Abstract
The extent to which closely related species share similar niches remains highly debated. Ecological niches are increasingly analysed by combining distribution records with broad-scale climatic variables, but interactions between species and their environment often occur at fine scales. The idea that macroscale analyses correctly represent fine-scale processes relies on the assumption that average climatic variables are meaningful predictors of processes determining species persistence, but tests of this hypothesis are scarce. We compared broad- and fine-scale (microhabitat) approaches by analyzing the niches of European plethodontid salamanders. Both the microhabitat and the macroecological approaches identified niche differences among species, but the correspondence between micro- and macroecological niches was weak. When exploring niche evolution, the macroecological approach suggested a close relationship between niche and phylogenetic history, but this relationship did not emerge in fine-scale analyses. The apparent pattern of niche evolution emerging in broad-scale analyses likely was the by-product of related species having closely adjacent ranges. The environment actually experienced by most of animals is more heterogeneous than what is apparent from macro-scale predictors, and a better combination between macroecological and fine-grained data may be a key to obtain robust ecological generalizations.
Similar content being viewed by others
Introduction
The idea that phylogenetically related species also tend to be ecologically similar has intrigued researchers since Darwin’s Origin of Species1. Phylogenetic conservatism is the tendency of closely related species to be more similar than expected under randomness1,2. Phylogenetic signal is often observed for morphological and life history traits (e.g. refs1,2,3,4), and has also been detected for traits representing species niche, such as eco-physiological features, climatic niche, diet and habitat1,5. Nevertheless, signal for niche traits is not ubiquitous, as many studies have actually found a high evolutionary lability of realized niches1,5. There is thus a growing interest in the study of phylogenetic signal of niches, and of the conditions and traits for which effects of phylogenetic signal on niche are stronger or can be better detected1,5.
The evolution of niches is often analysed through a broad-scale (bioclimatic) approach, i.e. by combining species distribution data with coarse-resolution, ‘scenopoetic’ variables5. These macroecological approaches have had increasing appeal given the availability of broad-scale information (e.g. species distribution data, climatic information, environmental data from remote sensing, phylogenies), and the impressive progress of ecological informatics6. The broad geographical scale of these studies is both a strength and a limitation. Working over macro-scales allows drawing general patterns that are hardly recovered using local analyses, but the data available over broad scales generally have a coarse resolution. For instance, most of analyses of relationships between animals and climate are performed at scales that are ~10,000 times larger than the study organisms6,7. However, it is widely recognized that species distributions are the product of multi-scalar processes, and many interactions between species and the environment occur at fine scales8,9. Thus, abiotic conditions actually experienced by individuals do not necessarily correspond to such macro-predictors10,11,12, and bioclimatic predictors often are just surrogates of the fine-scale environmental features actually experienced by individuals11.
Until now, many studies have implicitly assumed that broad-scale variables are meaningful predictors of the parameters influencing species (mean field approximation)13, without comparing the effects of micro- and macro-scale conditions. In order to assess how climate determines the distribution of species we need testing the appropriateness of the mean field approximation, and thus comparing the outcome of micro- and macroclimate analyses13. Such comparison can be performed using statistical downscaling11,14,15 or explicit modelling of microclimate16, but these approaches suffer some limitations15, and do not empirically assess the actual microclimates exploited by organisms.
Alternatively, the comparison can be performed using microclimate data from real observations10. Microhabitat selection and thermoregulation through behaviour are major processes allowing animals to maintain body conditions within their physiological limits, i.e. within the range of conditions imposed by the fundamental niche of the species12. Microhabitat selection by species in the wild can provide accurate data on species requirements, thus allowing us to draw measures of species niche, with a rationale analogous to analyses of operative temperature12 or to habitat preference experiments in which organisms are exposed to a variety of environmental conditions and can select those within their suitability range (Fig. 1; see e.g.17). Bioclimatic and microhabitat data can provide insights about different aspects of species niches. Hierarchical approaches, integrating analyses at multiple levels, can thus greatly enhance understanding of niches and help to evaluate under which conditions the different approaches are most appropriate18,19, but there are few multi-scalar analyses (for examples, see9,19).
How microhabitat selection can mirror habitat selection experiments. At increasing depths, temperature decreases and humidity increases: salamanders are only found when conditions are within the species range. The figure represents the microhabitat and salamander distribution actually observed in the cave “Brecca su Fenugu” (39°42′N, 9°25′E). The salamander image is by N. Sinegina. The image was obtained from (http://www.supercoloring.com/silhouettes/salamander) under a Creative Commons Attribution-Share Alike 4.0 Licence. https://creativecommons.org/licenses/by-sa/4.0.
Terrestrial salamanders have been a frequent focus of analyses of bioclimatic niche. Niche analyses have been used to infer distribution changes and declines caused by climate change, to identify broad-scale drivers of biodiversity patterns, to analyse niche evolution in a phylogenetic context and even as a tool to describe new species (e.g.)19,20,21,22,23. In this study we analysed niches of eight species of terrestrial salamanders (genus Hydromantes, subgenera Speleomantes and Atylodes; see Wake)24 using the microhabitat selection and bioclimatic approaches, and assessed the phylogenetic signal of niches with the two approaches. Despite being sometimes named “cave salamanders”, these are not true cave-dwelling organisms: underground environments just are the habitats where salamander detection is easiest25.
European terrestrial salamanders are an interesting group for niche analyses. First, salamanders have superficial activity during cool and wet periods (from autumn to spring), but move to underground environments during summer, when external conditions would be too harsh (e.g., dry, hot). In these environments, they select sectors having microclimatic features within their physiological limits (Fig. 1; see Methods). Their microhabitat selection is similar to what is done in habitat preference experiments, in which organisms are placed in a gradient where they select environmental conditions within their suitability range17, and is thus particularly appropriate to identify the tolerance of species. Actually, previous analyses have shown that microhabitat selection provides reliable information on the operative conditions of individuals, thus allowing a good characterization of species biophysical niches26. Second, the features of underground habitats are different but strongly dependent on conditions outside the cave (epigean). For instance, far from the surface, the mean temperature approximately corresponds to the average local temperature of the atmosphere, and underground conditions are heavily influenced by epigean variation of temperature and precipitation27,28,29. Underground environments are not a unique case, as there are many environments in which microclimate might be imperfectly modelled by macroclimate, such as streams, ponds, forests with dense understory and topographically complex landscapes10,14,16,30,31, thus insights of our analyses can be relevant for many species and habitats. Finally, the fauna living underground and in the soil is rarely investigated by macroecological studies6, even though it includes a major proportion of terrestrial biodiversity.
We analysed the niche of salamander species using both a fine-grained (microhabitat, representing the operative conditions actually experienced by individuals) and a broad-scale perspective (i.e. combining presence localities with broad-scale bioclimatic variables). We tested to what extent information on niche features and evolution is conserved between these two scales of analysis, and identified the geographical and evolutionary factors determining the mismatch between fine-grained and coarse-grained analyses of niche evolution.
Results
In field surveys, we detected >2700 salamanders in 521 out of the 1251 cave sectors; the number of sectors in which we detected salamanders was heterogeneous among species (Table 1, Fig. 2a).
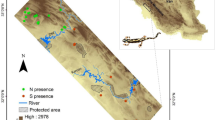
Distribution of (a) caves sampled for the microhabitat analyses; (b) presence localities used for the broad scale, macroecological analyses. The map was created using QGis 2.18 (www.qgis.org).
Niche analyses at the microhabitat level
Relationships between species presence and abiotic variables were similar across the eight salamander species. All species were significantly associated with the sectors having highest humidity, lowest temperature, and lack of light. Relationships with spiders were generally weak (Fig. 3, Table 2). The relationship between humidity and two species (H. flavus and H. italicus) was non-linear, as the probability of presence quickly decreased when humidity was <80% (Fig. S1). Furthermore, a non-linear relationship between temperature and H. strinatii indicated a sharp drop of suitability above 20 °C (Fig. S1). Multiple regression models confirmed the univariate analyses: all species were associated with dark sectors characterized by high humidity and/or low temperature (Table S1).
Nevertheless, similarity tests showed significant niche differences for nearly all the species pairs. Niche overlap ranged between 0.165 and 0.799. Niche equivalency was rejected in 21/26 pairwise tests, and remained significant after sequential Bonferroni’s correction in 19/26 tests (Table S2a). The majority of non-significant comparisons involved the species with most restricted range and smallest sample size (H. sarrabusensis). According to the microhabitat analyses, H. ambrosii and H. strinatii were the species most tolerant to light and to dry conditions, H. sarrabusensis was the species associated with warmest temperatures, while H. genei, H. italicus and H. supramontis were restricted to the darkest, wettest and coldest sectors (Figs 3 and 4, Fig. S2).
Bioclimatic analysis
We obtained 597 presence localities, widely covering the range of all the species (5–179 records per species; Table 1, Fig. 2b). Niche overlap measured at the bioclimatic level was generally limited (range: 0.001–0.504), and was lower than the overlap measured at the microhabitat level (paired samples t-test for unequal variances: t54 = −6.1, p < 0.0001). Niche equivalency was rejected in 25 out of 26 pairwise tests (Table S2b), and the single non-significant test involved the two species with smallest sample size (H. sarrabusensis and H. supramontis). According to the bioclimatic analyses, H. ambrosii and H. strinatii were associated with the coldest and wettest climates, while H. sarrabusensis, H. supramontis, H. genei and H. flavus were associated with warm and dry conditions (Figs 4 and S3).
Microhabitat, bioclimatic niche and phylogenetic relationships
The correspondence between microhabitat and bioclimatic niches was weak. For instance, the microhabitat analysis identified H. strinatii and H. ambrosii among the species with the highest tolerance to dry sectors, while in the bioclimatic analyses they were associated with the wettest climates. Similarly, in the microhabitat analysis H. genei was associated with the coldest sectors, while in bioclimatic analyses it was among the species living in the warmest climates (Fig. 4). Overall, we found no relationship between niche dissimilarities calculated using the fine- and the coarse-scale approaches (Mantel’s test: r = −0.17, p = 0.36, Fig. 5a).
Phylogenetic analyses32 showed that the eight study species form a monophyletic group. H. genei was the most basal species; two well supported monophyletic groups included (1) H. flavus, H. supramontis, H. imperialis and H. sarrabusensis and (2) H. italicus, H. ambrosii and H. strinatii32 (Fig. S4).
Microhabitat distances were unrelated to genetic distances (r = −0.06, p = 0.95, Fig. 4b), while genetically distant species showed the largest bioclimatic distances (r = 0.53, p = 0.001, Fig. 4c). However, the relationship between bioclimatic distance and evolutionary history was complicated by the fact that species genetically distant also live in distant geographical areas (r = 0.47, p = 0.013), and bioclimatic distance was positively related to geographical distance between species ranges (r = 0.52, p = 0.01). Altogether, geographical and genetic distances explained bioclimatic distance well (MRDM: R2 = 0.39, p < 0.003), but disentangling their relative role was difficult. In a commonality analysis, both variables showed a limited unique effect (genetic distance: unique effect = 0.12; geographical distance: unique effect = 0.11), while more explanatory power was shared between these two parameters (Table S3). These results were robust to different approaches to the calculation of niches at both the microhabitat and bioclimatic level, to the incorporation of parameters representing spatial autocorrelation, and to the use of only a subset of localities for analyses (Supplementary Results).
Discussion
Both microhabitat (i.e. fine-scale) and bioclimatic (i.e. coarse-scale) analyses identified clear niche differences between species. However, the bioclimatic and microhabitat approaches showed dissimilar patterns, as the bioclimatic analyses suggested a close relationship between niche and evolutionary divergence, i.e. a strong phylogenetic signal of niches, while the microhabitat divergence was unrelated to either phylogeny or to the bioclimatic pattern.
Theory clearly acknowledges the multi-scalar nature of niches, and several studies have shown that species distribution is the product of processes acting at both broad and fine scale (reviewed in ref.33). An increasing number of studies has tested whether ecological niches retain a signal of phylogenetic history, and many of them have used a bioclimatic approach for niche definition5. However, the geographical distribution of organisms is strongly related to their evolutionary history, and recent work suggests that complex interplay between present-day distribution, evolutionary history, and the spatial autocorrelation of bioclimatic variables may complicate the reconstruction of niche evolution34. Warren et al.34 proposed a conceptual framework, in which diversification mostly occurs through allopatric speciation. Sister-species are thus generally allopatric, and only phylogenetically distant species may have overlapping ranges, because they have limited competition. Under this framework, closely related clades may show the strongest apparent niche divergence, even if the opposite may be true (e.g., unrelated species exist in sympatry because of limited competition, i.e. small niche overlap)35. Our study shows that a similar interplay between evolutionary history and geography may even determine the opposite pattern. Allopatric speciation was the most likely driver of the differentiation between terrestrial salamander species36,37, but strong interspecific competition38 and barriers likely cause the absence of sympatry between closely-related species (Fig. 1), while poor dispersal limits their geographical spread. Under these conditions, closely related species often have proximate ranges, and this may cause a pattern with closely related species sharing similar niches (Fig. 5c) just as a by-product of geographical proximity. As a consequence, niche comparisons on the basis of bioclimatic data only can miss the full history: the geography of speciation might be the actual driver of most observed patterns on niche evolution, instead of the inferred ecological processes34. The niche comparison method used here39 is considered to be able to correct at least in part for the similarity determined by spatial autocorrelation34, yet, a clear effect of geographical distance on bioclimatic niche differentiation remained evident. Actually, it was hard to tell whether the niche similarity between closely related species was the result of niche conservatism, or whether it was just the by-product of related species having nearby ranges (Table S3).
The analyses of niches can be improved by the explicit integration of multiple approaches. Measures more closely related to the fundamental niche (e.g. performance, microhabitat selection, tolerance limits, operational conditions), if available, can be used to test the reliability of bioclimatic analyses40. For instance, in terrestrial salamanders, the average operational temperature measured at the microhabitat level was unrelated to the average air temperature during the activity season, obtained from global gridded data (Fig. S7), and such discrepancy casts doubts on the reliability of the bioclimatic results alone. On the other hand, the growing availability of spatial datasets and analytical tools allows quickly extracting information that would be much harder to obtain at the microhabitat level, and this has likely helped the fast progress of macroecological studies. Joint availability of broad-scale and fine-grained data is limited6, and researchers need to assess the validity of macroecological analyses, even in the absence of information on performance at the small-scale. If the relationship between niche and history abruptly changes when taking into account geography, or if we cannot tease apart their relative role, then Warren’s34 hypothesis that we are mistaking geography for biology is a likely explanation. Spatial patterns are inherently linked to ecological processes; thus researchers must utilize approaches that allow explicitly take into account the spatial structure of their data. For instance, the simple effect of geographical distance may be considered as a null-model, over which the phylogenetic history can be compared41, even though the spatial effect of past geography, topographical and ecological barriers may be complex, and it is not so easy to explicitly take them into account.
Microhabitat and macroecological analyses certainly characterize non-identical aspects of the niche, still parameters such as thermal preferences have relevant implications on broad scale species distribution42, and thus we expected some relationships between them. We suggest that in our study system the microhabitat approach might represent adequately species niches because (i) at least for some parameters (e.g. temperature), microhabitat is an excellent proxy of operative eco-physiological conditions of salamanders26, which are a major approach to the measurement of fundamental niches42; (ii) the microhabitat approach is not biased by dispersal limitations or biotic interactions and (iii) within each cave, a full range of conditions generally exists, from the harshest to the most suitable, enabling a parallel with habitat preference experiments (Fig. 1). However, it is important to recall that the observed distribution and niche exploitation of species is determined by the joint effect of environmental suitability and dispersal43,44. In suboptimal habitats populations can have low fitness, but can be maintained demographically by immigration from nearby source habitats45,46. At the macroecological scale, flow of individuals can occur from the centre to the edge of geographical ranges47. Similarly, at the microhabitat scale salamanders can be present in suboptimal sectors just because they are nearby sectors with very high abundance, and these issues can complicate the interpretation of niche analyses. Our conclusions are probably robust to these issues, as we obtained identical results both removing observations in sectors with extreme conditions, and removing many distribution records at the boundary of species ranges (Tables S4 and S5). Nevertheless, taking into account variation in abundance across sites can provide a more complete understanding of niche variation in these species, and on the links between environmental suitability at broad scale, and population processes occurring at the local scale48.
Niche analyses are increasingly used to answer multiple ecological and evolutionary questions, such as predictions of species’ responses to climate change, analyses of biodiversity drivers and even to analyse local adaptations and identify species. Studies combining distribution data with macroecological predictors can be extremely effective, and some of them have been able to analyse thousands of species at the continental or even global scale. Such broad scale analyses are based on the assumption that grid-cell average climatic conditions provide a good prediction of the probability of species persistence in a site13 but, in many cases, this assumption is untested. A few studies have evaluated whether species fitness can be actually predicted by broad-scale analyses (e.g.)19,49, and found mixed results. For instance, Searcy and Shaffer19 tested whether climatic variables important in broad-scale species distribution models are also related to salamander recruitment, and observed some match between the two approaches. However, the strength of the match was strongly dependent on metrics and methods used to develop the distribution models, and different approaches yielded non-identical predictions of species responses to climate change19.
Differences between micro- and macrohabitat approaches might be particularly relevant for animals living in complex landscapes and specific microhabitats (e.g. underground, in freshwater habitats, within plants…) where conditions can be very different from the commonly used measures of climate, such as mean air temperature10,14,16,30,31. Actually, such organisms include many amphibians, insects12 and likely other terrestrial invertebrates. These taxa are not those most studied in macroecology6, but comprise the majority of terrestrial animals, thus the discrepancy between microhabitat and bioclimatic analyses may be present for many organisms. It should also be noted that there are systems in which this pattern was not observed, as some studies on surface-living salamanders found concordance between fine-scale (microclimate, body temperature) and bioclimatic data20,50.
It might also be argued that animals associated with underground environments are special cases, if they shelter in microhabitats that are independent from macrohabitat conditions. However, underground temperature and water availability are tightly linked to outdoor temperature and precipitation27,28,29. In the study system, the temperature measured inside caves sectors is strongly related to the surface average annual temperature values (such as the ones used in macroecological analyses) (Fig. S8). The similarity between air temperature inside caves and the average annual outdoor temperature is strikingly high in sectors far from the surface (Fig. S8b), except than in a few outlier caves, which probably have particular air circulation51. Underground environments receive a much lower interest in the macroecological/biogeographical literature than more visible aboveground habitats, but host a major portion of Earth biodiversity52. Additional analyses are required to assess how frequent are the differences between micro and macrohabitat patterns of niche similarity, and whether our results can apply to different systems.
Macroecology has allowed us to move from reductionist, small scale ecology to a much broader approach with great potential for generalization, which can provide key responses to the global biodiversity crisis53,54. Nevertheless, when laying the foundations of macroecology, Brown53 described himself as an oddball that continues combining reductionist and holistic approaches. The microhabitat and bioclimatic approaches provide insights about different aspects of species niches, and should be integrated for a more complete understanding of niche variation. The integration of multiple approaches certainly requires more time and investments, but the urgency to obtain answers should not preclude the need of robust, biologically sound data55. The integration of studies at multiple scales allows to take into account a broader spectrum of processes influencing populations, thus providing more accurate inference on niche evolution19. A better combination between bioclimatic and fine-grained data56, and also considering additional niche components such as diet and other biotic interactions, may be a key to obtain robust generalizations that can help us to address the consequences of global changes.
Methods
Study system
In summer, underground environments show a continuous microclimatic gradient: the superficial sectors have conditions similar to the outdoor ones (light, high temperature, low humidity). However, far from the surface the microhabitat becomes wetter, colder and dark (Fig. 1). Salamanders move underground because they must reach the sectors where conditions are within the tolerance limits of the species12 but, as food is more abundant in superficial sectors28,57,58, they are restricted to a few tens of meters from the surface. Generally, the realized niche does not correspond to the fundamental niche because of dispersal limitations and biotic interactions43. These issues exist for all the environments33 but, within this system, they are alleviated because the full environmental gradient exist within a few meters, well within the dispersal ability of individuals, and because of the lack of predators and competitors within these environments (Hydromantes species are allopatric, Fig. 2, no other terrestrial salamanders are present, and they are apex predators in these environments)59. Movements are limited and home ranges small (6–22 m2)25, therefore observations are unlikely to represent transient individuals. The study system thus can be viewed as a natural habitat selection experiment, in which individuals are exposed to continuous environmental gradients, within which they select the favourable conditions (i.e., the conditions within their fundamental niche). Furthermore, previous studies showed that the microhabitat conditions selected by salamanders are consistent through the year, and niche estimates from summer surveys are generally similar to estimates for the other seasons28. Summer is the period in which salamander detection is easiest, thus analyses performed on summer observation allow an appropriate characterization of species niche. Finally, terrestrial salamanders are generally at equilibrium with their environment for temperature and water and, in the field, the average temperature difference between air and body temperature is <0.5 °C26,60. Thus, air conditions are an excellent proxy of operative conditions of individuals12,26.
Ethics statement
Samples were collected in accordance with regulations for the protection of terrestrial wild animals (authorization by the Italian Ministry of the Environment, prot. 0040002).
Surveys and data collection
To measure species distribution and habitat at fine spatial scale (microhabitat) we surveyed caves in Mediterranean Italy and France, widely covering the range of all European Hydromantes species (Fig. S1a). We excluded caves from the narrow hybrid zone between H. ambrosii and H. italicus61. Surveys were performed in early summer (June–July 2011–2014), when the conditions outside the cave are unfavourable and underground detection is highest28. All surveys were performed during the central hours of sunny and dry days. Each cave was subdivided in 3-m longitudinal intervals (hereafter: sectors); the size of sectors approximately corresponds to home ranges size25,57, covering the whole cave or until the first empty sector after the last salamander. Overall, we surveyed 278 caves and 1251 cave sectors. In each sector we used visual encounter surveys to detect the presence of active salamanders, and measured four abiotic variables known to influence salamander distribution: air temperature (°C; accuracy: 0.1 °C) and relative humidity (%; accuracy: 0.1%) were recorded with a EM882 multi-function device, waiting until the measurement was stable (variation <0.1 °C or <0.1% for >60 seconds). Minimum and maximum incident light (illuminance, measured in lux, accuracy 0.01 lux) were recorded using the EM882 by performing at least 10 measures of illuminance in the portions of the sector receiving more and less light, respectively. Furthermore, as a biotic parameter, we counted the number of adult large Meta spiders (M. menardi or M. bourneti). These spiders are the major predators of arthropods in the study caves, and have been proposed as indicators of prey availability for salamanders57,62.
To analyse the bioclimatic niche, we obtained distribution records covering the whole range of all the Hydromantes species from the present study and from the literature25,37,61,63,64,65,66. We only considered localities with accuracy of 1-km or better. To match the number of microhabitat predictors, we considered five bioclimatic parameters: mean temperature and summed precipitation during the period in which salamanders are active outside the cave (from September to May), temperature seasonality, precipitation seasonality, and normalized difference vegetation index (NDVI). Climatic variables were extracted at the 30 arc-second resolution from Worldclim67, while NDVI was extracted from the ESA Land Cover CCI (mean NDVI over the 1999–2012 period; http://maps.elie.ucl.ac.be/CCI/viewer/download.php). Tolerance to these parameters is assumed to directly influence animals, particularly during the periods in which they perform outdoor activity. To assess the robustness of our conclusions to the selection of parameters, we also repeated analyses using annual climatic features.
Microhabitat preferences of species
We used generalized linear mixed models (GLMMs) with binomial error to assess the within-cave relationships between each species and the features of cave sectors. In GLMMs, cave identity was included as random effect, salamander presence as dependent, and the five microhabitat variables were the predictors. First, for each species we built the univariate models relating salamander presence to the five microhabitat variables. We tested both linear and quadratic relationships; quadratic terms were retained if they significantly improved fit. We then used the Akaike’s Information Criterion (AIC) to build the minimum adequate models, best describing the occurrence pattern of each species on the basis of multiple predictors68. We built models considering all possible combinations of microhabitat variables, and ranked them using AIC. Some microhabitat variables were strongly correlated: minimum illuminance was related to maximum illuminance, while temperature data were negatively correlated with humidity (in the datasets of most species, |r| > 0.7). Models including highly correlated variables were excluded from the candidate models. The lowest-AIC model, i.e. the one explaining more variation with fewer predictors, was considered as the minimum adequate model for each species68.
A species is certainly present where it is detected, while non-detection may represent either real absences or failure of detecting the present species; not taking into account misdetection can influence regression results69. Previous analyses on a subset of species showed that, with our sampling protocol, detection probability is high but imperfect (approx. 0.75 per visit)28,70. Therefore, in our models we weighted absences with a weight of 0.75 following71. We calculated significance of variables using likelihood-ratio tests. For all species the residual deviance was similar or lower than the residual degrees of freedom (variance inflation factor of best-AIC models always ≤1.06), therefore overdispersion was not an issue. Before running analyses, illuminance was log-transformed, while humidity % was transformed using square-root-arcsine to improve normality and reduce skewness.
Niche overlap and equivalency among species
We used an approach based on Principal Component Analyses of environmental variables (PCA-env) to perform multivariate comparisons of niche overlap between pairs of species following39. PCA-env measures niche overlap between pairs of species or populations on the basis of occurrence and environmental data, is among the most reliable techniques for niche comparisons, and shows better performance than approaches based on species distribution modelling39. PCA-env uses a kernel density function to compute the density of occurrences in the multivariate PCA space, in order to take potential bias into account that stems from unequal sampling effort. We calculated niche overlap and equivalency using the Schoener’s D metric72. Schoener’s D ranges between 0 (lack of overlap) and 1 (complete overlap), and is among the most widespread metrics of niche overlap in ecological, evolutionary and biogeographical studies (e.g.72,73). For the niche comparison of a species pair, PCA-env performs a non-phylogenetic principal component analysis (PCA) on the environmental spaces available to the two species39. In the micro-habitat analysis, the “available space” of each species corresponded to the sectors of all the surveyed caves within the range of that species. In the bioclimatic analysis, the available space corresponded to the grid cells within 150 km from known presence points. This distance is three times the largest gap within a species range, thus likely includes all the areas potentially available to species dispersal (see44). Preliminary analyses using different distance buffers yielded highly consistent results (see also74).
Species distribution data and bioclimatic variables often show strong spatial autocorrelation, and this can influence the outcome of ecological analyses, but no formal approaches are currently available to incorporate autocorrelation into PCA-env. To assess the robustness of PCA-env to spatial autocorrelation, we repeated the bioclimatic analysis including an additional predictor representing spatial autocorrelation. For each species, we first built a spatial generalized additive model (GAM) with binomial error, using species presence/absence as dependent variable, and incorporating geographic coordinates of sites as tensor product smooth terms, using thin plate regression splines75. We then used the spatial predictions of GAMs as an additional covariate in PCA-env. Even though the incorporation of spatial predictions as covariates is not a perfect approach to deal with autocorrelation, simulations showed that this implementation of GAMs helps to correctly estimate relationships in spatially structured datasets with relatively good performance75. For both the microhabitat and bioclimatic analyses, significance of niche differences between species was assessed using the niche equivalency tests through 1999 permutations.
Relationships between microhabitat, bioclimatic niche and evolutionary history
Genetic distance between species pairs was calculated on the basis of three mitochondrial (12S, 16S and cyt-b) and two nuclear (RAG-1 and BNDF) genes, amplified by van der Meijden et al.32. We considered the 49 individuals for which data from all five genes were available (2–15 individuals per species). The concatenated genetic dataset contained 3494 base pairs32. The Tamura-Nei distance was calculated for each species pair, using the between group mean distance function in Mega 6. To calculate the geographical distances among species, we generated the polygon of the range of each species on the basis of presence records using α-hulls76, and then calculated the Euclidean distances between the centroids of the ranges.
Microhabitat and bioclimatic niche distances between species were calculated as 1 - Shoener’s D. We then evaluated the relationships between microhabitat, bioclimatic and genetic distances. First, we used non-metric multidimensional scaling (NMDS) for the graphical representation of niche distances among species77. For the graphical representation of among-species differences in habitat relationships, we calculated the mean values of environmental variables in the presence localities, and then fitted them to the NMDS space using vector fitting78. Vector fitting returned essentially the same niche differences between species obtained with PCA-env (Figs S2, S3), with the advantage of synthetically illustrating the relationships between all the species pairs in one single plot. Relationships between niche dissimilarity at micro- and macro-ecological level and genetic differentiation were analysed with Mantel’s test for ranked data (bivariate analyses) or with rank multiple regressions on distance matrices (MRDM; multivariate analyses)77, using 9999 permutations to assess significance. Previous studies have shown that the Mantel test and other metrics of phylogenetic signal (e.g. Abouheif index, Bolmberg’s K) are closely related to each other because they are all based on a cross-product statistic, and the Mantel test is thus appropriate to assess phylogenetic signal for dissimilarity matrices79,80,81. After MRDM, we used commonality analysis to assess the unique and common contribution of intercorrelated independent variables82. Statistical analyses were run using the packages lme4, MuMIn, raster, vegan, ecodist and hyat in R 3.1 (www.r-project.org).
Data availability
Raw data are available as Data table S1. To avoid illegal poaching on protected species, we degraded the quality of distribution records. The reported coordinates have a randon error of up to 3 km, compared to the true ones. The correct coordinates were used for analyses.
References
Losos, J. B. Phylogenetic niche conservatism, phylogenetic signal and the relationship between phylogenetic relatedness and ecological similarity among species. Ecol. Lett. 11, 995–1003, https://doi.org/10.1111/j.1461-0248.2008.01229.x (2008).
Blomberg, S. P. & Garland, T. Tempo and mode in evolution: phylogenetic inertia, adaptation and comparative methods. J. Evol. Biol. 15, 899–910, https://doi.org/10.1046/j.1420-9101.2002.00472.x (2002).
Enriquez-Urzelai, U., Montori, A., Llorente, G. A. & Kaliontzopoulou, A. Locomotor Mode and the Evolution of the Hindlimb in Western Mediterranean Anurans. Evol. Biol. 42, 199–209, https://doi.org/10.1007/s11692-015-9311-1 (2015).
Mesquita, D. O. et al. Life-History Patterns of Lizards of the World. Am. Nat. 187, 689–705, https://doi.org/10.1086/686055 (2016).
Peterson, A. T. Ecological niche conservatism: a time-structured review of evidence. J. Biogeogr. 38, 817–827, https://doi.org/10.1111/j.1365-2699.2010.02456.x (2011).
Beck, J. et al. What’s on the horizon for macroecology. Ecography 35, 673–683, https://doi.org/10.1111/j.1600-0587.2012.07364.x (2012).
Potter, K. A., W.H., A. & Pincebourde, S. Microclimatic challenges in global change biology. Global Change Biol. 19, 2932–2939, https://doi.org/10.1111/gcb.12257 (2013).
Soberon, J. Grinnellian and Eltonian niches and geographic distributions of species. Ecol. Lett. 10, 1115–1123, https://doi.org/10.1111/j.1461-0248.2007.01107.x (2007).
Fraterrigo, J. M., Wagner, S. & Warren, R. J. Local-scale biotic interactions embedded in macroscale climate drivers suggest Eltonian noise hypothesis distribution patterns for an invasive grass. Ecol. Lett. 17, 1447–1454, https://doi.org/10.1111/ele.12352 (2014).
Scheffers, B. R., Edwards, D. P., Diesmos, A., Williams, S. E. & Evans, T. A. Microhabitats reduce animal’s exposure to climate extremes. Global Change Biol. 20, 495–503, https://doi.org/10.1111/gcb.12439 (2014).
Kearney, M. R. et al. Microclimate modelling at macro scales: a test of a general microclimate model integrated with gridded continental-scale soil and weather data. Methods Ecol. Evol. 5, 273–286, https://doi.org/10.1111/2041-210X.12148 (2014).
Sunday, J. M. et al. Thermal-safety margins and the necessity of thermoregulatory behavior across latitude and elevation. Proc. Natl. Acad. Sci. USA 111, 5610–5615 (2014).
Bennie, J., Wilson, R. J., Maclean, I. M. D. & Suggitt, A. J. Seeing the woods for the trees – when is microclimate important in species distribution models? Global Change Biol. 20, 2699–2700, https://doi.org/10.1111/gcb.12525 (2014).
Fridley, J. D. Downscaling Climate over Complex Terrain: High Finescale (<1000 m) Spatial Variation of Near-Ground Temperatures in a Montane Forested Landscape (Great Smoky Mountains). J. Appl. Meteorol. Climatol. 48, 1033–1049, https://doi.org/10.1175/2008jamc2084.1 (2009).
Bucklin, D. N. et al. Climate downscaling effects on predictive ecological models: a case study for threatened and endangered vertebrates in the southeastern United States. Reg. Envir. Chang. 13, S57–S68, https://doi.org/10.1007/s10113-012-0389-z (2013).
Sears, M. W., Raskin, E. & Angilletta, M. J. The World Is not Flat: Defining Relevant Thermal Landscapes in the Context of Climate Change. Integr. Comp. Biol. 51, 666–675, https://doi.org/10.1093/icb/icr111 (2011).
Freidenburg, L. K. & Skelly, D. K. Microgeographical variation in thermal preference by an amphibian. Ecol. Lett. 7, 369–373 (2004).
Gallien, L., Douzet, R., Pratte, S., Zimmermann, N. E. & Thuiller, W. Invasive species distribution models – how violating the equilibrium assumption can create new insights. Global Ecol. Biogeogr. 21, 1126–1136 (2012).
Searcy, C. A. & Shaffer, H. B. Do Ecological Niche Models Accurately Identify Climatic Determinants of Species Ranges? Am. Nat. 187, 423–435, https://doi.org/10.1086/685387 (2016).
Kozak, K. H. & Wiens, J. J. Climatic zonation drives latitudinal variation in speciation mechanisms. Proc. R. Soc. B 274, 2995–3003 (2007).
Fisher-Reid, M. C., Kozak, K. H. & Wiens, J. J. How is the rate of climatic-niche evolution related to climatic-niche breadth? Evolution 66, 3836–3851, https://doi.org/10.1111/j.1558-5646.2012.01729.x (2012).
Kozak, K. H. & Wiens, J. J. What explains patterns of species richness? The relative importance of climatic-niche evolution, morphological evolution, and ecological limits in salamanders. Ecol. Evol. 6, 5940–5949, https://doi.org/10.1002/ece3.2301 (2016).
Rissler, L. J. & Apodaca, J. J. Adding more ecology into species delimitation: Ecological niche models and phylogeography help define cryptic species in the black salamander (Aneides flavipunctatus). Syst. Biol. 56, 924–942, https://doi.org/10.1080/10635150701703063 (2007).
Wake, D. B. The enigmatic history of the European, Asian and American plethodontid salamanders. Amphibia-Reptilia 34, 323–336, https://doi.org/10.1163/15685381-00002893 (2013).
Lanza, B., Pastorelli, C., Laghi, P. & Cimmaruta, R. A review of systematics, taxonomy, genetics, biogeography and natural history of the genus Speleomantes Dubois, 1984 (Amphibia Caudata Plethodontidae). Atti Mus. Civ. St. Nat. Trieste 52(Suppl), 5–135 (2006).
Lunghi, E. et al. Thermal equilibrium and temperature differences among body regions in European plethodontid salamanders. J. Therm. Biol. 60, 79–85, https://doi.org/10.1016/j.jtherbio.2016.06.010 (2016).
Badino, G. Cave temperatures and global climatic change. Int. J. Speleol. 33, 103–114 (2004).
Lunghi, E., Manenti, R. & Ficetola, G. F. Seasonal variation in microhabitat of salamanders: environmental variation or shift of habitat selection? PeerJ 3, e1122, https://doi.org/10.7717/peerj.1122 (2015).
Sánchez-Fernández, D. et al. The deep subterranean environment as a potential model system in ecological, biogeographical and evolutionary research. Subterranean Biol. 25, 1–7 (2018).
De Frenne, P. et al. Microclimate moderates plant responses to macroclimate warming. Proc. Natl. Acad. Sci. USA 110, 18561–18565, https://doi.org/10.1073/pnas.1311190110 (2013).
Scheffers, B. R., Evans, T. A., Williams, S. E. & Edwards, D. P. Microhabitats in the tropics buffer temperature in a globally coherent manner. Biol. Lett. 10, 4, https://doi.org/10.1098/rsbl.2014.0819 (2014).
van der Meijden, A. et al. Phylogenetic relationships of Sardinian cave salamanders, genus Hydromantes, based on mitochondrial and nuclear DNA sequence data. Mol. Phylogenet. Evol. 51, 399–404, https://doi.org/10.1016/j.ympev.2008.12.011 (2009).
Peterson, A. T. et al. Ecological niches and geographic distributions. (Princeton University Press, 2011).
Warren, D. L., Cardillo, M., Rosauer, D. F. & Bolnick, D. I. Mistaking geography for biology: inferring processes from species distributions. Trends Ecol. Evol. 29, 572–580, https://doi.org/10.1016/j.tree.2014.08.003 (2014).
Gutiérrez, E. E., Boria, R. A. & Anderson, R. P. Can biotic interactions cause allopatry? Niche models, competition, and distributions of South American mouse opossums. Ecography 37, 741–753, https://doi.org/10.1111/ecog.00620 (2014).
Carranza, S., Romano, A., Arnold, E. N. & Sotgiu, G. Biogeography and evolution of European cave salamanders, Hydromantes (Urodela: Plethodontidae), inferred from mtDNA sequences. J. Biogeogr. 35, 724–738, https://doi.org/10.1111/j.1365-2699.2007.01817.x (2008).
Chiari, Y. et al. Phylogeography of Sardinian cave salamanders (genus Hydromantes) is mainly determined by geomorphology. Plos One 7, e32332 (2013).
Cimmaruta, R., Forti, G., Nascetti, G. & Bullini, L. Spatial distribution and competition in two parapatric sibling species of European plethodontid salamanders. Ethol. Ecol. Evol. 11, 383–398 (1999).
Broennimann, O. et al. Measuring ecological niche overlap from occurrence and spatial environmental data. Global Ecol. Biogeogr. 21, 841–897 (2012).
Grandcolas, P., Nattier, R., Legendre, F. & Pellens, R. Mapping extrinsic traits such as extinction risks or modelled bioclimatic niches on phylogenies: does it make sense at all? Cladistics 27, 181–185, https://doi.org/10.1111/j.1096-0031.2010.00324.x (2011).
McIntire, E. J. B. & Fajardo, A. Beyond description: the active and effective way to infer processes from spatial patterns. Ecology 90, 46–56 (2009).
Kearney, M. & Porter, W. Mechanistic niche modelling: combining physiological and spatial data to predict species’ ranges. Ecol. Lett. 12, 334–350 (2009).
Soberon, J. & Nakamura, M. Niches and distributional areas: Concepts, methods, and assumptions. Proc. Natl. Acad. Sci. USA 106, 19644–19650, https://doi.org/10.1073/pnas.0901637106 (2009).
Godsoe, W. I can’t define the niche but I know it when I see it: a formal link between statistical theory and the ecological niche. Oikos 119, 53–60 (2010).
Gomulkiewicz, R., Holt, R. D. & Barfield, M. The Effects of Density Dependence and Immigration on Local Adaptation and Niche Evolution in a Black-Hole Sink Environment. Theor. Popul. Biol. 55, 283–296, https://doi.org/10.1006/tpbi.1998.1405 (1999).
Osorio-Olvera, L. A., Falconi, M. & Soberon, J. On the relationship between habitat suitability and population abundance under different dispersal scenarios. Rev. Mex. Biodivers. 87, 1080–1088, https://doi.org/10.1016/j.rmb.2016.07.001 (2016).
Pironon, S. et al. Geographic variation in genetic and demographic performance: new insights from an old biogeographical paradigm. Biol. Rev. 92, 1877–1909, https://doi.org/10.1111/brv.12313 (2017).
Lunghi, E. et al. Environmental suitability models predict population density, performance and body condition for microendemic salamanders. Sci. Rep. 8, 7527, https://doi.org/10.1038/s41598-018-25704-1 (2018).
Brambilla, M. & Ficetola, G. F. Species distribution models as a tool to estimate reproductive parameters: a case study with a passerine bird species. J. Anim. Ecol. 81, 781–787 (2012).
Fisher-Reid, M. C., Engstrom, T. N., Kuczynski, C. A., Stephens, P. R. & Wiens, J. J. Parapatric divergence of sympatric morphs in a salamander: incipient speciation on Long Island? Mol. Ecol. 22, 4681–4694, https://doi.org/10.1111/mec.12412 (2013).
Luetscher, M. & Jeannin, P. Y. Temperature distribution in karst systems: the role of air and water fluxes. Terra Nova 16, 344–350, https://doi.org/10.1111/j.1365-3121.2004.00572.x (2004).
Brandmayr, P. et al. Hypogean carabid beetles as indicators of global warming? Environ. Res. Lett. 8, 11, https://doi.org/10.1088/1748-9326/8/4/044047 (2013).
Brown, J. H. Macroecology. 284 (The University of Chicago Press, 1995).
Kerr, J. T., Kharouba, H. M. & Currie, D. J. The macroecological contribution to global change solutions. Science 316, 1581–1584 (2007).
Bernardo, J. Biologically grounded predictions of species resistance and resilience to climate change. Proc. Natl. Acad. Sci. USA 111, 5450–5451 (2014).
Sandel, B. Towards a taxonomy of spatial scale-dependence. Ecography 38, 358–369, https://doi.org/10.1111/ecog.01034 (2015).
Ficetola, G. F., Pennati, R. & Manenti, R. Spatial segregation among age classes in cave salamanders: habitat selection or social interactions? Popul. Ecol. 55, 217–226, https://doi.org/10.1007/s10144-012-0350-5 (2013).
Lunghi, E., Manenti, R. & Ficetola, G. F. Cave features, seasonality and subterranean distribution of non-obligate cave dwellers. PeerJ, e3169, https://doi.org/10.7717/peerj.3169 (2017).
Lunghi, E., Manenti, R. & Ficetola, G. F. Do cave features affect underground habitat exploitation by non-troglobite species? Acta Oecol. 55, 29–35 (2014).
Spotila, J. R. Role of temperature and water in the ecology of lungless salamanders. Ecol. Monogr. 42, 95–125 (1972).
Ruggi, A. Descrizione di una zona di contatto e ibridazione tra Speleomantes italicus e S. ambrosii bianchii (Amphibia: Plethodontidae) sulle Alpi Apuane, mediante marcatori nucleari e mitocondriali. 109 (Università degli Studi della Tuscia di Viterbo. http://dspace.unitus.it/handle/2067/240, 2007).
Manenti, R., Lunghi, E. & Ficetola, G. F. Distribution of spiders in cave twilight zone depends on microclimatic features and trophic supply. Invertebr. Biol. 134, 242–251, https://doi.org/10.1111/ivb.12092 (2015).
Lanza, B., Caputo, V., Nascetti, G. & Bullini, L. Morphologic and genetic studies of the European plethodontid salamanders: taxonomic inferences (genus Hydromantes). (Monografie XVI. Museo Regionale di Scienze Naturali, 1995).
Pasmans, F. et al. Resistance to Chytridiomycosis in European Plethodontid Salamanders of the Genus Speleomantes. Plos One 8, e63639, https://doi.org/10.1371/journal.pone.0063639 (2013).
Fiacchini, D., Carotti, G. & Fusco, G. Biospeleologia nell’Appennino. Studi e ricerche su Anfibi e Invertebrati, con particolare riferimento all’Appennino Umbro-Marchigiano. (Tecnostampa Edizioni, 2008).
Lanza, B., Arntzen, J. W. & Gentile, E. Vertebral numbers in the Caudata of the Western Palaeartic (Amphibia). Atti Mus. Civ. St. Nat. Trieste 54, 3–114 (2009).
Hijmans, R. J., Cameron, S. E., Parra, J. L., Jones, P. G. & Jarvis, A. High resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978 (2005).
Burnham, K. P. & Anderson, D. R. Model selection and multimodel inference: a practical information-theoretic approach. (Springer Verlag, 2002).
MacKenzie, D. I. et al. Occupancy estimation and modeling: inferring patterns and dynamics of species occurrence. (Academic Press, 2006).
Ficetola, G. F., Pennati, R. & Manenti, R. Do cave salamanders occur randomly in cavities? An analysis with Hydromantes strinatii. Amphibia-Reptilia 33, 251–259 (2012).
Gómez-Rodríguez, C., Bustamante, J., Díaz-Paniagua, C. & Guisan, A. Integrating detection probabilities in species distribution models of amphibians breeding in Mediterranean temporary ponds. Divers. Distrib. 18, 260–272 (2012).
Warren, D. L., Glor, R. E. & Turelli, M. Environmental niche equivalency versus conservatism: Quantitative approaches to niche evolution. Evolution 62, 2868–2883, https://doi.org/10.1111/j.1558-5646.2008.00482.x (2008).
Rödder, D. & Engler, J. O. Quantitative metrics of overlaps in Grinnellian niches: advances and possible drawbacks. Global Ecol. Biogeogr. 20, 915–927 (2011).
Ficetola, G. F. & Stöck, M. Do hybrid-origin polyploid amphibians occupy transgressive or intermediate ecological niches compared to their diploid ancestors? J. Biogeogr. 43, 703–715, https://doi.org/10.1111/jbi.12667 (2016).
Beale, C. M., Lennon, J. J., Yearsley, J. M., Brewer, M. J. & Elston, D. A. Regression analysis of spatial data. Ecol. Lett. 13, 246–264 (2010).
Ficetola, G. F. et al. An evaluation of the robustness of global amphibian range maps. J. Biogeogr. 41, 211–221 (2014).
Legendre, P. & Legendre, L. Numerical Ecology. (Elsevier, 2012).
Borcard, D., Gillet, F. & Legendre, P. Numerical Ecology with R. (Springer, 2011).
Hardy, O. J. & Pavoine, S. Assessing phylogenetic signal with measurement error: a comparison of Mantel tests, Blomberg et al.‘s K, and phylogenetic distograms. Evolution 66, 2614–2621, https://doi.org/10.1111/j.1558-5646.2012.01623.x (2012).
Pavoine, S. et al. Life history traits, but not phylogeny, drive compositional patterns in a butterfly metacommunity. Ecology 95, 3304–3313 (2014).
Pavoine, S. & Ricotta, C. Testing for phylogenetic signal in biological traits: the ubiquity of cross-product statistics. Evolution 67, 828–840, https://doi.org/10.1111/j.1558-5646.2012.01823.x (2013).
Prunier, J. G., Colyn, M., Legendre, X., Nimon, K. F. & Flamand, M. C. Multicollinearity in spatial genetics: separating the wheat from the chaff using commonality analyses. Mol. Ecol. 24, 263–283, https://doi.org/10.1111/mec.13029 (2015).
Acknowledgements
We thank two anonymous reviewers for comments on an early version of this manusciript, and many speleologists for helping us during fieldwork. This study was funded by Univ. Milano-Bicocca, by Labex OSUG@2020 (Investissements d’avenir – ANR10 LABX56) and by Mohamed bin Zayed Species Conservation Fund (150511222).
Author information
Authors and Affiliations
Contributions
Conceived and designed the study: G.F.F. and R.M. Performed the field work: G.F.F., E.L., C.C., E.P.S., R.P., R.M. Analysed the data: G.F.F. Prepared the figures: G.F.F. G.F.F. wrote the first version of the paper, with subsequent contribution by all the co-authors.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ficetola, G.F., Lunghi, E., Canedoli, C. et al. Differences between microhabitat and broad-scale patterns of niche evolution in terrestrial salamanders. Sci Rep 8, 10575 (2018). https://doi.org/10.1038/s41598-018-28796-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-28796-x
This article is cited by
-
Evolution of seasonal land surface temperature trend in pond-breeding newt (Neurergus derjugini) in western Iran and eastern Iraq
Ecological Processes (2023)
-
Habitat and local climate influence the activity and abundance of Baron’s Mantella frog (Mantella baroni)
Evolutionary Ecology (2023)
-
The trophic niche of subterranean populations of Speleomantes italicus
Scientific Reports (2022)
-
Updating salamander datasets with phenotypic and stomach content information for two mainland Speleomantes
Scientific Data (2021)
-
Habitat selection of cave-restricted fauna in a new hotspot of subterranean biodiversity in Neotropics
Biodiversity and Conservation (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.