2014.11.008 (2015).
Pravosudov, V. V., Roth, T. C. II, LaDage, L. D. & Freas, C. A. Environmental influences on spatial memory and the hippocampus in food-caching chickadees. Comp. Cogn. Behav. Rev. 10, 25–43 (2015).
Brenowitz, E. A. & Larson, T. A. Neurogenesis in the adult avian song-control system. Cold Spring Harb. Perspect, Biol. 7, a019000, https://doi.org/10.1101/cshperspect.a019000 (2015).
Brenowitz E. A. Plasticity of the song control system in adult birds in Neuroscience of birdsong 332–349 (Cambridge University Press, 2008).
Ball, G. F. et al. Seasonal plasticity in the song control system: multiple brain sites of steroid hormone action and the importance of variation in song behavior. Ann N Y Acad Sci. 1016, 586–610 (2004).
Hoshooley, J. S. & Sherry, D. F. Neuron production, neuron number, and structure size are seasonally stable in the hippocampus of the food-storing blackcapped chickadee (Poecile atricapillus). Behav. Neurosci. 118, 345–355 (2004).
Zann, R. A. The Zebra Finch: A Synthesis of Field and Laboratory Studies (Oxford University Press, 1996).
Morton, S. R. & Davies, P. H. Food of the Zebra Finch (Poephila guttata), and an examintation of granivory in birds of the Australia arid zone. Aust. J Ecol. 8, 235–243 (1983).
Zann, R. A. & Straw, B. Feeding ecology and breeding of Zebra Finches in farmland in northern Victoria. Aust. Wildlife. Res. 11, 533–552 (1984).
Adar, E., Nottebohm, F. & Barnea, A. The relationship between nature of social change, age, and position of new neurons and their survival in adult zebra finch brain. J. Neurosci. 28, 5394–5400, https://doi.org/10.1523/JNEUROSCI.5706-07.2008 (2008).
Tokarev, K., Boender, A. J., Claßen, G. A. & Scharff, C. Young, active and well-connected: adult-born neurons in the zebra finch are activated during singing. Brain Struct. Funct. 221, 1833–1843, https://doi.org/10.1007/s00429-015-1006-y (2016).
Immelmann, K. Song development in the zebra finch and other Estrildid finches in Bird vocalization (ed. R.A. Hinde) 61–74 (Cambridge University Press, 1969).
Katz, A., Mirzatoni, A., Zhen, Y. & Schlinger, B. A. Sex differences in cell proliferation and glucocorticoid responsiveness in the zebra finch brain. Eur J Neurosci. 28, 99–106 (2008).
Mirzatoni, A. et al. Steroidal and gonadal effects on neural cell proliferation in vitro in an adult songbird. Brain Res. 1351, 41–49 (2010).
Krebs, J. R., Sherry, D. F., Healy, S. D., Perry, V. H. & Vaccarino, A. L. Hippocampal specialization of food-storing birds. Proc. Natl. Acad. Sci. USA 86, 1388–1392 (1989).
Sherry, D. F. & Vaccarino, A. L. Hippocampus and memory for food caches in black-capped chickadees. Behav. Neurosci. 103, 308 (1989).
Shettleworth, S. J., Krebs, J. R., Healy, S. D. & Thomas, C. M. Spatial memory of food-storing tits (Parus ater and P. atricapillus): Comparison of storing and nonstoring tasks. J. Comp. Psychol. 104, 71 (1990).
Smulders, T. V. The Avian Hippocampal Formation and the Stress Response. Brain. Behav. Evol. 90(1), 81–91, https://doi.org/10.1159/000477654. (2017).
Vates, G. E., Broome, B. M., Mello, C. V. & Nottebohm, F. Auditory pathways of caudal telencephalon and their relation to the song system of adult male zebra finches (Taenopygia guttata). J. Comp. Neurol. 366, 613–642 (1996).
Mello, C. V. & Clayton, D. F. Song-induced ZENK gene expression in auditory pathways of songbird brain and its relation to the song control system. J. Neurosci. 14, 6652–6666 (1994).
Mello, C. V., Vates, E., Okuhata, S. & Nottebohm, F. Descending auditory pathways in the adult male zebra finch (Taeniopygia guttata). J. Comp. Neurol. 395, 137–160 (1998).
Nottebohm, F. Song learning in birds offers a model for neuronal replacement in adult brain in Neurogenesis in the Adult Brain I: Neurobiology (Springer Science & Business Media)74–75; 10.1007/978-4-431-53933-9 (2011).
Güntürkün, O. The avian “prefrontal cortex” and cognition. Curr Opin Neurobiol. 15, 686–693 (2005).
Veit, L., Hartmann, K. & Nieder, A. Neuronal correlates of visual working memory in the corvid endbrain. J. Neurosci. 34, 7778–7786 (2014).
Reiner, A., Perkel, D. J., Mello, C. V. & Jarvis, E. D. Songbirds and the revised avian brain nomenclature. Ann. N. Y. Acad. Sci. 1016, 77–108 (2004).
Watanabe, S. Effects of lobus parolfactorius lesions on repeated acquisition of spatial discrimination in pigeons. Brain Behav. Evol. 58, 333–342 (2001).
Durstewitz, D., Kröner, S. & Güntürkün, O. The dopaminergic innervation of the avian telencephalon. Prog. Neurobiol. 59, 161–195 (1999).
Matsushima, T., Izawa, E., Aoki, N. & Yanagihara, S. The mind through chick eyes: memory, cognition and anticipation. Zoolog. Sci. 20, 395–408 (2003).
Heldmaier, G. & Werner, D. (eds) Environmental signal processing and adaptation 269 (Springer-Verlag, 2003).
Maldonado, K. E., Cavieres, G., Veloso, C., Canals, M. & Sabat, P. Physiological responses in rufous-collared sparrows to thermal acclimation and seasonal acclimatization. J Comp Physiol B. 179(3), 335–343, https://doi.org/10.1007/s00360-008-0317-1 (2009).
McKechnie, A. E., Chetty, K. & Lovegrove, B. G. Phenotypic flexibility in the basal metabolic rate of laughing doves: responses to short-term thermal acclimation. J Exp Biol. 210(Pt 1), 97–106 (2007).
Wang, J. Q., Wang, J. J., Wu, X. J., Zheng, W. H. & Liu, J. S. Short photoperiod increases energy intake, metabolic thermogenesis and organ mass in silky starlings Sturnus sericeus. Zool Res. 37(2), 75–83, https://doi.org/10.13918/j.issn.2095-8137.2016.2.75 (2016).
Cattan, A., Ayali, A. & Barnea, A. The cell birth marker BrdU does not affect recruitment of subsequent cell divisions in the adult avian brain. BioMed Res. Int. https://doi.org/10.1155/2015/126078 (2015).
Pytte, C. L., Gerson, M., Miller, J. & Kirn, J. R. Increasing stereotypy in adult zebra finch song correlates with a declining rate of adult neurogenesis. Dev. Neurobiol. 67, 1699–1720; https://doi.org/10.1002/dneu.20520(2007).
Vistoropsky, Y., Heiblum, R., Smorodinsky, N. I. & Barnea, A. Active immunization against vasoactive intestinal polypeptide decreases neuronal recruitmetnt and inhibits reproduction in zebra finches. J. Comp. Neurol. 524, 2516–2528, https://doi.org/10.1002/cne.23971 (2016).
Alvarez-Buylla, A. & Nottebohm, F. Migration of young neurons in adult avian brain. Nature 335, 353–354 (1988).
Kirn, J. R., Fishman, Y., Sasportas, K., Alvarez-Buylla, A. & Nottebohm, F. Fate of new neurons in adult canary high vocal center during the first 30 days after their formation. J. Comp. Neurol. 411, 487–494 (1999).
Stokes, T. C., Leonard, C. M. & Nottebohm, F. A stereotaxic atlas of the telencephalon, diencephalon, and mesencephalon of the canary, Serinus canarius. J. Comp. Neurol. 156, 337–374 (1974).
Guillery, R. W. & Herrup, K. Quantification without pontification: choosing a method for counting objects in sectioned tissues. J. Comp. Neurol. 386, 2–7 (1997).
Sokal R. R., Rholf J. F. Biometry. (Freeman, 1995).
Carey, G. Quantitative methods in neuroscience. http://psych.colorado.edu/~carey/qmin/QMIN_2013_03_17.pdf (2013).
Tramontin, A. D. & Brenowitz, E. A. A field study of seasonal neuronal incorporation into the song control system of a songbird that lacks adult song learning. J. Neurobiol. 40, 316–326 (1999).
Ming, G. L. & Song, H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70, 687–702, https://doi.org/10.1016/j.neuron.2011.05.001 (2011).
Cavegn, N. et al. Habitatspecific shaping of proliferation and neuronal differentiation in adult hippocampal neurogenesis of wild rodents. Front. Neurosci. 7; https://doi.org/10.3389/fnins.2013.00059 (2013).
Migaud, M., Butrille, L. & Batailler, M. Seasonal regulation of structural plasticity and neurogenesis in the adult mammalian brain: focus on the sheep hypothalamus. Front. Neuroendocrinol. 37, 146–157, https://doi.org/10.1016/j.yfrne.2014.11.004 (2015).
Radmilovich, M., Fernández, A. & Trujillo-Cenóz, O. Environment temperature affects cell proliferation in the spinal cord and brain of juvenile turtles. J. Exp. Biol. 206, 3085–3093 (2003).
Ramirez, C. et al. Photoperiod–temperature and neuroblast proliferation– migration in the adult lizard cortex. Neuroreport 8, 2337–2342 (1997).
Delgado-Gonzalez, F. J. et al. Seasonal differences in ventricular proliferation of adult Gallotia galloti lizards. Brain Res. 1191, 39–46, https://doi.org/10.1016/j.brainres.2007.10.092 (2008).
Maine, A. R., Powers, S. D. & Lutterschmidt, D. I. Seasonal variation in cell proliferation and cell migration in the brain of adult red-sided garter snakes (Thamnophis sirtalis parietalis). Brain Behav. Evol. 84, 181–196, https://doi.org/10.1159/000364778 (2014).
Barnea, A., Mishal, A. & Nottebohm, F. Social and spatial changes induce multiple survival regimes for new neurons in two regions of the adult brain: An anatomical representation of time? Behav. Brain Res. 167, 63–74 (2006).
Barkan, S., Ayali, A., Nottebohm, F. & Barnea, A. Neuronal recruitment in adult zebra finch brain during a reproductive cycle. Develop. Neurobiol. 67, 687–701 (2007).
Kirn, J. R. The relationship of neurogenesis and growth of brain regions to song learning. Brain Lang. 115, 29–44, https://doi.org/10.1016/j.bandl.2009.09.006 (2010).
Reiner, A., Medina, L. & Veenman, C. L. Structural and functional evolution of the basal ganglia in vertebrates. Brain Res. Brain Res. Rev. 28, 235–285 (1998).
Lawrence, A. D. et al. Executive and mnemonic functions in early Huntington’s disease. Brain 119, 1633–1645 (1996).
Owen, A. M., Roberts, A. C., Hodges, J. R. & Robbins, T. W. Contrasting mechanisms of impaired attentional set-shifting in patients with frontal lobe damage or Parkinson’s disease. Brain 116, 1159–1175 (1993).
Mizumori, S. J., Ragozzino, K. E. & Cooper, B. G. Location and head direction representation in the dorsal striatum of rats. Psychobiology 28, 441–462 (2000).
He, X., Lin Xiao, A. & Nan Sui, A. Effects of SCH23390 and spiperone administered into medial striatum and intermediate medial mesopallium on rewarding effects of morphine in day-old chicks. Eur. J.Pharmacol. 627, 136–141, https://doi.org/10.1016/j.ejphar.2009.10.041 (2009).
Mayer, U., Pecchia, T., Bingman, V. P., Flore, M. & Vallortigara, G. Hippocampus and Medial Striatum Dissociation During Goal Navigation by Geometry or Features in the Domestic Chick: An Immediate Early Gene Study. Hippocampus 26, 27–40, https://doi.org/10.1002/hipo.22486 (2016).
Ragozzino, M. E., Ragozzino, K. E., Mizumori, S. J. & Kesner, R. P. Role of the dorsomedial striatum in behavioral flexibility for response and visual cue discrimination learning. Behav. Neurosci. 116, 105 (2002).
Sicard, B., Oliver, J. & Baylé, J. D. Gonadotropic and photosensitive abilities of the lobus paraolfactorius: electrophysiological study in quail. Neuroendocrinology 36, 81–87 (1983).
Stevenson, T. J. et al. Disrupted seasonal biology impacts health, food security and ecosystems. Proc. Biol.l Sci. 282, 20151453, https://doi.org/10.1098/rspb.2015.1453 (2015).
Schoenfeld, T. & Gould, E. Stress, Stress Hormones, and Adult Neurogenesis. Exp Neurol. 233(1), 12–21, https://doi.org/10.1016/j.expneurol.2011.01.008 (2012).
Koch, C. E., Leinweber, B., Drengberg, B. C., Blaum, C. & Oster, H. Interaction between circadian rhythms and stress. Neurobiol Stress. 6, 57–67, https://doi.org/10.1016/j.ynstr.2016.09.001 (2017).
Bedrosian, T. A. & Nelson, R. J. Timing of light exposure affects mood and brain circuits. Transl Psychiatry 7, e1017, https://doi.org/10.1038/tp.2016.262 (2017).
Samuels, B. A. & Hen, R. Neurogenesis and affective disorders. Eur J Neurosci. 33, 1152–1159, https://doi.org/10.1111/j.1460-9568.2011.07614.x (2011).
Meyer, C. et al. Seasonality in human cognitive brain responses. Proc Natl Acad Sci. 113, 3066–3071, https://doi.org/10.1073/pnas.1518129113 (2016).
Calisi, R. M. & Bentley, G. E. Lab and field experiments: are they the same animal? Horm. Behav. 56, 1–10, https://doi.org/10.1016/j.yhbeh.2009.02.010 (2009).
Dickens, M. J. & Bentley, G. E. Stress, captivity, and reproduction in a wild bird species. Horm. Behav. 66, 685–693, https://doi.org/10.1016/j.yhbeh.2014.09.011 (2014).
Acknowledgements
Thanks are due to the staff of the Meier Segals Garden for Zoological Research at Tel Aviv University for their technical help. We thank the anonymous reviewer whose comments significantly improved this manuscript. This work was supported by The Open University Research Fund.
Author information
Authors and Affiliations
Contributions
A.B. and T.P. conceived this work, T.P. and Y.V. conducted the experiments, S.M. helped with the acquisition and interpretation of data, R.H. conducted the statistical analysis, All authors wrote the paper and contributed to ideas.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) an d the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pozner, T., Vistoropsky, Y., Moaraf, S. et al. Questioning Seasonality of Neuronal Plasticity in the Adult Avian Brain. Sci Rep 8, 11289 (2018). https://doi.org/10.1038/s41598-018-29532-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-29532-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.