Abstract
The majority of cervical cancer (CC) cases are attributable to HPV infection. Altered Notch pathway signals and HPV are believed to modify clinicopathogenesis of CC, however, the involvement of each molecular player and its mechanism is still not known. Jagged-1 (JAG1) is one of the ligands that induce Notch pathway. The involvement of JAG1 in the modulation of a disease condition is not very clear. Hence, this study aims to study the role of JAG1 in HPV-16/18 associated different histological sub-types of CC, especially ADC. 40 non-neoplastic cervical tissues, 30 precancer and 118 tumor specimens (total 188 tissue biopsies) were studied for the expression of the JAG1 protein through immunohistochemistry, immunoblotting and for HPV infection. Two folds increase of cytoplasmic (Mean ± S.E, 3.67 ± 0.33; p = 0.0001) and nuclear (3.70 ± 0.38, p = 0.0001) JAG1 expression was identified in normal (N) vs precancer and three folds cytoplasmic (4.44 ± 0.17, p = 0.0001) and nuclear (4.64 ± 0.17; p = 0.0001) in N vs. ISCC. Total 85% of ADC patients were found to be infected with HPV, which were 100% infected with HPV-16. These findings suggest the complex synergistic interplay between JAG1 and HPV in regulating clinicopathological progression of CC through its deregulation.
Similar content being viewed by others
Introduction
Cervical cancer (CC) ranks 4th in women related malignancies and overall 7th most globally reported carcinoma with high incidence rate (5,28,000 new cases) in 20121. Histologically, CC can be characterized by two different sub-categories i.e. (i) invasive squamous cell carcinomas (ISCC) which are frequent and covers 85–90% of CC cases (ii) adenocarcinomas (ADC) which are relatively rare and comprises only 10–15% cases2. The CC develops progression of precancerous lesions; called cervical intraepithelial neoplasia (CIN) grade 1–3 or squamous intraepithelial lesion (SIL)3.
Human Papillomavirus (HPV) has emerged as a fundamental regulator of CC4,5 and more than 90% of detected infections get cleared within two years6. Therefore, the major risk factor of CC is exposure to HPV infection especially HPV-16/18, but to generate and to maintain the malignant processes, HPV infection needs other cofactors; as only few cells infected with carcinogenic HPV develops into CC7. Thus, the current thrust area in the field of CC is to illustrate the molecular mechanism/s involved in the initiation and progression of CC, as well as to develop therapeutic targets.
Studies revealed an interaction of HPV proteins with Notch signaling pathway4. The Notch is a complex transmembrane pathway involved in a) cell proliferation, differentiation and development8, b) organogenesis, c) maintains stem cell viability9 and d) renewal in the adult10. There are four Notch homologues (Notch 1–4) and five ligands (three Delta-like and two Jagged/Serrate)11. Among all ligands, animal studies showed that a null mice for Jagged-1 (JAG1) encoded genes exhibit distinct embryonic defects12, suggesting its inimitability independent of canonical Notch pathway. JAG1 binding with Notch-3 dissociates its extracellular unit from the transmembrane unit, sequentially cleaved by proteases, translocating the cleaved intracellular Notch C-terminal fragment to the nucleus and recruits CSL (CBF1, Suppressor of Hairless, Lag1), Mastermind-like proteins (MAML1, 2 and 3) and p300 proteins to transcriptionally regulate target genes.
Till date, there are five studies (Supplement S1) available with respect to JAG1 induced Notch signaling in HPV associated CC. Hence, this study aims to fill the research gap by measuring the expression of JAG1 in various degrees of HPV linked cervical precancer, ISCC and ADC patients by immunohistochemistry, in order to understand its biological role in activation of Notch signaling and to further evaluate its clinical utility in this cancer.
Results
Immunohistochemical analysis of JAG1 expression in cervical precancer and cancer
The expression pattern (Fig. 1a–f) and total expression scores (Table 1) of JAG1 were observed in N, precancer, ISCC and ADC tissues.
Immunohistochemical analysis of JAG1 in normal, precancer and cancer tissues of uterine cervix. (a) negative control in normal tissue, 200X (b) mild nuclear and cytoplasmic expression of JAG1 in normal cervix, 200X (c) moderate nuclear expression of JAG1 in precancerous tissue, 200X (d) negative control in cancer tissue (ISCC, 200X) (e) intense cytoplasmic and nuclear localization of JAG1 in ISCC, 200X (f) intense nuclear expression of JAG1 in ADC, 200X (g) western blots depicting expression pattern of JAG1 protein during the progression of cervical cancer in tissues (normal, precancer, ISCC, ADC) (h) western blots showing expression pattern of JAG1 protein in Caski and C-33A cell lines. Protein extracts from cervical tumor biopsies, normal tissues and HPV-16 positive (Caski) and HPV-16 negative (C-33A) cell lines were separated in 10% SDS-PAGE and detected by specific antibody of JAG1. All the blots were stripped and reprobed for β-actin levels to confirm equal loading and the quantitation of bands was performed densitometrically as indicated in materials and methods section.
Cytoplasm (C)
Two folds (Mean ± S.E, 3.67 ± 0.33; p = 0.0001) increase of cytoplasmic JAG1 expression was identified in N vs. precancer, three folds (4.44 ± 0.17; p = 0.0001) in N vs. ISCC. However, only two folds (3.05 ± 0.40; p = 0.0001) increase was found in N vs. ADC.
Nucleus (Nu)
JAG1 expression in the nucleus was found to be increased two folds (Mean ± S.E, 3.70 ± 0.38; p = 0.0001) in N vs. precancer, three folds (4.64 ± 0.17; p = 0.0001) in N vs. ISCC and N vs. ADC (4.20 ± 0.40; p = 0.0001).
C + Nu
In accordance, two folds (3.68 ± 0.33; p = 0.0001) increase was observed in N vs. precancer and three folds (4.56 ± 0.16; p = 0.0001) elevation in N vs. ISCC. Only two folds (3.63 ± 0.23; p = 0.0001) increase was observed in N vs. ADC.
Hence, we hypothesize that JAG1, while binding to Notch-3, gives a signal which results in the cleavage of NICD domain (Fig. 2). The cleaved part translocates to the nucleus where it gives activating signal to transcription factor/s (TFs) of JAG1 gene. Moreover, HPV-16 gives an additional signal to the TFs of JAG1 gene (based on other experimental results on HPV given below) which cumulatively activates the transcription of JAG1 and further results to the increase production of JAG1 protein in the nucleus. The JAG1 protein once formed translocate to the cytoplasm, hence, increasing its level in the cytoplasm.
Evaluation of JAG1 potential to distinguish precancer, invasive squamous cell carcinoma and adenocarcinoma from normal cervix tissue
Receiver Operating Characteristic (ROC) analysis was done to estimate the JAG1 potential as a diagnostic marker for all three groups (precancer, ISCC and ADC) of the uterine cervix.
Cytoplasm
The values for AUC (area-under-the-curve) for JAG1 in precancer (0.86; p = 0.01), ISCC (0.91; p = 0.0001) and ADC (0.81; p = 0.0001) was found to be significant.
Nucleus
Similarly, the significant AUC values for nuclear JAG1 in precancer (0.84; p = 0.0001), ISCC (0.93; p = 0.0001) and ADC (0.90; p = 0.0001) were observed. The sensitivity and specificity for cytoplasmic JAG1 were 76.7%, 84.7%, 75%; and 80%, 80%, 80% respectively. In accordance, for nuclear JAG1 these were 73.3%, 86.7%, 85% and 77.5, 77.5%, 80% respectively {Fig. 3(a–c) and Table S1}.
(a–c) Receiver operating characteristic curves of JAG1 nuclear and cytoplasmic in (a) Normal vs. Precancer, (b) Normal vs. ISCC and (c) Normal vs. ADC. The blue line shows ROC analysis for cytoplasmic JAG1. The green line shows ROC analysis for nuclear JAG1 respectively. Y-axis of the plot shows true-positive fraction and X-axis shows the false positive fraction.
High sensitivity and specificity of JAG1 in precancer, ISCC and ADC support the clinical utility of JAG1 for early detection and progression of cervical cancer.
Relation of immunohistochemical expression of JAG1 with clinicopathological parameters of ISCC
An association of JAG1 expression with clinicopathological parameters of ISCC has been described as follows (Table 2).
Cytoplasm
JAG1 overexpression showed an association with lymph nodes metastasis (90.8%; p = 0.01), vaginal involvement of tumor (92.3%; p = 0.003), as well as with Figo stage (92.3%; p = 0.003) respectively.
Nucleus
Tumor vaginal involvement (93.8%; p = 0.004), lymph nodes metastasis (90.8%; p = 0.05) and Figo stage (93.8%; p = 0.004) were found to be associated with JAG1 expression.
C + Nu
Similarly, JAG1 was associated with tumor vaginal involvement (93.8%; p = 0.001), lymph nodes metastasis (92.3%; p = 0.02) as well as with Figo stage (93.8%; p = 0.004) respectively.
The results imply the association of JAG1 with the aggressive behavior of the CC.
Immunoblotting
A gradual increase in the expression of JAG1 (134 kDa) was identified in precancer, ISCC, and ADC patient samples (Fig. 1g, S1a,b). Immunoblotting results were also validated in Caski and C-33A cell lines. Caski showed increased expression as compared to C-33A (Fig. 1h, S2). The above results validate the findings of immunohistochemistry.
Prevalence of HPV infection in ADC and its correlation with clinicopathological parameters
Previously, we identified the prevalence of HPV infection in precancer and ISCC tissues13. In this study, 85% (17/20) of ADC patients were identified to be infected with HPV. Among them, all the patients were found to be infected with HPV-16 (17/17) and 5.8% (01/17) were found to be co-infected with HPV-18. All the ADC patients had 100% Grade II + III and Figo stage III + IV, and hence all HPV infected ADC were of higher pathological grades.
Hence, clinicopathological progression of ADC confirms the involvement of HPV-16.
Association of HPV-16/18 infection and Jagged-1 in precancer, ISCC, and ADC
Association of HPV with JAG1 expression in all tissues is defined as follows (Table 3):
Precancer
HPV-16 positive precancer patients showed 91.7% (p = 0.0001) cytoplasmic, 87.5% (p = 0.0002) nuclear and 91.7% (p = 0.0007) cytoplasmic + nuclear JAG1 positivity (Table 3). None of the precancer patients were found to be infected with HPV-18. Among precancer patients, HPV-16 positive CIN-1 and CIN-2/3 cases showed 84.2%, 78.9%; 63.6%, 63.6% JAG1 cytoplasmic and nuclear positivity respectively (Table S2).
ISCC
HPV-16 positive ISCC patients showed 94% nuclear and 94% cytoplasmic JAG1 positivity. This showed that in ISCC patients, nuclear (p = 0.0001) and cytoplasmic (p = 0.0001) JAG1 were observed to be correlated significantly with HPV-16.
ADC
No significant association of JAG1 with HPV-16 positive ADC patients was observed.
Cell lines
Increased expression of JAG1 was depicted in Caski (HPV-16 positive) as compared to C-33A (HPV-16 negative) cell lines.
This strengthens the hypothesis that HPV-16 associated precancer and cervical tumorigenesis is synergized with altered Notch signaling.
Associations between JAG1 and Notch-3 Protein in Precancer and ISCC patients
Interprotein correlations was analyzed between Notch-3 and JAG1 proteins. In precancer patients, significant positive associations were found between JAG1 nuclear (r = 0.530, p = 0.003) and Notch-3 nuclear proteins (Table S3). Similarly, in ISCC patients significant positive associations were observed between JAG1 nuclear (r = 0.379, p = 0.0001), cytoplasmic (r = 0.479, p = 0.0001) and cytoplasmic + nuclear (r = 0.453, p = 0.0001) and Notch-3 proteins (Table S4).
Discussion
The development and advancement of tumor from precancer to ISCC and ADC of uterine cervix remains a major clinical problem for decades. Evidence suggests that HPV infection alone is inadequate to induce malignant changes14. The network of altered signaling pathways are also important for the development of CC13. Therefore, for defining an effective treatment strategy, it is essential to understand the molecular mechanism/s involved in CC cells infected with oncogenic HPV types. Knowledge of this may improve the diagnostic modalities for precancer and cancer patients of uterine cervix and defining treatment strategies. This study is the first step towards this direction, where the role of JAG1 in the etio-pathogenesis of HPV infected different histological sub-types of CC is defined. Along with ISCC, we have also included a very rare form of CC i.e. ADC in our study. The outcome of this study will aid clinicians in deciding the targeted therapeutic strategy for CC patients and researchers in understanding the involvement of JAG1 in the pathogenesis of CC.
The results of JAG1 protein expression profile through immunohistochemistry suggest the JAG1 binding with Notch-3 triggers Notch signaling in HPV associated CC, which in-turn activates TFs for JAG1 expression. The JAG1 protein produced in the nucleus accumulates and translocates from nucleus to cytoplasm. Our JAG1 expression results demonstrate altered expression levels of JAG1 protein which results in deregulation of the Notch pathway. This overall may support the infection of the cervical mucosa by HPV-16. Together, this may lead to the acceleration of the cell cycle with an acquisition of more genetic damage. Our results are in agreement with a study15 who showed an intense immunoreactivity of JAG1. However, this study elaborated and showed that cytoplasmic and nuclear expression of JAG1 in HPV associated precancer, ISCC and ADC cases. Based on cytoplasmic and nuclear expression pattern we have explained the probable mechanism of the JAG1 feedback loop.
The ROC curve analysis of JAG1 showed its high sensitivity and specificity suggesting its clinical utility in early detection and progression of CC patients.
Immunohistochemical expression of JAG1, with respect to clinicopathological parameters, showed its association with the tumor vaginal involvement, Figo stage and lymph node metastasis, highlighting its clinical utility in ISCC. The upregulated JAG1 expression may interact with aggressive tumor behaviour, tumor progression and expansion, predicting as a potential candidate for biomarker of disease progression. This supports the findings of Yousif et al.16 who also identified the correlation of JAG1 expression levels in tumor patients with variable tumor clinicopathological parameters but through western blotting.
The IHC results corroborated well with immunoblotting and showed concordance with Yousif et al.16 who identified increased JAG1 levels in CC tissues by real- time PCR and western blotting. This study expanded with respect to HPV associated precancer, ISCC and ADC patients by IHC as well as Immunoblotting.
We studied earlier the prevalence of HPV infection in precancer and in invasive CC biopsies, and observed the linkage of HPV positive cancers with clinicopathological parameters of the disease progression13. This indicates that E6 and E7 oncogenic proteins of HPV can interact with the activated JAG1 which in-turn activate various pathways associated in cancers and altogether synergizes with each other, inhibiting apoptosis, promoting cell proliferation and tumorigenesis17. This study did not find any significant association of JAG1 with HPV-16 positive ADC subjects which could be due to the limited number of ADC samples (n = 20) being analyzed.
We validated the increased expression of JAG1 in Caski, as compared to C-33A cell lines, invasive cervical tumor-derived cell lines. Similar expression pattern of JAG1 was also previously reported in Caski by Veeraraghavalu et al.18,19.
Human studies and in vitro cell line experiments strongly support the hypothesis that JAG1 is the major ligand in triggering activation of Notch signaling, which acts as a central player in HPV-16 associated CC development and progression. Therefore, cancer cells expressing JAG1 plays pivotal roles in two manners: (i) JAG1 acts as a ligand and complexed with its receptor activating neighboring cells of a tumor in a juxtacrine way. (ii) The ICD (intracellular domain) of JAG1 may initiate Notch pathway and propagate tumor cell growth, leading to deregulation of JAG1. This creates a favorable environment for the growth of early precancerous lesions, acting as a protagonist in the tumor development and proliferation. Hence, serves a probable transducer of this cancer.
A better understanding of crosstalk between Notch signaling and other pathways, such as Wnt, Hedgehog, and vascular endothelial growth factor (VEGF), as well as with the immune system can provide valuable clues how to target cancer survival by ablating them and will make JAG1, an attractive approach orchestrated for combination therapy. Furthermore, due to its anti-apoptotic and pro-“stemness” functions, JAG1 blockade epitomizes an alluring model for combination therapy orchestrated by standard chemotherapy as demonstrated in preclinical models of ovarian and pancreatic cancer and lymphoma20,21.
In conclusion, our findings excavate the understanding of JAG1 driven Notch signaling events in HPV associated progression of CC. JAG1 expression in the nucleus denotes the transcription of JAG1 gene due to NICD and HPV-16 signaling to TFs resulting in JAG1 protein in nucleus. However, JAG1 expression in cytoplasm clarifies its translocation from nucleus to cytoplasm which in-turn activates the Notch signaling via binding to Notch-3, hence, acting as a positive feedback loop. Thus, it provides a legitimate target for CC therapy in the hope it might lead to the development of such tailored individualized therapy, as well as characterizing its biological functions in the cells which may be sufficient to abolish the neoplastic phenotype. This may help clinicians in characterizing patients when selecting for treatment of such cancer, by developing chemotherapeutic and combination therapies to interfere with cancer invasion and metastasis. Further validations at an RNA transcript level by RNA Seq can be done in future studies. In addition, studies are desired to assess JAG1 ablation as an adjuvant treatment to existing and currently ineffective chemotherapeutic agents.
Methods
Study design and participants
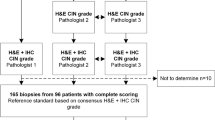
118 tumor specimens (98 ISCC, 20 ADC), 30 precancer and 40 non-neoplastic cervical tissues (total 188 tissue biopsies, Fig. 4) were collected after taking the written informed consent from all participating patients during the clinical procedures at Department of Obstetrics and Gynecology, Safdarjang Hospital, New Delhi, India. All patients in the study were recruited having no prior treatment and no family history of any disease or CC. This study was approved by the institutional ethics committee of ICMR-National Institute of Cancer Prevention and Research, Noida, as well as from Vardhman Mahavir Medical College and Safdarjang Hospital, New Delhi. Also, the study confirms that all experiments were performed in accordance with relevant guidelines and regulations.
The clinicopathological characteristics of patients with CC were recorded according to previous studies13,22. The staging of the tumors was carried out according to the criterion of the International Federation of Gynecology and Obstetrics (FIGO) classification of tumor staging23. Each slide was evaluated for its respective histopathological grade and clinical staging by two independent pathologists22,23. Each collected sample was distributed into two parts, one for histopathological diagnosis and other stored at −70 °C to perform molecular analysis.
IHC
All the collected specimens were fixed with 10% formalin, embedded in paraffin and then cut in 5μm sections, further mounted on priorly coated poly-L-lysine (Sigma, St. Louis, MO, USA) slides. Conventional H&E staining was done in each section followed by immunohistochemistry. Tris EDTA (pH 9.0) was used for antigen retrieval in the microwave and incubated overnight at 40C with the primary rabbit polyclonal antibody of JAG-1 (ab7771 Abcam) at a dilution of 1:200. After washing with tris buffer saline (TBS), pH 7.4, sections were incubated consequently with polymer based Envision™ (An Envision System peroxidase kit, DAKO, Carpinteria, CA) as described by us previously13. The images were captured using Olympus microscope (model BX-51, Olympus America, Inc., Melville, NY) using Olympus Bio-report software. 3,3-diaminobenzidine hydrochloride (DAB) was used for color development followed by its counter staining by Mayer’s Hematoxylin.
IHC Evaluation
The slides of IHC were independently reviewed by two authors along with one histopathologist independently without prior knowledge of patient’s identity, and were further scored as described previously13. Percentage positivity score was then combined with intensity scores in order to get the final score.
Immunoblotting
Immunoblotting was performed in representative cases from all categories (normal, precancer, ISCC and ADC) of collected biopsy tissues and cell lines according to our previous studies3,13,24. The primary antibodies used were rabbit polyclonal antibody of JAG-1 (ab7771 Abcam, 1:1000), and rabbit monoclonal β-actin (1:2000, Abcam, US). Blots were developed using enhanced chemiluminescence ECL detection system (Santa Cruz Biotech, USA). The quantitation and band intensity comparison of JAG1 was done between the subset of normal, precancer and cancer tissues, as well as between C-33A and Caski cell lines respectively by densitometry as mentioned previously3,24. Alpha Digidoc version 4.1.0 (Alpha Innotech Corporation, IL) was used for the evaluation of expression.
Cell culture
Human CC cell lines HPV-16 positive (Caski) and HPV-16 negative (C-33A) were purchased from NCCS, Pune (http://www.nccs.res.in/) in December 2017 and January 2018 respectively. These cell lines were tested and authenticated directly from NCCS, Pune from where they were purchased. Sixteen short tandem repeat (STR) loci were amplified using commercially available AmpFISTR® Identifier® Plus PCR amplification kit from Applied Biosystems. The cell line samples were processed using the Applied Biosystems® 3500 Genetic Analyser. Data was analyzed using Gene Mapper® ID-X v1.5 software (Applied Biosystems) along with appropriate positive and negative controls. The cells were last tested at the time of their purchase. They were culture in DMEM supplemented with 5% FBS, 100 U/mL penicillin, and 100 µg/mL streptomycin at 37 °C in an atmosphere of 5% CO2.
Genomic DNA isolation, and PCR detection of HPV isotypes
Genomic DNA was extracted from the ADC samples following phenol-chloroform method24,25. Duplex PCR assay was performed using the consensus sequence primers, for a conserved L1 gene of HPV genome, and by primers specifically designed for HPV-16 and HPV-18. The detailed protocol was mentioned earlier3.
Statistics
SPSS (Version 20) software package was used to perform statistical analysis. Chi-square test was used to determine the expression of the JAG1 protein with the clinicopathological parameters of cancer patients. Interprotein correlations was observed between Notch-3 and JAG1 proteins by Spearman’s rank correlation test. In doing so, the data of Notch-3 was obtained from our previous published report13. The p-value ≤ 0.05 was considered as statistically significant.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Summary
This study describes the role of Jagged-1 in context to HPV infection, in the pathogenesis of different histological subtypes of cervical carcinoma. It provides the molecular circuitry regulating Notch signaling and a novel therapeutic opportunity of JAG1 ablation, aiding clinicians.
References
Ferlay, J. et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. cancer 136, E359–86 (2015).
Das, B. C., Hussain, S., Nasare, V. & Bharadwaj, M. Prospects and prejudices of human papillomavirus vaccines in India. Vaccine 26, 2669–79 (2008).
Singh, N. et al. Downregulation of tumor suppressor gene PML in uterine cervical carcinogenesis: impact of human papillomavirus infection (HPV). Gynecol. Oncol. 128, 420–6 (2013).
Halim, T. A., Farooqi, A. A. & Zaman, F. Nip the HPV encoded evil in the cancer bud: HPV reshapes TRAILs and signaling landscapes. Cancer Cell Int. 13, 61 (2013).
zur Hausen, H. Papillomaviruses and cancer: from basic studies to clinical application. Nat. Rev. Cancer 2, 342–50 (2002).
Moscicki, A.-B., Schiffman, M., Kjaer, S. & Villa, L. L. Chapter 5: Updating the natural history of HPV and anogenital cancer. Vaccine 24(Suppl 3), S3/42–51 (2006).
Molano, M. et al. Determinants of clearance of human papillomavirus infections in Colombian women with normal cytology: a population-based, 5-year follow-up study. Am. J. Epidemiol. 158, 486–94 (2003).
Ranganathan, P., Weaver, K. L. & Capobianco, A. J. Notch signalling in solid tumours: a little bit of everything but not all the time. Nat. Rev. Cancer 11, 338–51 (2011).
Ortiz-Sánchez, E. et al. Characterization of cervical cancer stem cell-like cells: phenotyping, stemness, and human papilloma virus co-receptor expression. Oncotarget 7, 31943–54 (2016).
Le Borgne, R., Remaud, S., Hamel, S. & Schweisguth, F. Two distinct E3 ubiquitin ligases have complementary functions in the regulation of delta and serrate signaling in Drosophila. PLoS Biol. 3, e96 (2005).
Choi, K. et al. Distinct biological roles for the notch ligands Jagged-1 and Jagged-2. J. Biol. Chem. 284, 17766–74 (2009).
D’Souza, B., Miyamoto, A. & Weinmaster, G. The many facets of Notch ligands. Oncogene 27, 5148–67 (2008).
Tripathi, R. et al. Clinical impact of de-regulated Notch-1 and Notch-3 in the development and progression of HPV-associated different histological subtypes of precancerous and cancerous lesions of human uterine cervix. PLoS One 9, e98642 (2014).
Dixit, R., Bhavsar, C. & Marfatia, Y. S. Laboratory diagnosis of human papillomavirus virus infection in female genital tract. Indian J. Sex. Transm. Dis. 32, 50–2 (2011).
Yeasmin, S. et al. Expression of nuclear Notch3 in cervical squamous cell carcinomas and its association with adverse clinical outcomes. Gynecol. Oncol. 117, 409–16 (2010).
Yousif, N. G. et al. Notch1 ligand signaling pathway activated in cervical cancer: poor prognosis with high-level JAG1/Notch1. Arch. Gynecol. Obstet. 292, 899–904 (2015).
Rangarajan, A. et al. Activated Notch1 signaling cooperates with papillomavirus oncogenes in transformation and generates resistance to apoptosis on matrix withdrawal through PKB/Akt. Virology 286, 23–30 (2001).
Veeraraghavalu, K. et al. Complementation of human papillomavirus type 16 E6 and E7 by Jagged1-specific Notch1-phosphatidylinositol 3-kinase signaling involves pleiotropic oncogenic functions independent of CBF1;Su(H);Lag-1 activation. J. Virol. 79, 7889–98 (2005).
Veeraraghavalu, K. et al. Papillomavirus-mediated neoplastic progression is associated with reciprocal changes in JAGGED1 and manic fringe expression linked to notch activation. J. Virol. 78, 8687–700 (2004).
Cao, Z. et al. Angiocrine factors deployed by tumor vascular niche induce B cell lymphoma invasiveness and chemoresistance. Cancer Cell 25, 350–65 (2014).
Li, D., Masiero, M., Banham, A. H. & Harris, A. L. The notch ligand JAGGED1 as a target for anti-tumor therapy. Front. Oncol. 4, 254 (2014).
Bahnassy, A. A. et al. The role of cyclins and cyclins inhibitors in the multistep process of HPV-associated cervical carcinoma. J. Egypt. Natl. Canc. Inst. 18, 292–302 (2006).
Shepherd, J. H. Cervical and vulva cancer: changes in FIGO definitions of staging. Br. J. Obstet. Gynaecol. 103, 405–6 (1996).
Hussain, S. et al. Transcription factor AP-1 in esophageal squamous cell carcinoma: alterations in activity and expression during human Papillomavirus infection. BMC Cancer 9, 329 (2009).
Sobti, R. C. et al. Aberrant promoter methylation and loss of suppressor of cytokine signalling-1 gene expression in the development of uterine cervical carcinogenesis. Cell. Oncol. (Dordr). 34, 533–43 (2011).
Acknowledgements
This study was supported by Indian Council of Medical Research (ICMR, Grant no. 3/1/3/PDF-2010/HRD-11/2nd batch) grant New Delhi, India.
Author information
Authors and Affiliations
Contributions
R.T. designed the study, searched the literature, collected the samples, performed the experiments, analyzed data, interpreted the results, and wrote the draft manuscript. G.R. designed the study, contributed reagents/material/analysis tools, interpreted the results, and corrected the manuscript. S.H. performed the experiments, interpreted the results and corrected the manuscript. P.J. collected the samples. K.B. performed the experiments. V.S. interpreted the results, reviewed and edited the manuscript. M.B. designed the study, provided the reagents/material/analysis tools and corrected the manuscript. R.M. critically reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tripathi, R., Rath, G., Hussain, S. et al. Jagged-1 induced molecular alterations in HPV associated invasive squamous cell and adenocarcinoma of the human uterine cervix. Sci Rep 8, 9359 (2018). https://doi.org/10.1038/s41598-018-27699-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-27699-1
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.