Abstract
Traumatic brain injury (TBI) is caused by physical damage to the brain and it induces blood-brain barrier (BBB) breakdown and inflammation. To diminish the sequelae of TBI, it is important to decrease haemorrhage and alleviate inflammation. In this study, we aimed to determine the effects of 2-carba-cyclic phosphatidic acid (2ccPA) on the repair mechanisms after a stab wound injury as a murine TBI model. The administration of 2ccPA suppressed serum immunoglobulin extravasation after the injury. To elucidate the effects of 2ccPA on inflammation resulting from TBI, we analysed the mRNA expression of inflammatory cytokines. We found that 2ccPA prevents a TBI-induced increase in the mRNA expression of Il-1β, Il-6, Tnf-α and Tgf-β1. In addition, 2ccPA reduces the elevation of Iba1 levels. These data suggest that 2ccPA attenuates the inflammation after a stab wound injury via the modulation of pro-inflammatory cytokines release from microglial cells. Therefore, we focused on the function of 2ccPA in microglial polarisation towards M1 or M2 phenotypes. The administration of 2ccPA decreased the number of M1 and increased the number of M2 type microglial cells, indicating that 2ccPA modulates the microglial polarisation and shifts them towards M2 phenotype. These data suggest that 2ccPA treatment suppresses the extent of BBB breakdown and inflammation after TBI.
Similar content being viewed by others
Introduction
Traumatic brain injury (TBI) occurs following physical brain damage due to a variety of causes such as road traffic accidents and falls. It affects approximately 10 million people worldwide per year. TBI severity and prognosis are associated with the impact of the primary and secondary brain injury. Primary brain injury refers to the mechanical damage including axonal loss and haemorrhage. Secondary brain injury occurs after several minutes to days of the primary brain injury. The secondary brain injury is caused by oedema and ischemia due to inflammation, cell death, and gliosis1. The secondary brain injury has a significant impact on the TBI outcome. To attenuate negative consequences of TBI, it is very important to alleviate the impact of the secondary brain injury.
Lysophosphatidic acid (LPA) is a lipid mediator. LPA was found to be elevated in the cerebrospinal fluid of TBI patients and of mice in a TBI model2. In a zebrafish model of spinal cord injury (SCI), LPA administration increased glial cell proliferation and neuronal cell death, indicating that LPA exerts proinflammatory activity and plays an inhibitory role in neuroregeneration after nerve injury3. It has been demonstrated that blocking LPA signalling with LPA-antibodies reduces lesion volume and diminishes tissue damage in mouse models of SCI and TBI2,3. LPA is generated by enzymatic hydrolysis of lysophosphatidylcholine catalysed by autotaxin (ATX)4, and it has been reported that ATX-mediated LPA production is induced 2–3 hours after nerve injury5. Therefore, decreasing LPA production and/or inhibiting its function after brain injury may diminish the secondary injury after TBI and thus attenuate the sequelae of TBI.
Cyclic phosphatidic acid (cPA) is a lipid mediator, which is also generated by ATX6. Although the chemical formula of cPA is similar to that of LPA, cPA has a unique structure consisting of a cyclic phosphate ring at the sn-2 and sn-3 positions of its glycerol backbone7,8. These features provide cPA with biological functions that are distinct from or oppose the functions of LPA9,10. In the nervous system, cPA exhibits neuroprotective effects. For example, in vitro studies revealed that cPA elicits neurotrophin-like actions9,11 and protects neurons from mitochondrial dysfunction-induced apoptosis12. In an in vivo study, cPA attenuated ischemia-induced delayed neuronal death in rat hippocampal CA1 regions13. Additionally, cPA reduced the extent of demyelination, astrogliosis, microglial activation, and motor dysfunction in a cuprizone-induced multiple sclerosis mouse model14. Moreover, it is known that cPA inhibits ATX activity15. Thus, cPA could be an endogenous inhibitor of LPA production via ATX inhibition. These results led us to investigate the effects of cPA on TBI. In this study, we used a metabolically stabilised derivative of cPA; 2-carba-cyclic phosphatidic acid (2ccPA) is a compound in which the phosphate oxygen is replaced by a methylene group at the sn-2 position15,16. It has been demonstrated that 2ccPA efficiently replicates numerous biological functions of cPA. For instance, 2ccPA is a much more potent inhibitor of ATX than cPA15. In a cuprizone-induced multiple sclerosis mouse model, 2ccPA not only exhibits beneficial effects on neuroregeneration, it exerts also more pronounced effects than cPA14,17.
Using as a TBI model a cerebral stab wound lesion caused by a needle, we investigated the effects of 2ccPA on the progression of the wound and found that 2ccPA reduces the severity of blood-brain barrier (BBB) breakdown in the primary brain injury phase. Furthermore, 2ccPA suppresses the expression of the pro-inflammatory cytokines interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumour necrosis factor-α (TNF-α) and the anti-inflammatory cytokine transforming growth factor-β1 (TGF-β1) as well as the activation of microglial cells in the injured area. To further investigate the effects of 2ccPA in the stab wound model, we examined the function of 2ccPA in microglial polarisation towards a neurotoxic M1 or a neuroprotective M2 phenotype. We found that 2ccPA decreases the number of M1 microglial cells and increases the number of M2 microglial cells. These data demonstrate that 2ccPA is a promising therapeutic agent for primary and secondary traumatic brain injury.
Materials and Methods
Mice
Female ICR wild-type mice were obtained from Charles River Laboratory (Kanagawa, Japan). All animal experiments were approved by the Institutional Animal Care and Use Committee of Ochanomizu University, Japan (animal study protocols 15027, 16006, and 17006), and were performed in accordance with the guidelines established by the Ministry of Education, Science, and Culture in Japan. All mice were allowed free access to food and water in a clean environment.
Pharmacological agents
2ccPA 18:1 was chemically synthesised as previously described16. 2ccPA was dissolved in phosphate buffered saline (PBS).
Stab wound surgery
Stab wound surgery was performed using a modified version of the procedure previously described by Hashimoto et al.18. Mice (6 weeks old) were anaesthetised by a mixture of 0.75 mg/kg Domitor (Nippon Zenyaku Kogyo, Fukushima, Japan), 4 mg/kg midazolam (Sandoz, Holzkirchen, Germany), and 5 mg/kg Vetorphale (Meiji Seika Pharma, Tokyo, Japan). A 19-gauge needle was then used to penetrate the skull in the occipital region of the right hemisphere, and a 27-gauge needle was inserted through this hole along the rostrocaudal axis, and the needle was pulled out gently to induce just one stab wound to the right cerebral cortex. After the operation, the Domitor antagonist Antisedan (0.75 mg/kg; Nippon Zenyaku Kogyo) was injected intraperitoneally to facilitate recovery from anaesthesia. A sample size calculation was based on our previous study18.
Postoperative treatment with 2ccPA
Wounded and naïve mice were randomly assigned to receive either 2ccPA or vehicle (PBS). They received shortly after the administration of Antisedan either 500 µg/ml 2ccPA (1.8 mg/kg/day) or PBS by intraperitoneal injection. Successive injections of the same dose of PBS or 2ccPA were performed every day until the collection of the brain samples (see Fig. 1A). Naïve mice had the same drug regimen. The delivery route and dosing regimen were based on previous work and preliminary tests14.
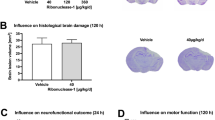
Effects of 2ccPA administration on the recovery from BBB breakdown caused by stab wound injuries. The extent of BBB breakdown near the lesion was assessed by the extravasation of serum IgG. (A) The dose, delivery route, and treatment regimen of 2ccPA. After the stab wound surgery, 500 µg/ml 2ccPA (1.8 mg/kg/day) or PBS as a vehicle were injected intraperitoneally. Successive injections of the same dose of 2ccPA or PBS were administered every day until the collection of the brain samples. Uninjured mice that received PBS or 2ccPA were regarded as naïve. (B) Cortical sections were immunoenzymatically stained with an antibody against mouse IgG. Regions near the stab wound injury are shown from naïve and stab-wounded mice treated with PBS (control) or 2ccPA. Scale bar: 100 µm. (C) Quantification of mouse IgG staining intensities near stab wounded injuries in PBS- and 2ccPA-treated mice on days 0, 1, 3, 5, and 7. All values were normalised to the mean staining intensity of the uninjured, contralateral hemisphere (n = 3 female mice/group). *p < 0.05, **p < 0.01, ***p < 0.001 vs. control groups, two-way ANOVA and Tukey-Kramer test. Data represent the mean ± SEM of three independent experiments using three pairs of mice at each specified day. A total of 36 female mice (6 weeks old) were subjected to this experiment.
Immunohistochemistry
Immunohistochemistry was performed as described by Hashimoto et al. with modifications18. Briefly, naïve and stab-wounded mice at postoperative days 0, 1, 3, 5, and 7 were perfused with PBS and fixed with 4% paraformaldehyde (PFA), and the collected brains were fixed with 4% PFA overnight. After cryoprotection with 30% sucrose, serial coronal sections (20 μm thick) of the cerebral cortex were obtained using a cryostat (Leica CM1850; Leica Microsystems, Wetzlar, Germany).
For immunofluorescence staining of microglial cells, the sections were incubated with primary antibodies overnight followed by secondary antibodies for 1 h. The primary antibodies in this study included anti-Iba1 (1:500 dilution; 019–19741; Wako, Osaka, Japan), anti-CD86 (1:200 dilution; 14-0862-82; Thermo Fisher Scientific), and anti-CD206 (1:200 dilution; AF2535; R&D Systems, Minneapolis, MN). The secondary antibodies were as follows: Alexa Fluor 568 goat anti-rabbit immunoglobulin G (IgG), Alexa Fluor 488 goat anti-rat IgG, and Alexa Fluor 488 chicken anti-goat IgG (all Thermo Fisher Scientific). Additionally, 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI; 1:2500 dilution, Roche Diagnostics) was used for nuclear staining. Images were captured with a confocal microscope (LSM710; Carl Zeiss, Oberkochen, Germany). To determine the frequency of stained cells, fields (600 × 600 μm2) were chosen arbitrarily near stab wound regions in each cerebral cortex section. A minimum of five sections from each mouse was analysed.
For immunoenzymatic stainings, the sections were incubated with the primary antibody overnight, with secondary antibodies for 1 h, and additionally with the avidin-biotin complex (Vectastain Elite ABC Standard Kit; Vector Laboratories, Burlingame, CA) for 1 h, followed by incubation in 3,3′-diaminobenzidine solution to detect signals. For the staining of mouse IgG, the sections were incubated with only secondary antibody for 1 h. Images of the sections were captured using a microscope (FSX100; Olympus, Tokyo, Japan). For the quantification of the mouse IgG staining, staining intensities were quantified using ImageJ by the measurement of mean grey values in five fields (8 × 8 µm2), which were selected randomly from immunoenzymatically stained regions in each section. To quantify the Iba1 staining intensity, staining intensities were quantified by the measurement of mean grey values in ten fields (8 × 8 µm2), which were selected randomly in each section. The mean grey value in each contralateral, uninjured region was subtracted from those of the ipsilateral region to correct for background values. A minimum of five sections was analysed from each mouse.
Real-time RT-PCR
RNA was extracted from cerebral cortices near the site of the injury or cultured microglial cells. The RNA (1 μg) was reverse-transcribed using ReverTra Ace qPCR RT Kit (Toyobo Co., Ltd., Osaka, Japan). Real-time RT-PCR analysis was performed to quantify Iba1, Il-1β, Il-6, Tnf-α, Tgf-β1, Lpar1–6, Gpr87, and P2ry10 mRNA expression levels. The primer sequences used in the present study are listed in Table 112,19,20,21,22,23. mRNA expression levels were quantified using a KOD SYBR qPCR Mix (Toyobo Co., Ltd.) with an ABI 7300 real-time PCR machine (Thermo Fisher Scientific, Waltham, MA). Analyses of relative gene expression levels were performed using the 2−Delta-Delta-Ct method between contralateral and injured cortices to reduce individual differences.
Microglial cell cultures
Cortical microglial cells were cultured using a modified version of the procedure described by Milner and Campbell24,25. Microglial cells and astrocytes were collected from the cerebral cortices of ICR mice on postnatal day 2. Cultures were maintained in T75 flasks in Dulbecco’s Modified Eagle’s Medium (Sigma-Aldrich, St. Louis, MO) with 10% fetal bovine serum, 50 units/ml penicillin and 50 μg/ml streptomycin for 10–14 days, before being shaken to yield microglial cells. Microglial cells were plated into 24-well plates (2.0 × 105 cells/well) and cultured for 24 h with astrocyte-derived supernatants. Finally, 1 μg/ml lipopolysaccharide (LPS) (Sigma-Aldrich) or 1, 5 and 10 μM 2ccPA were added for 4 h. PBS was used as the vehicle for LPS.
Statistical analyses
In the experiments using tissue sections, three pairs of 2ccPA treated mice and control mice were used. For real-time RT-PCR analysis in vivo, eight pairs of mice were used. The experiments using cultured microglial cells were performed a minimum of four times. The values are expressed as the mean ± standard error of the mean (SEM). Changes were considered statistically significant if the p-value obtained from the one-way or two-way ANOVA followed by Tukey-Kramer test or Student’s t-test reached one of the following significance levels: *p < 0.05, **p < 0.01, and ***p < 0.001.
Results
Administration of 2ccPA reduces the extent of BBB breakdown resulting from stab wounds to the mouse cerebral cortex
To elucidate whether 2ccPA affects the wound-healing process and recovery from BBB breakdown in injured regions, stab wounds were made in murine cerebral cortices using needles, and 2ccPA or PBS as a vehicle control was administered intraperitoneally immediately after the operation. Furthermore, successive injections of 2ccPA or PBS were given every 24 h until the collection of the brain samples (Fig. 1A). In the injured regions of the collected brains, the extent of BBB breakdown was assessed by measuring extravasation of serum IgG at days 0, 1, 3, 5, and 7. Serum IgG extravasation was not detected in the cortices of naïve mice but was observed around the lesions in cortices from injured mice on days 0–7 (Fig. 1B). IgG extravasation in the lesion area transiently increased after the operation until day 1 and decreased steadily afterwards from day 3 to day 7. Extravasation of serum IgG in contralateral hemispheres was not significantly induced by the stab wound injury (Fig. 1C). The administration of 2ccPA significantly decreased IgG extravasation in the lesions on days 0, 1, 3, 5, and 7 compared to control mice but did not affect extravasation in the contralateral regions (Fig. 1B,C). In addition, we profiled the mRNA expression levels of LPA receptors 1–6 (Lpar1–6), G protein-coupled receptor 87 (Gpr87), and the purinergic receptor P2Y10 (P2ry10), which also serve as cPA receptors, in mouse cerebral cortices after stab wound injuries. We found that the mRNA expression levels of most LPA receptors were increased and peaked around day 5 after the application of the stab wound, while the administration of 2ccPA suppressed this increase of mRNA expression (Supplementary Fig. S1).
2ccPA suppresses the expression of inflammatory cytokines after a traumatic injury to the cerebral cortex
To understand the mechanisms underlying 2ccPA-induced attenuation of serum IgG extravasation in the injured cerebral cortex, an investigation was carried out to determine whether 2ccPA suppresses the expression of inflammatory cytokines in the vicinity of cortical stab wounds. It has been reported that mRNA expression levels of Il-1β, Il-6, Tnf-α, and Tgf-β1 are increased in mouse cerebral cortices after application of a stab wound20,26. In the present study, mRNA expression levels of Il-1β, Il-6, Tnf-α, and Tgf-β1 were quantified in TBI model mice receiving 2ccPA. Near the cortical lesions, the mRNA expression of the pro-inflammatory cytokines Il-1β, Il-6, and Tnf-α was increased in both control and 2ccPA-treated mice, reaching maximal values at day 1. However, the administration of 2ccPA significantly suppressed the mRNA expression of Il-1β on days 3–7, Il-6 on days 5 and 7, and Tnf-α on day 5 (Fig. 2A–C). In addition, 2ccPA administration inhibited the injury-induced up-regulation of Tgf-β1 mRNA expression on day 3 (Fig. 2D). In naïve mice, the cortical mRNA expression of Il-1β, Il-6, and Tnf-α was not significantly affected by the administration of 2ccPA at any investigated time point (Supplementary Fig. S2A–C).
mRNA expression levels of pro- and anti-inflammatory cytokines near the stab wound injury in the cerebral cortices of 2ccPA-treated mice. (A–D) Real-time RT-PCR analyses of Il-1β, Il-6, Tnf-α, and Tgf-β1 mRNA expression on days 1, 3, 5, and 7 in PBS (control)- and 2ccPA-treated mice. mRNA expression levels were normalised to those of Gapdh and were subsequently normalised to the corresponding expression levels of the contralateral hemisphere (n = 8 female mice/group). Each expression level in PBS-administered naïve mice was regarded as 1, and all other values were normalized relative to this value. *p < 0.05, **p < 0.01 vs. control group, two-way ANOVA and Tukey-Kramer test. Data represent the mean ± SEM of eight pairs of mice at each specified postoperative day. A total of 80 female mice (6 weeks old) were subjected to this experiment.
2ccPA suppresses the activation of microglial cells after a stab wound injury
Microglial cells are the major source of pro- and anti-inflammatory cytokines in the central nervous system27,28. It has been reported that the administration of cPA effectively attenuates the activation of microglial cells in the corpus callosum of mice in a multiple sclerosis model14. To determine whether 2ccPA also affects the activation of microglial cells in the cerebral cortex after a TBI, the effects of 2ccPA administration on cortical Iba1 mRNA expression were examined. The administration of 2ccPA significantly suppressed the mRNA expression of Iba1, a microglial marker, on day 3 (Fig. 3A) but did not significantly affect the expression of Iba1 mRNA in the cortices of naïve mice (Supplementary Fig. S2D). Next, the expression of Iba1 protein in the vicinity of stab wounds was examined using an immunoenzymatic staining technique. The administration of 2ccPA markedly suppressed the expression of Iba1 protein near stab wound regions on days 1, 3, and 5 (Fig. 3B,C). Furthermore, the number of Iba1-positive cells near injured regions was counted on day 3 using immunofluorescence-stained sections. The ratio of Iba1-positive cells to DAPI-positive cells was significantly decreased by 2ccPA (Fig. 3D,E).
Effects of 2ccPA on Iba1 expression in microglial cells after a stab wound injury. (A) Real-time RT-PCR analysis of the Iba1 mRNA levels near the injury site at days 1, 3, 5, and 7 in 2ccPA-treated mice. mRNA levels for Iba1 were normalised to those for Gapdh and were subsequently normalised to the values in the corresponding contralateral region (n = 3 female mice/group). *p < 0.05, **p < 0.01 vs. control group, two-way ANOVA and Tukey-Kramer test. (B–E) Analysis of Iba1 expression near the stab wound site in mice receiving 2ccPA. (B) Cortical sections of PBS (control)- and 2ccPA-treated mice from postoperative days 0, 1, 3, 5, and 7 were immunoenzymatically stained with an antibody against Iba1. (C) Quantification of Iba1 staining intensities near the lesions. The mean grey values for Iba1 were normalised to those of the corresponding region in the contralateral hemisphere. After background normalisation, Iba1 mean grey values were normalised to those of control naïve mice (n = 3 female mice/group). *p < 0.05, **p < 0.01 vs. control group, two-way ANOVA and Tukey-Kramer test. (D) Representative examples of sections from PBS- and 2ccPA-treated mice on postoperative day 3 stained with anti-Iba1 antibodies shown in red. (E) Ratio of Iba1-positive cells to the total number of cells near stab wound regions on day 3 in PBS- and 2ccPA-treated mice. Scale bar: 100 µm. *p < 0.05, **p < 0.01 vs. control group, t-test. The RT-PCR data represent the mean ± SEM of eight pairs of mice at each specified day after the traumatic injury. All other data represent the mean ± SEM of three independent experiments using three pairs of mice at each postoperative day. A total of 110 female mice (6 weeks old) were subjected to this experiment.
2ccPA suppresses the expression of inflammatory cytokines in primary cultured microglial cells
To determine whether 2ccPA exerts its effects on the production of inflammatory cytokines via modulation of microglial cell behaviour, the effects of 2ccPA on the LPS-induced production of inflammatory cytokines in primary cultured microglial cells were examined. First, to confirm the expression of LPA receptors, the mRNA levels of the 2ccPA receptor candidates Lpar1–6, Gpr87, and P2ry10 were measured in microglial cells with and without exposure to LPS. Lpar1, Lpar2, Lpar3, Lpar4, Lpar5, Lpar6, Gpr87, and P2ry10 were expressed in cultured microglial cells in the presence and absence of LPS (Fig. 4), indicating that several candidates for 2ccPA receptors are expressed in microglial cells. The addition of LPS significantly increased the expression of Lpar1, Lpar2 and Gpr87 mRNA, and decreased Lpar5 and Lpar6 mRNA levels (Fig. 4). Next, the effect of 2ccPA on the mRNA expression of inflammatory cytokines was studied in cultured microglial cells. The addition of LPS changed the microglial cell morphology from ramified to amoeboid, the latter being considered a sign for microglial activation, and the presence of 10 μM 2ccPA did not appear to suppress this LPS-induced change (Fig. 5A). However, 10 μM 2ccPA suppressed the LPS-induced up-regulation of Il-1β, Il-6, and Tnf-α mRNA expression in microglial cells (Fig. 5B–D). The addition of 5 μM 2ccPA to LPS-treated microglial cells did not affect the Il-1β, Il-6, Tnf-α mRNA levels (Fig. 5B–D). Moreover, the expression of Tgf-β1 mRNA was also decreased by the addition of 1 and 5 μM 2ccPA (Fig. 5E).
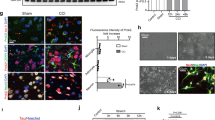
mRNA expression of putative 2ccPA receptors in primary cultured microglial cells. Real-time RT-PCR analysis of Lpar1, Lpar2, Lpar3, Lpar4, Lpar5, Lpar6, Gpr87, and P2ry10 mRNA levels in cultured microglial cells exposed to LPS or PBS as a vehicle. Each mRNA expression level was normalised to that of Gapdh. All values are presented relative to the mRNA expression level of Lpar1 in untreated microglial cells (control) (n = 3 cultures/group). *p < 0.05, **p < 0.01 vs. vehicle group, t-test. Data represent the mean ± SEM of three independent experiments. A total of 36 newborn mice (postnatal day 2) were subjected to this experiment.
Effects of 2ccPA on mRNA expression of pro- and anti-inflammatory cytokines in primary cultured microglial cells. (A) LPS- and 2ccPA-stimulated microglial cells stained with anti-Iba1 antibody (green) and DAPI (blue). (B,C,D,E) Real-time RT-PCR analysis of Il-1β, Il-6, Tnf-α, and Tgf-β1 mRNA expression in primary cultured microglial cells exposed to LPS and 2ccPA. The mRNA expression levels of Il-1β, Il-6, Tnf-α, and Tgf-β1 were normalised to that of Gapdh. All mRNA levels were normalised to those from cultured microglial cells without LPS or 2ccPA (n = 3 cultures/group). Scale bar: 50 µm. *p < 0.05, **p < 0.01, ***p < 0.001 vs. control group, one-way ANOVA and Tukey-Kramer test. Data represent the mean ± SEM of five independent experiments. A total of 36 newborn mice (postnatal day 2) were subjected to this experiment.
2ccPA promotes the progression from the M1 to the M2 phenotype in microglial cells after TBI
Activated microglial cells can be categorised into two opposite types, an M1 phenotype, which promotes inflammation, and an M2 phenotype, which produces anti-inflammatory cytokines and protects neurons29,30,31. To determine whether 2ccPA modulates microglial polarisation in the vicinity of a stab wound towards the M1 or M2 phenotype, the numbers of CD86− and CD206−positive cells, which are the markers of M1 and M2 phenotype, respectively, were quantified near the lesion site on day 3. The ratio of CD86+/Iba1+ cells to DAPI+ cells near the site of the injury was significantly decreased by administration of 2ccPA. Additionally, the ratio of CD206+/Iba1+ cells to DAPI+ cells was significantly increased by 2ccPA administration (Fig. 6).
Effects of 2ccPA on microglial polarisation towards M1 or M2 phenotypes after a stab wound injury. (A) Immunofluorescent images of cerebral cortices from PBS (control)- and 2ccPA-treated mice on postoperative day 3. Sections were stained with anti-CD86 (green), anti-Iba1 (red), and DAPI (blue). (B) Immunofluorescence staining of cerebral cortex sections from PBS- and 2ccPA-treated mice on day 3 with anti-CD206 (green), anti-Iba1 (red), and DAPI (blue). The arrowheads and arrows indicate CD86+/Iba1+ and CD206+/Iba1+ cells, respectively. (C) Ratios of CD86+/Iba1+ or CD206+/Iba1+ cell numbers to DAPI+ cell numbers near the stab wounded injury on day 3 in PBS- and 2ccPA-treated mice (n = 3 female mice/group). Scale bar: 100 µm. *p < 0.05, ***p < 0.001 vs. control group, t-test. Data represent the mean ± SEM of three independent experiments using three pairs of mice. A total of 6 female mice (6 weeks old) were subjected to this experiment.
Discussion
We studied the effects of 2ccPA administration on wound healing in the murine cerebral cortex after a stab wound injury using as parameters the degree of haemorrhage, inflammation, and activation of microglial cells. First, we observed that the administration of 2ccPA significantly attenuates the extent of BBB breakdown caused by a stab wound (Fig. 1), suggesting that 2ccPA promotes recovery from an injury-induced breakdown of the BBB. Second, 2ccPA suppresses the TBI-induced up-regulation of mRNA for inflammatory cytokines such as Il-1β, Il-6, Tnf-α, and Tgf-β1 (Fig. 2), suggesting that 2ccPA attenuates the inflammation after a stab wound injury. Third, we determined that the administration of 2ccPA markedly attenuates the injury-induced up-regulation of the microglial cell number and the Iba1 protein level (Fig. 3B–E). In our study, 2ccPA was not only administered shortly after the induction of the traumatic injury but also repeatedly at intervals of 24 h until the collection of the brains. Therefore, a continuous administration of 2ccPA might be needed to maintain its effects during wound healing. Fourth, 10 μM 2ccPA suppresses the LPS-induced up-regulation of mRNA levels from inflammatory cytokines in cultured microglial cells (Fig. 5). This suggests that 2ccPA attenuates the inflammation via regulation of microglial activation in the cerebral cortex after a traumatic injury. Fifth, the administration of 2ccPA in injured mice decreases the number of M1 microglial cells, while increasing simultaneously the number of microglial cells with an M2 phenotype (Fig. 6), suggesting that 2ccPA modulates microglial polarisation towards M1 or M2 phenotypes. These data suggest that 2ccPA administration leads to a suppression of BBB breakdown extent and inflammation caused by a stab wound injury.
Regarding the observed 2ccPA-induced suppression of BBB breakdown in the stab wound region, it has been suggested that 2ccPA accelerates the recovery from BBB breakdown. However, the underlying mechanisms are unknown. The extracellular matrix is one of the basic components of the BBB and plays a critical role in maintaining its integrity. Several studies have reported that proteolytic enzymes, such as matrix metalloproteinases (MMPs) and urokinase plasminogen activator (uPA), are up-regulated after brain injury and degrade the BBB32. Studies have shown that LPA increases MMP-9 and uPA expression and thus increases the permeability of BBB32. Furthermore, it has been reported that LPA levels are not only elevated in a mouse model of TBI, but also in the cerebrospinal fluid of TBI patients2. Since cPA is an endogenous inhibitor of LPA production through ATX15, 2ccPA might suppress LPA activity and thereby offer protection against BBB breakdown.
We found that 2ccPA suppresses not only the severity of BBB breakdown but also the inflammation in the vicinity of the stab wound injury. In the present study, it was determined that the mRNA expression of the pro-inflammatory cytokines Il-1β, Il-6, and Tnf-α were suppressed by 2ccPA administration in TBI model mice (Fig. 2). We also found that the activation of microglial cells induced by the stab wound was attenuated by 2ccPA (Fig. 3). These findings suggest that 2ccPA prevents the up-regulation of pro-inflammatory cytokine expression via a suppression of microglial cell activation. This is supported by the fact that 2ccPA also suppresses the mRNA expression of pro-inflammatory cytokines in primary cultures of LPS-stimulated microglial cells (Fig. 5B–D). Since pro-inflammatory cytokines are also produced by astrocytes27,28, it is possible that the administration of 2ccPA suppresses the activation of astrocytes. Further experiments are required to determine the effects of 2ccPA on the activation of astrocytes after a stab wound injury.
It is possible that 2ccPA plays a role in the modulation of microglial polarisation towards the M1 or M2 phenotype. Our results suggest that 2ccPA modulates microglial polarisation towards the M2 phenotype rather than M1 phenotype. It is known that 2ccPA and cPA protect oligodendrocytes and neuroblastoma Neuro2A cells via suppression of apoptosis pathways12,17,33, suggesting that 2ccPA exerts a neuroprotective activity. Since M2 microglial cells play roles in attenuating inflammation and promoting tissue repair34, our data suggest that 2ccPA contributes to the suppression of inflammation after the stab wound injury via promotion of microglial polarisation towards the M2 phenotype in the mouse cerebral cortex. It is known that M2 microglial cells release anti-inflammatory cytokines such as TGF-β135. In the present study, 2ccPA did not increase Tgf-β1 mRNA expression in vivo or in vitro (Figs 2D, 5E) despite the observed increase in the number of M2 microglial cells. In control mice, the injury-induced increase of mRNA levels from pro-inflammatory cytokines peaked on day 1. While the pro-inflammatory mRNA expression began to decrease on day 3, the mRNA level of the anti-inflammatory cytokine TGF-β1 reached its maximum values (Fig. 2), suggesting that TGF-β1 expression is up-regulated by inflammation in regions near the stab wound. This is in support of the notion that the lack of TGF-β1 up-regulation in TBI model mice might be caused by a 2ccPA-induced attenuation of the inflammation.
We found that mouse cerebral cortices and microglial cells express receptors for 2ccPA including LPA1–6, GPR87, and P2Y10, which are known to be activated by cPA15,36,37,38,39. LPA receptors mRNA expression levels were increased until day 5 after the stab wound trauma and declined to basal levels at day 7 after the injury (Supplementary Fig. S1). These increases in mRNA levels of LPA receptors were suppressed by 2ccPA treatment (Supplementary Fig. S1). In this study, we were not able to establish, whether 2ccPA administration decreases the mRNA expression of LPA receptors directly or indirectly by suppression of the inflammation. To our knowledge, there is no report that 2ccPA and cPA regulate the expression of LPA receptors. Therefore, it might be necessary to investigate in future studies whether 2ccPA controls the expression of LPA receptors directly. Furthermore, the addition of LPS altered the expression of these receptors for 2ccPA (Fig. 4). Our data indicate that LPS increases Lpar1, Lpar2, and Gpr87 mRNA expression levels in cultured microglial cells. The selective blockade of LPA1 results in reduced demyelination and improvement in locomotor recovery after spinal cord contusion in mice40. It is possible that 2ccPA binds to LPA1, LPA2, GPR87 in LPS-stimulated microglial cells and thereby suppresses inflammation.
The mechanisms underlying 2ccPA suppression of pro-inflammatory cytokine production are unclear. LPS binds to toll-like receptor 4 (TLR4) and promotes the transcription of pro-inflammatory cytokines via nuclear factor-kappa B (NF-κB) and activator protein 1 (AP-1) pathways41,42. The transcription of inflammatory cytokines might be suppressed by 2ccPA via inhibition of the activation of signal transduction factors in the NF-”κB and AP-1 pathways. In addition, it might be an important topic for future studies to elucidate the mechanisms by which 2ccPA regulates the microglial polarisation towards an M1 or M2 phenotype. NF-κB regulates the expression of M1-related genes43. Knockout of TLR4 decreases the phosphorylation of NF-κB and increases the number of M2 type microglial cells, indicating that the activity of NF-κB is involved in microglial polarisation44. Therefore, it is possible that 2ccPA decreases the polarisation into M1 type microglia by the inhibition of NF-κB activity. Moreover, it is known that signal transducers and activators of transcription 3 (STAT3) regulates the microglial polarisation into the M1 type, while signal transducers and activators of transcription 6 (STAT6) promotes microglial M2 polarisation43. It might be of interest to study the influence of 2ccPA on the nuclear translocation of the STAT family during M1 and M2 polarisation of microglial cells.
In summary, we examined the effects of 2ccPA on the wound healing of the cerebral cortex in a mouse model of TBI. This study indicates that administration of 2ccPA significantly attenuates the severity of BBB breakdown, suppresses the up-regulation of mRNA expression from pro-inflammatory cytokines, attenuates the number of neurotoxic M1 microglial cells, and increases the number of neuroprotective M2 microglial cells. These findings demonstrate that 2ccPA suppresses primary and secondary traumatic brain injury via regulation of microglial activation in the cerebral cortex.
References
Rosenfeld, J. V. et al. Early management of severe traumatic brain injury. Lancet 380, 1088–1098, https://doi.org/10.1016/S0140-6736(12)60864-2 (2012).
Crack, P. J. et al. Anti-lysophosphatidic acid antibodies improve traumatic brain injury outcomes. J. Neuroinflammation 11, 37, https://doi.org/10.1186/1742-2094-11-37 (2014).
Goldshmit, Y. et al. Blockage of lysophosphatidic acid signaling improves spinal cord injury outcomes. Am. J. Pathol. 181, 978–992, https://doi.org/10.1016/j.ajpath.2012.06.007 (2012).
Umezu-Goto, M. et al. Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J. Cell Biol. 158, 227–233, https://doi.org/10.1083/jcb.200204026 (2002).
Ma, L., Nagai, J. & Ueda, H. Microglial activation mediates de novo lysophosphatidic acid production in a model of neuropathic pain. J. Neurochem. 115, 643–653, https://doi.org/10.1111/j.1471-4159.2010.06955.x (2010).
Tsuda, S. et al. Cyclic phosphatidic acid is produced by autotaxin in blood. J. Biol. Chem. 281, 26081–26088, https://doi.org/10.1074/jbc.M602925200 (2006).
Murakami-Murofushi, K., Shioda, M., Kaji, K., Yoshida, S. & Murofushi, H. Inhibition of eukaryotic DNA polymerase alpha with a novel lysophosphatidic acid (PHYLPA) isolated from myxoamoebae of Physarum polycephalum. J. Biol. Chem. 267, 21512–21517 (1992).
Murakami-Murofushi, K. et al. A novel lipid mediator, cyclic phosphatidic acid (cPA), and its biological functions. Ann. N. Y. Acad. Sci. 905, 319–321 (2000).
Murakami-Murofushi, K. et al. Biological functions of a novel lipid mediator, cyclic phosphatidic acid. Biochim. Biophys. Acta 1582, 1–7 (2002).
Fujiwara, Y. Cyclic phosphatidic acid - a unique bioactive phospholipid. Biochim. Biophys. Acta 1781, 519–524, https://doi.org/10.1016/j.bbalip.2008.05.002 (2008).
Fujiwara, Y. et al. Cyclic phosphatidic acid elicits neurotrophin-like actions in embryonic hippocampal neurons. J. Neurochem. 87, 1272–1283 (2003).
Gotoh, M., Sano-Maeda, K., Murofushi, H. & Murakami-Murofushi, K. Protection of neuroblastoma Neuro2A cells from hypoxia-induced apoptosis by cyclic phosphatidic acid (cPA). PLoS One 7, e51093, https://doi.org/10.1371/journal.pone.0051093 (2012).
Gotoh, M., Hotta, H. & Murakami-Murofushi, K. Effects of cyclic phosphatidic acid on delayed neuronal death following transient ischemia in rat hippocampal CA1. Eur. J. Pharmacol. 649, 206–209, https://doi.org/10.1016/j.ejphar.2010.09.052 (2010).
Yamamoto, S. et al. Cyclic phosphatidic acid treatment suppress cuprizone-induced demyelination and motor dysfunction in mice. Eur. J. Pharmacol. 741, 17–24, https://doi.org/10.1016/j.ejphar.2014.07.040 (2014).
Baker, D. L. et al. Carba analogs of cyclic phosphatidic acid are selective inhibitors of autotaxin and cancer cell invasion and metastasis. J. Biol. Chem. 281, 22786–22793, https://doi.org/10.1074/jbc.M512486200 (2006).
Uchiyama, A. et al. Inhibition of transcellular tumor cell migration and metastasis by novel carba-derivatives of cyclic phosphatidic acid. Biochim. Biophys. Acta 1771, 103–112, https://doi.org/10.1016/j.bbalip.2006.10.001 (2007).
Yamamoto, S. et al. Protective and therapeutic role of 2-carba-cyclic phosphatidic acid in demyelinating disease. J. Neuroinflammation 14, 142, https://doi.org/10.1186/s12974-017-0923-5 (2017).
Hashimoto, K., Ikeda, N., Nakashima, M., Ikeshima-Kataoka, H. & Miyamoto, Y. Vitronectin Regulates the Fibrinolytic System during the Repair of Cerebral Cortex in Stab-Wounded Mice. J. Neurotrauma 34, 3183–3191, https://doi.org/10.1089/neu.2017.5008 (2017).
Amin, D. N. et al. Identification of stage biomarkers for human African trypanosomiasis. Am. J. Trop. Med. Hyg. 82, 983–990, https://doi.org/10.4269/ajtmh.2010.09-0770 (2010).
Ikeshima-Kataoka, H., Abe, Y., Abe, T. & Yasui, M. Immunological function of aquaporin-4 in stab-wounded mouse brain in concert with a pro-inflammatory cytokine inducer, osteopontin. Mol. Cell. Neurosci. 56, 65–75, https://doi.org/10.1016/j.mcn.2013.02.002 (2013).
Zanier, E. R. et al. Bone marrow mesenchymal stromal cells drive protective M2 microglia polarization after brain trauma. Neurotherapeutics 11, 679–695, https://doi.org/10.1007/s13311-014-0277-y (2014).
Jiang, J. et al. Therapeutic window for cyclooxygenase-2 related anti-inflammatory therapy after status epilepticus. Neurobiol. Dis. 76, 126–136, https://doi.org/10.1016/j.nbd.2014.12.032 (2015).
Wei, J. et al. Microglia activation: one of the checkpoints in the CNS inflammation caused by Angiostrongylus cantonensis infection in rodent model. Parasitol. Res. 114, 3247–3254, https://doi.org/10.1007/s00436-015-4541-9 (2015).
Milner, R. & Campbell, I. L. Cytokines regulate microglial adhesion to laminin and astrocyte extracellular matrix via protein kinase C-dependent activation of the alpha6beta1 integrin. J. Neurosci. 22, 1562–1572 (2002).
Milner, R. & Campbell, I. L. The extracellular matrix and cytokines regulate microglial integrin expression and activation. J. Immunol. 170, 3850–3858 (2003).
Wang, Y., Moges, H., Bharucha, Y. & Symes, A. Smad3 null mice display more rapid wound closure and reduced scar formation after a stab wound to the cerebral cortex. Exp. Neurol. 203, 168–184, https://doi.org/10.1016/j.expneurol.2006.08.006 (2007).
Lieberman, A. P., Pitha, P. M., Shin, H. S. & Shin, M. L. Production of tumor necrosis factor and other cytokines by astrocytes stimulated with lipopolysaccharide or a neurotropic virus. Proc. Natl. Acad. Sci. USA 86, 6348–6352 (1989).
Welser-Alves, J. V. & Milner, R. Microglia are the major source of TNF-alpha and TGF-beta1 in postnatal glial cultures; regulation by cytokines, lipopolysaccharide, and vitronectin. Neurochem. Int. 63, 47–53, https://doi.org/10.1016/j.neuint.2013.04.007 (2013).
Ransohoff, R. M. & Perry, V. H. Microglial physiology: unique stimuli, specialized responses. Annu. Rev. Immunol. 27, 119–145, https://doi.org/10.1146/annurev.immunol.021908.132528 (2009).
Saijo, K. & Glass, C. K. Microglial cell origin and phenotypes in health and disease. Nat. Rev. Immunol. 11, 775–787, https://doi.org/10.1038/nri3086 (2011).
Zhao, X. et al. Neuronal Interleukin-4 as a Modulator of Microglial Pathways and Ischemic Brain Damage. J. Neurosci. 35, 11281–11291, https://doi.org/10.1523/JNEUROSCI.1685-15.2015 (2015).
Yu, Y. et al. Role of Rho kinase in lysophosphatidic acid-induced altering of blood-brain barrier permeability. Int. J. Mol. Med. 33, 661–669, https://doi.org/10.3892/ijmm.2014.1618 (2014).
Nakajima, S., Gotoh, M., Fukasawa, K., Murofushi, H. & Murakami-Murofushi, K. 2-O-Carba-oleoyl cyclic phosphatidic acid induces glial proliferation through the activation of lysophosphatidic acid receptor. Brain Res. 1681, 44–51, https://doi.org/10.1016/j.brainres.2017.12.031 (2018).
Cherry, J. D., Olschowka, J. A. & O’Banion, M. K. Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J. Neuroinflammation 11, 98, https://doi.org/10.1186/1742-2094-11-98 (2014).
Lee, Y. et al. Therapeutically targeting neuroinflammation and microglia after acute ischemic stroke. Biomed Res Int 2014, 297241, https://doi.org/10.1155/2014/297241 (2014).
Lee, C. W., Rivera, R., Dubin, A. E. & Chun, J. LPA(4)/GPR23 is a lysophosphatidic acid (LPA) receptor utilizing G(s)-, G(q)/G(i)-mediated calcium signaling and G(12/13)-mediated Rho activation. J. Biol. Chem. 282, 4310–4317, https://doi.org/10.1074/jbc.M610826200 (2007).
Williams, J. R. et al. Unique ligand selectivity of the GPR92/LPA5 lysophosphatidate receptor indicates role in human platelet activation. J. Biol. Chem. 284, 17304–17319, https://doi.org/10.1074/jbc.M109.003194 (2009).
Yanagida, K. et al. Identification and characterization of a novel lysophosphatidic acid receptor, p2y5/LPA6. J. Biol. Chem. 284, 17731–17741, https://doi.org/10.1074/jbc.M808506200 (2009).
Konakazawa, M., Gotoh, M., Murakami-Murofushi, K., Hamano, A. & Miyamoto, Y. The effect of cyclic phosphatidic acid on the proliferation and differentiation of mouse cerebellar granule precursor cells during cerebellar development. Brain Res. 1614, 28–37, https://doi.org/10.1016/j.brainres.2015.04.013 (2015).
Santos-Nogueira, E. et al. Activation of Lysophosphatidic Acid Receptor Type 1 Contributes to Pathophysiology of Spinal Cord Injury. J. Neurosci. 35, 10224–10235, https://doi.org/10.1523/JNEUROSCI.4703-14.2015 (2015).
Muller, J. M., Ziegler-Heitbrock, H. W. & Baeuerle, P. A. Nuclear factor kappa B, a mediator of lipopolysaccharide effects. Immunobiology 187, 233–256, https://doi.org/10.1016/S0171-2985(11)80342-6 (1993).
Lawrence, T. & Natoli, G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat. Rev. Immunol. 11, 750–761, https://doi.org/10.1038/nri3088 (2011).
Lan, X., Han, X., Li, Q., Yang, Q. W. & Wang, J. Modulators of microglial activation and polarization after intracerebral haemorrhage. Nat. Rev. Neurol. 13, 420–433, https://doi.org/10.1038/nrneurol.2017.69 (2017).
Yao, X. et al. TLR4 signal ablation attenuated neurological deficits by regulating microglial M1/M2 phenotype after traumatic brain injury in mice. J. Neuroimmunol. 310, 38–45, https://doi.org/10.1016/j.jneuroim.2017.06.006 (2017).
Acknowledgements
The authors gratefully acknowledge the work of past and present members of Miyamoto laboratory and Murofushi laboratory. This work was supported by grant from Institute for Human Life Innovation, Ochanomizu University.
Author information
Authors and Affiliations
Contributions
All authors revised the manuscript and approved its final version. Specific contributions are as follows: K.H., M.N. and M.G. wrote the main manuscript. K.H., M.N. and A.H. prepared Figures 1–6 and supplementary dataset. K.H. and Y.M. designed the experiments and provided experimental oversight. M.N. and A.H. carried out the mouse experiments. M.N. performed the immunohistochemistry. A.H. performed the real-time RT-PCR analysis of injured brains and primary cultured microglial cells. A.H. and H.I.-K. developed the primary culture of microglial cells. H.I.-K. and K.M.-M. contributed to the analysis and interpretation of the data.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hashimoto, K., Nakashima, M., Hamano, A. et al. 2-carba cyclic phosphatidic acid suppresses inflammation via regulation of microglial polarisation in the stab-wounded mouse cerebral cortex. Sci Rep 8, 9715 (2018). https://doi.org/10.1038/s41598-018-27990-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-27990-1
This article is cited by
-
Two-carba cyclic phosphatidic acid treatment promotes phenotypic switch from M1 to M2 microglia and prevents behavioral abnormalities in a mouse model of neuropsychiatric systemic lupus erythematosus
Human Cell (2023)
-
Apelin alleviated neuroinflammation and promoted endogenous neural stem cell proliferation and differentiation after spinal cord injury in rats
Journal of Neuroinflammation (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.