Abstract
Large (>10 kb) palindromic sequences are enriched on mammalian sex chromosomes. In mice, these palindromes harbor gene families (≥2 gene copies) expressed exclusively in post-meiotic testicular germ cells, a time when most single-copy sex-linked genes are transcriptionally repressed. This observation led to the hypothesis that palindromic structures or having ≥2 gene copies enable post-meiotic gene expression. We tested these hypotheses by using CRISPR to precisely engineer large (10’s of kb) inversions and deletions of X-chromosome palindrome arms for two regions that carry the mouse 4930567H17Rik and Mageb5 palindrome gene families. We found that 4930567H17Rik and Mageb5 gene expression is unaffected in mice carrying palindrome arm inversions and halved in mice carrying palindrome arm deletions. We assessed whether palindrome-associated genes were sensitive to reduced expression in mice carrying palindrome arm deletions. Male mice carrying palindrome arm deletions are fertile and show no defects in post-meiotic spermatogenesis. Together, these findings suggest palindromic structures on the sex chromosomes are not necessary for their associated genes to evade post-meiotic transcriptional repression and that these genes are not sensitive to reduced expression levels. Large sex chromosome palindromes may be important for other reasons, such as promoting gene conversion between palindrome arms.
Similar content being viewed by others
Introduction
In humans and mice, the sex chromosomes are enriched for large (>10 kb), nearly identical (>99% nucleotide identity) segmental duplications in palindromic orientation1,2,3,4. In mice, genes within large X-chromosome palindromes are expressed predominantly or exclusively in post-meiotic testicular germ cells3. This specific expression pattern is surprising, because most single-copy X-linked genes are transcriptionally repressed post-meiosis5,6,7,8. The mechanism by which palindrome-associated genes escape transcriptional repression is unknown. Two hypotheses have been suggested to explain this distinct expression pattern. First, palindromes may form secondary structures (e.g. palindrome arms pairing to form a hairpin) enabling their associated genes to evade transcriptional repression3. Intrachromosomal synapsis of palindrome arm pairing could facilitate the evasion of post-meiotic gene repression, which itself is a consequence of asynapsis-triggered meiotic sex chromosome inactivation9,10,11. Second, X-palindromic genes may be sensitive to reduced expression levels and thus require ≥2 gene copies3. Consistent with this, the mouse X chromosome also carries non-palindromic multicopy genes that are expressed specifically in post-meiotic cells3. To test the two hypotheses, individual palindrome arms must be genetically manipulated, in vivo.
To rigorously test whether palindrome structure or gene copy number are required for post-meiotic expression, we genetically dissected two mouse X-palindromes. We utilized CRISPR to generate large-scale (10’s of kb) inversions and deletions in mice of two X-palindrome arms harboring the 4930567H17Rik and Mageb5 (Melanoma antigen gene family member b5) gene families. We chose these two X-palindromes because they possess the canonical features of palindromes across mammals; they have >99% percent nucleotide identity between the two arms, the arms are >10 kb in length, and they harbor a gene family expressed predominantly in post-meiotic testicular germ cells. We also selected these two gene families because they have nucleotide variants that differ between the two gene copies, enabling detection of palindrome arm-specific expression of each gene copy. We found that for the 4930567H17Rik and Mageb5 palindromic gene families, palindrome structure is not necessary for regulating their associated post-meiotic gene expression, since mice containing palindrome arm inversions exhibit wild-type expression levels. We observed that deletion of a single palindrome arm, for both the 4930567H17Rik and Mageb5 gene families, reduces gene expression levels by half. Reduced expression levels did not lead to male infertility or spermatogenic defects in either case. This suggests that palindromes enrichment on the sex chromosomes is important for other reasons and that there are alternative, unknown mechanisms for palindrome-associated genes to evade post-meiotic repression.
Results
The mouse X chromosome harbors eight singleton palindromes
Large palindromes on mammalian sex chromosomes are typically found as isolated pairs of palindrome arms (singleton palindromes) or in complex arrays of palindromes. We investigated singleton palindromes, because they are more commonly found across mammalian sex chromosomes and can be genetically manipulated in vivo more precisely. Of the eight singleton palindromes on the mouse X chromosome (Table 1 and Fig. 1A), we selected two harboring the 4930567H17Rik and Mageb5 gene families, because they share canonical features of sex chromosome palindromes: >10 kb, >99% nucleotide identity between palindrome arms, harbor genes expressed specifically in testicular germ cells, and have a spacer sequence between the palindrome arms (Table 1 and Fig. 1B). Additionally, the palindrome carrying the 4930567H17Rik gene family has the longest palindrome arm (65 kb), for a singleton palindrome, which will serve as a proof of principle for the manipulation of shorter palindrome arms.
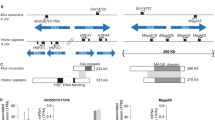
Singleton palindromes on the mouse X chromosome. (A) The location of the eight singleton palindromic regions on the mouse X chromosome are labeled by arm size, the sequence features of which can be found in Table 1. (B) Self-symmetry triangular dot plots of the two singleton X-palindromes to be studied, carrying the 4930567H17Rik and Mageb5 gene families, respectively. Each dot plot represents the palindromic X chromosome sequence (4930567H17Rik = chrX:70385921–70553920 and Mageb5 = chrX:91624421–91790420) plotted against itself with a sliding window of 100 nucleotides (step size = 1 nucleotide). When the window of 100 nucleotides is identical to the sequence it is compared to, a dot is plotted. Segmental duplications in an inverted orientation are visualized as vertical lines. A visual representation of the palindrome arms (arrows) and the gene copies (squares) are plotted at the base of the triangular plots across the 168 kb 4930567H17Rik and 166 kb Mageb5 palindromic regions. (C) Expression levels of 4930567H17Rik and Mageb5 genes in adult tissues and juvenile testis, shown as FPKMs (number of fragments per kilobase per million mapped fragments). D) Sanger sequencing of RT-PCR products displaying the nucleotide differences that distinguish the two palindromic gene copies. In 4930567H17RikDelArm/Y and Mageb5DelArm/Y mice, expression is detected only from the remaining copy. WT = wild-type.
We validated the exclusive expression of the 4930567H17Rik and Mageb5 gene families in post-meiotic round spermatids by reanalyzing previously published RNA-seq datasets from a tissue panel, juvenile testis (Fig. 1C) and sorted testicular germ cells (Supplementary Fig. 1). To determine if both gene copies of 4930567H17Rik and Mageb5 are expressed, we utilized individual nucleotide differences between gene copies. Sequencing of RT-PCR products for both 4930567H17Rik and Mageb5 show that both gene copies are expressed (Fig. 1D). Having confirmed that both gene copies are expressed exclusively in post-meiotic round spermatids, we proceeded to delete or invert individual palindrome arms to assess the importance of palindrome structure and gene copy number.
Generation of mice carrying precise inversions and deletions of individual X-palindrome arms via CRISPR
We utilized CRISPR/Cas9 technology to precisely invert or delete large X-palindrome arms in mice. Pronuclear injection of mouse zygotes with dual single guide RNAs (sgRNAs), targeting unique flanking regions of each palindrome arm, and use of a single stranded oligonucleotide donor enabled us to generate large (29 kb and 65 kb) inversions and deletions of individual arms for the 4930567H17Rik and Mageb5 X-palindromes (Fig. 2A). The single stranded oligonucleotide donors were used to promote inversions and deletions. We screened founder mouse lines with combinations of primers flanking the sgRNA cut sites in order to detect and distinguish inversions and deletions (Fig. 2A,B). We validated inversion and deletion junctions by PCR followed by Sanger sequencing (Fig. 2C). After ~300 pronuclear injections, we obtained 2 and 3 independent mouse lines carrying deletions of a single palindrome arm for the 4930567H17Rik and Mageb5 gene families, respectively. Similarly, after ~300 pronuclear injections we obtained 4 and 2 independent mouse lines carrying inversions of a single palindrome arm for the 4930567H17Rik and Mageb5 gene families, respectively. The independently obtained inversion and deletion junctions differed from each other by only a few nucleotides at the junctions. We also confirmed the deletions via Sanger sequencing of RT-PCR products to show that only a single sequence family variant was expressed in each mouse line (Fig. 1D). Eight independent mouse lines, backcrossed onto a C57BL/6J genetic background, were selected for further study, two lines carrying deletions and two lines carrying inversions for each of the two X-palindromic regions. Throughout this study, the genotypes for the eight deletion and inversion carrying lines are designated 4930567H17RiKInvArm, 4930567H17RikDelArm, Mageb5InvArm, and Mageb5DelArm, followed by a ‘1’ or ‘2’ to denote individual mouse lines. Our dual sgRNAs combined with single stranded oligonucleotides approach was successful in generating 29 kb and 65 kb inversions and deletions of single palindrome arms.
CRISPR strategy to generate large inversions and deletions of individual palindrome arms. (A) Schematic of the mouse X chromosome with a diagram of a singleton palindrome shown below. Palindrome arms are shown as blue arrows, sgRNA sites as red vertical lines and primers as black arrows. (B) PCR genotyping of DNA from the two independent mouse lines for each deletion and inversion of 4930567H17Rik and Mageb5 palindrome arms. Numbered primers from panel A used to amplify deletion (1&4) and inversion (1&3, 2&4) junctions (Supplementary Table 1). Full-length agarose gels are in Supplementary Fig. 4. WT = wild-type. (C) Representative example of Sanger sequencing chromatograms of the deletion and inversion junctions for 4930567H17Rik palindrome arm rearrangements. Junction sites are shown with a vertical red line. The mm10 coordinates for the sequence removed in the 4930567H17RikDelArm1/Y line is ChrX:70389542–70457358 and coordinates for the sequence inverted in the 4930567H17RiKInvArm1/Y are ChrX:70389544–70457357.
Disruption of palindrome structure, via inverting a single palindrome arm, does not affect the gene expression levels of the palindrome associated gene family
With the generation of mice carrying precise inversions of two different X-palindrome arms that disrupt palindrome structure, we tested whether palindrome structure is necessary for post-meiotic gene expression. If palindrome structure is necessary for post-meiotic gene expression, then we expected to abolish gene expression of 4930567H17Rik and Mageb5 in 4930567H17RiKInvArm/Y and Mageb5InvArm/Y mice, respectively. We compared the testis gene expression levels of 4930567H17Rik and Mageb5 gene families via quantitative RT-PCR (RT-qPCR). Expression was normalized to Trim42 and compared to wild-type controls. 4930567H17RiKInvArm/Y and Mageb5InvArm/Y mice, in two independent mouse lines, express their associated genes at levels similar to wild-type mice (Fig. 3A). Consistent with their similar gene expression levels, 4930567H17RiKInvArm/Y and Mageb5InvArm/Y mice are fertile and do no exhibit detectable spermatogenic defects. Assuming the post-meiotic-specific gene expression of 4930567H17Rik and Mageb5 is maintained in mice carrying palindrome arm inversions, our data demonstrate that palindrome structure is not required for gene expression of 4930567H17Rik and Mageb5.
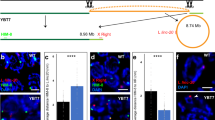
Male mice carrying 4930567H17Rik and Mageb5 palindrome arm inversions or deletions express their associated genes at wild-type and half of wild-type levels, respectively. (A) 4930567H17Rik and Mageb5 gene expression levels from testes of male mice carrying palindrome arm inversions as compared to wild-type controls. (B) 4930567H17Rik and Mageb5 gene expression levels from testes of male mice carrying palindrome arm deletions as compared to wild-type controls. For A and B, the gene expression values are shown as fold changes normalized to wild-type (WT = 1) and to the standard reference gene Trim42 for two independent mouse lines, per genotype (inversion or deletion of Mageb5 or 4930567H17Rik palindrome arms). Error bars represent the standard error of the mean across the biological replicates. The number of biological replicates (n) examined per line is shown above each bar graph. Unpaired two-tailed t-tests, with a Welch correction, were performed to compare mean gene expression (n.s. = not significant, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).
Deleting a single palindrome arm reduces the gene expression level of the palindrome-associated gene family by half
Using mice carrying single palindrome arm deletions, we reduced the gene copy number of the palindrome-associated 4930567H17Rik and Mageb5 genes by half (from 2 gene copies to 1). We expected 4930567H17Rik and Mageb5 gene expression levels would be reduced by half in 4930567H17RikDelArm/Y and Mageb5DelArm/Y mice. We compared the testis gene expression levels of 4930567H17Rik and Mageb5 gene families via quantitative RT-PCR (RT-qPCR). Expression was normalized to Trim42 and compared to wild-type controls. We found that 4930567H17RikDelArm/Y and Mageb5DelArm/Y mice, in two independent mouse lines, expressed their associated genes at approximately half the levels of wild-type males (Fig. 3B). The reduction of gene expression by half, in mice carrying palindrome arm deletions, is consistent with reducing gene copy number from 2 to 1.
Male mice carrying deletions of individual palindrome arms do not exhibit defects in fecundity, testis histology, or number of round spermatids
The reduction of gene expression by half allows us to test whether male mice are sensitive to reduced gene expression of the 4930567H17Rik and Mageb5 gene families. We performed a systematic characterization of fecundity and post-meiotic spermatogenic development of 4930567H17RikDelArm/Y and Mageb5DelArm/Y mice. We found that 4930567H17RikDelArm/Y and Mageb5DelArm/Y mice are fertile and produce litter sizes and relative testis weights similar to wild-type controls (Fig. 4A,B). To detect potential defects in post-meiotic spermatid development, we examined histological sections of testis from 4930567H17RikDelArm/Y and Mageb5DelArm/Y mice. We did not observe defects in spermatid morphology, formation of the acrosome, or spermatid elongation (Supplementary Fig. 2). To assess whether the number of round spermatids were affected in 4930567H17RikDelArm/Y and Mageb5DelArm/Y mice, we quantified the number of round spermatids per testis as the ratio of round spermatids/spermatocytes (control) via FACs (Supplementary Fig. 3). The ratio of round spermatids/spermatocytes of 4930567H17RikDelArm/Y and Mageb5DelArm/Y mice was similar to wild-type males (Fig. 4C). Altogether, 4930567H17RikDelArm/Y and Mageb5DelArm/Y mice do not exhibit detectable defects in fecundity or post-meiotic spermatid development.
Male mice carrying 4930567H17Rik and Mageb5 palindrome arm deletions are fertile and do not display defects in spermatogenic cell population frequencies. (A) Multiple males from each 4930567H17RikDelArm/Y and Mageb5DelArm/Y line and control males (wild-type littermates) were mated to multiple CD1 females to assess fertility and fecundity. (B) Total testis weight (g) from 4930567H17RikDelArm/Y and Mageb5DelArm/Y lines were normalized to total body weight (g). (C) The frequency of post-meiotic round spermatids was assessed by normalizing the percentage of round spermatids (1n) to spermatocytes (4n). The spermatocyte population serves as a control and should be unaffected, because they do not express 4930567H17Rik and Mageb5. Error bars represent the standard error of the mean of biological replicates. Statistical significance was determined using an unpaired T-test assuming the same standard deviation between populations and correction for multiple comparisons using Holm-Sidak correction.
Discussion
Our findings suggest that palindrome structure is not necessary for regulating post-meiotic gene expression levels for the 4930567H17Rik and Mageb5 gene families. Our findings also show that a reduced gene expression level of the 4930567H17Rik and Mageb5 gene families, via deletion of a single palindrome arm, does not result in spermatogenic defects or male infertility. It will be important to compare our studies with complete knockouts of the 4930567H17Rik and Mageb5 gene families. If either gene family is necessary for spermatogenesis, then it would support our findings that palindrome structure or copy number are not necessary for spermatogenesis.
Our findings suggest there are alternative mechanisms for X-palindromic genes to be expressed on the otherwise transcriptionally repressed X chromosome. A small number of X-linked single-copy genes are expressed in round spermatids6,12, indicating that multiple gene copies are not a strict requirement for post-meiotic gene expression from the sex chromosomes. Specific enhancers and transcription factors may have evolved to overcome the transcriptional repression associated with post-meiotic sex chromosomes. Two transcription factors that may facilitate post-meiotic sex-linked gene expression are Heat Shock Transcription Factor 1 (HSF1), which localizes to sex chromatin13 and HSF2, which preferentially binds chromatin of Y-palindromic genes14. Consistent with this, the testis is known to have specialized transcription regulation strategies in post-meiotic testicular germ cells15 that appear to also apply to palindromic and multicopy X- and Y-linked genes.
This work leaves open the question as to why palindromes are heavily enriched on both the mammalian X and Y chromosomes. It is possible that X- and Y-chromosome palindromes are necessary for the long-term evolutionary stability of the genes they harbor in order to rapidly purge deleterious mutations via gene conversion16. Alternatively, the presence of two or more palindromic gene copies provides a larger substrate for new beneficial mutations to arise and become fixed, via gene conversion17. For both purging or fixing new mutations in palindrome arms, it will be important to assess arm-to-arm gene conversion rates.
Materials and Methods
Generation of 4930567H17Rik and Mageb5 palindrome arm inversions and deletions
To generate mice carrying palindrome arm inversions and deletions we used CRISPR with dual single guide RNAs (sgRNAs). sgRNAs were designed to unique sequences flanking the targeted palindrome arm, as close to the edge of the arm as possible (Supplementary Table 1). Since sgRNA cutting efficiency varies between sgRNAs, we tested their activity via pronuclear injection of an sgRNA/Cas9 expression plasmid, pSpCas9(BB)-2A-GFP (pX458)18 in mouse zygotes. The surviving mouse zygotes were allowed to develop into 64–128 cell blastocysts and the cutting efficiency of each sgRNA was determined by PCR of the cut sites (Supplementary Table 1) and Sanger sequencing of purified PCR products to identify edits at the target sites.
After active sgRNAs were identified for both sides of each targeted palindrome arm, two pX458M plasmids encoding the sgRNAs and Cas9 together with a single-stranded oligonucleotide were injected into the pronucleus of (C57BL/6JXSJL) F1 mouse zygotes. For 4930567H17Rik and Mageb5 palindrome arm inversions and for the 4930567H17Rik palindrome arm deletion, we added single-stranded oligonucleotides with sequence homology flanking the junction boundaries, as listed in Supplementary Table 1, to promote the events. For the Mageb5 palindrome arm deletion, we only used dual sgRNAs. The zygotes were generated from mating C57BL/6J females to (C57BL/6JXSJL) F1 males so that all targeted X chromosomes were C57BL/6J. Blastocysts from the pronuclear injection were implanted into pseudopregnant females. Genomic DNA from resulting pups was screened by PCR and Sanger sequencing of the inversion and deletion junctions. At least two independent mouse lines were obtained for inversions and deletions of the 4930567H17Rik and Mageb5 palindrome arms. Male and female mice were able to transmit both the 4930567H17Rik and Mageb5 deletions and inversions through the germline. Thus, their overall health was unaffected by CRISPR-mediated chromosome engineering.
Mice and testis sample collection
Females heterozygous for an inversion or deletion of a single palindrome arm were repeatedly backcrossed to C57BL/6J. Backcrossing was performed up to seven generations, for two independent lines per genotype, to minimize any CRISPR-mediated off-target effects. The heterozygous females mating scheme resulted in wild-type and deletion/inversion male littermates which were directly compared in all experiments. Use of wild-type littermates as controls minimized the effects of genetic background and age. If wild-type littermates were not available, then we used age-matched controls from the same breeding line. To assess fecundity (average litter size per male), we mated mutant males to CD1 females. Testes were collected from 2–6 month old males for all experiments and weighed, along with total body, in order to determine relative testis weight. The alleles for the first mouse lines of each type were named 4930567H17RikInvArm1, 4930567H17RikDelArm1, Mageb5InvArm1, Mageb5DelArm1 with respective registered symbols In(X4930567H17Rik)1Jbmu, Del(X4930567H17Rik)1Jbmu, In(XMageb5)1Jbmu and Del(XMageb5)1Jbmu. All experiments performed on mice were approved by the University of Michigan Committee on Use and Care of Animals, and all experiments followed the National Institutes of Health Guidelines of the Care and Use of Experimental Animals.
Preparation of adult testis cDNA and quantitative real-time PCR (qRT-PCR)
Intron-spanning primers (Supplementary Table 1), when possible, were used to perform qRT-PCR on adult testis cDNA preparations. One testis per mouse was used to isolate RNA using Trizol (Invitrogen) following the manufacturers recommendations. Five μg of total RNA was DNase treated with TurboDNase (Ambion) and reversed transcribed using Superscript II (Invitrogen) and oligonucleotide (dT) primers following the manufacturers protocol. qRT-PCR was performed in triplicate using Power SYBR Green master mix (Thermo Fisher Scientific) on a 7500 Real-time PCR thermalcycler (Applied Biosystems). Reactions were performed in 25 µl volumes, with 200 nM of each primer, 40 cycles of 95 °C for 15 seconds followed by 60 °C for 1 minute, and completed with a melt curve analysis. All PCR products produced a single peak based upon the melt curve analysis, indicating a single, specific, product is amplified in each reaction. We performed three technical replicates, per animal, for each qRT-PCR reaction. All comparisons of gene expression used the same standard reference control gene, Trim42 (Tripartite motif-containing 42), because it is expressed specifically in the same post-meiotic testicular cells and at similar levels as 4930567H17Rik and Mageb5. Normalized gene expression (∆CT) was calculated by subtracting Trim42 crossing thresholds (CT) values from 4930567H17Rik or Mageb5 CT values, for each biological replicate. Control biological replicates (wild-type littermates from the same transgenic line) ∆CT values were averaged. ∆∆CT values were determined by subtracting the average ∆CT of control samples from the ∆CT values of mice carrying either a deletion or inversion. Fold changes in 4930567H17Rik and Mageb5 gene expression were determined via the 2−∆∆CT method19. Statistical tests of differential gene expression were performed by comparing mean gene expression via unpaired, one-tailed, moderated t-tests.
Testis Histology
Testes were fixed overnight in Bouin’s solution, paraffin embedded, sectioned to 5 μm, and stained with Periodic acid Schiff (PAS) and hematoxylin. Sections were visualized under a light microscope and specific germ cell populations were identified by their location within a tubule, nuclear size, and the nuclear staining pattern of chromatin20.
FACs-based estimates of round spermatid frequencies
We largely followed a previously published protocol21 to isolate round spermatids (1n) and spermatocytes (4n). Briefly, we disassociated cells from a single testis by enzymatic treatment with Collagenase type I, DNase I (Worthington Biochemical Corporation), and Trypsin (Life Technologies). The cell suspension was passed through cell strainers (100 µm and 40 µm) and incubated with Hoechst 33342 (Life Technologies) to determine DNA content and propidium iodide (Acros Organics) to evaluate cell viability. Cell sorting was performed on a FACSAria II cell sorter (BD Biosciences). The purity of each sort was determined via fluorescence microscopy visual inspection of 100 cells morphology and nuclear staining with DAPI for round spermatids and with gH2AX antibody staining for pacyhtene spermatocytes. The purity of round spermatids was typically >85%, which is consistent with previous studies21.
RNA-seq analyses
RNA-seq analyses were conducted by analyzing previously published datasets. Specifically, mouse tissue panel data were analyzed from SRP01650122, ovary data from SRP01795923, juvenile testis data from SRP01869524 and sorted testicular germ cell populations from SRP06508225 and SRP01812426. Alignments were performed using Tophat27 with the mm10 mouse reference genome, a refFlat file with RefSeq gene annotations and–max-multihits set to 240; otherwise standard default parameters were used. We used Cufflinks27, using the refFlat gene annotation file, to estimate expression levels as fragments per kilobase per millions of mapped fragments (FPKM).
Dot plots
Self-symmetry triangular dot plots that show repeats within a sequenced region were generated from a custom Perl script that can be found at http://pagelab.wi.mit.edu/material-request.html.
Data availability
All RNA-seq data analyzed in this study has been previously published and can be found in the NCBI short read archive (SRA).
References
Mueller, J. L. et al. Independent specialization of the human and mouse X chromosomes for the male germ line. Nat Genet 45, 1083–1087 (2013).
Soh, Y. Q. et al. Sequencing the mouse Y chromosome reveals convergent gene acquisition and amplification on both sex chromosomes. Cell 159, 800–813 (2014).
Mueller, J. L. et al. The mouse X chromosome is enriched for multicopy testis genes showing postmeiotic expression. Nat Genet 40, 794–799 (2008).
Skaletsky, H. et al. The male-specific region of the human Y chromosome is a mosaic of discrete sequence classes. Nature 423, 825–837 (2003).
Turner, J. M., Mahadevaiah, S. K., Ellis, P. J., Mitchell, M. J. & Burgoyne, P. S. Pachytene asynapsis drives meiotic sex chromosome inactivation and leads to substantial postmeiotic repression in spermatids. Dev Cell 10, 521–529 (2006).
Namekawa, S. H. et al. Postmeiotic sex chromatin in the male germline of mice. Curr Biol 16, 660–667 (2006).
Greaves, I. K., Rangasamy, D., Devoy, M., Marshall Graves, J. A. & Tremethick, D. J. The X and Y chromosomes assemble into H2A.Z-containing [corrected] facultative heterochromatin [corrected] following meiosis. Mol Cell Biol 26, 5394–5405 (2006).
Khil, P. P., Smirnova, N. A., Romanienko, P. J. & Camerini-Otero, R. D. The mouse X chromosome is enriched for sex-biased genes not subject to selection by meiotic sex chromosome inactivation. Nat Genet 36, 642–646 (2004).
Turner, J. M. et al. Silencing of unsynapsed meiotic chromosomes in the mouse. Nat Genet 37, 41–47 (2005).
Homolka, D., Ivanek, R., Capkova, J., Jansa, P. & Forejt, J. Chromosomal rearrangement interferes with meiotic X chromosome inactivation. Genome Res 17, 1431–1437 (2007).
Baarends, W. M. et al. Silencing of unpaired chromatin and histone H2A ubiquitination in mammalian meiosis. Mol Cell Biol 25, 1041–1053 (2005).
Sin, H. S. & Namekawa, S. H. The great escape: Active genes on inactive sex chromosomes and their evolutionary implications. Epigenetics 8, 887–892 (2013).
Akerfelt, M. et al. Heat shock transcription factor 1 localizes to sex chromatin during meiotic repression. J Biol Chem 285, 34469–34476 (2010).
Akerfelt, M. et al. Promoter ChIP-chip analysis in mouse testis reveals Y chromosome occupancy by HSF2. Proc Natl Acad Sci USA 105, 11224–11229 (2008).
Sassone-Corsi, P. Unique chromatin remodeling and transcriptional regulation in spermatogenesis. Science 296, 2176–2178 (2002).
Rozen, S. et al. Abundant gene conversion between arms of palindromes in human and ape Y chromosomes. Nature 423, 873–876 (2003).
Betran, E., Demuth, J. P. & Williford, A. Why chromosome palindromes? Int J Evol Biol 2012, 207958 (2012).
Brault, V., Pereira, P., Duchon, A. & Herault, Y. Modeling chromosomes in mouse to explore the function of genes, genomic disorders, and chromosomal organization. PLoS Genet 2, e86 (2006).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods 25, 402–408 (2001).
Russell, L. D. E., Ettlin, R. A., Sinha Hikim, A. P. & Clegg, E. D. Histological and Histopathological Evaluation of the Testis. (Cache River Press, Vienna, Austria, 1990).
Gaysinskaya, V., Soh, I. Y., van der Heijden, G. W. & Bortvin, A. Optimized flow cytometry isolation of murine spermatocytes. Cytometry A 85, 556–565 (2014).
Merkin, J., Russell, C., Chen, P. & Burge, C. B. Evolutionary dynamics of gene and isoform regulation in Mammalian tissues. Science 338, 1593–1599 (2012).
Necsulea, A. et al. The evolution of lncRNA repertoires and expression patterns in tetrapods. Nature 505, 635–640 (2014).
Margolin, G., Khil, P. P., Kim, J., Bellani, M. A. & Camerini-Otero, R. D. Integrated transcriptome analysis of mouse spermatogenesis. BMC Genomics 15, 39 (2014).
Larson, E. L. et al. Contrasting Levels of Molecular Evolution on the Mouse X Chromosome. Genetics 203, 1841–1857 (2016).
Soumillon, M. et al. Cellular source and mechanisms of high transcriptome complexity in the mammalian testis. Cell Rep 3, 2179–2190 (2013).
Trapnell, C. et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc 7, 562–578 (2012).
Acknowledgements
We thank Sundeep Kalantry and Miriam Meisler for comments on the manuscript, Dirk de Rooij, Thomas Wilson and Michael Pihalja for technical advice, Thomas Saunders and the University of Michigan Transgenic Animal Model Core for pronuclear injections to generate the 4930567H17Rik and Mageb5 palindrome arm deletion and inversion mouse lines, University of Michigan Flow Cytometry Shared Resource Laboratory for FACs, DNA Sequencing Core for Sanger Sequencing, and Cancer Center Tissue Core for generating testis histological sections. These studies were supported by National Institutes of Health grants R00HD064753 to JLM, T32GM007544 to ANK and T32HD079342 to QE, a National Science Foundation Graduate Research Fellowship DGE 1256260 to ANK and a Cell and Molecular Biology Graduate Program Fellowship to QE.
Author information
Authors and Affiliations
Contributions
Q.E. and J.L.M. designed the study; Q.E., A.N.K., M.A.B. and E.R.G. conducted the experiments; A.N.K., Q.E. and J.L.M. analyzed and interpreted the results; A.N.K. and J.L.M. wrote the paper.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kruger, A.N., Ellison, Q., Brogley, M.A. et al. Male mice with large inversions or deletions of X-chromosome palindrome arms are fertile and express their associated genes during post-meiosis. Sci Rep 8, 8985 (2018). https://doi.org/10.1038/s41598-018-27360-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-27360-x
This article is cited by
-
X-linked palindromic gene families 4930567H17Rik and Mageb5 are dispensable for male mouse fertility
Scientific Reports (2022)
-
The X chromosome and male infertility
Human Genetics (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.