Abstract
Neuropathic pain (NP), defined as pain initiated or caused by a primary lesion or dysfunction in the nervous system, is a debilitating chronic pain condition often resulting from cancer treatment. Among cancer patients, neuropathy during cancer treatment is a predisposing event for NP. To identify genetic variants influencing the development of NP, we conducted a genome-wide association study in 1,043 patients with squamous cell carcinoma of the head and neck, based on 714,494 tagging single-nucleotide polymorphisms (SNPs) (130 cases, 913 controls). About 12.5% of the patients, who previously had cancer treatment, had neuropathy-associated diagnoses, as defined using the ICD-9/ICD-10 codes. We identified four common SNPs representing four genomic regions: 7q22.3 (rs10950641; SNX8; P = 3.39 × 10−14), 19p13.2 (rs4804217; PCP2; P = 2.95 × 10−9), 3q27.3 (rs6796803; KNG1; P = 6.42 × 10−9) and 15q22.2 (rs4775319; RORA; P = 1.02 × 10−8), suggesting SNX8, PCP2, KNG1 and RORA might be novel target genes for NP in patients with head and neck cancer. Future experimental validation to explore physiological effects of the identified SNPs will provide a better understanding of the biological mechanisms underlying NP and may provide insights into novel therapeutic targets for treatment and management of NP.
Similar content being viewed by others
Introduction
Head and neck cancer (HNC) is the sixth-most-common malignancy worldwide. Patients with HNC live longer relative to patients with other cancers. The treatment and management of pain is a significant clinical concern in this population. Pain is a common symptom reported by head and neck cancer patients, resulting from tumor invasion or compression of anatomic structures. Acute pain is extremely common during and after surgery, chemo- and/or radiotherapy. An estimated 80% of head and neck cancer patients report pain during treatment, and pain persists for some 36% of patients beyond treatment1. Neuropathic pain (NP), defined as pain initiated or caused by a primary lesion or dysfunction in the nervous system, is a debilitating chronic pain condition often resulting from cancer treatment. Patients typically describe it as a burning, shooting, or lancinating pain. It is often associated with allodynia and hyperalgesia. Various nerve damaging stimuli in the peripheral or central nervous system can lead to NP. Among cancer patients, clinical studies suggest that neuropathy during cancer treatment is a predisposing event for NP2. Chemotherapeutic agents, including vinca alkaloids, taxanes, and platinum-based compounds, may damage peripheral nerves resulting in aberrant somatosensory processing of the peripheral or central nervous system. Surgery may also result in NP, with hypoesthesia as a significant predictive factor for the development of NP3. Radiation therapy may also cause NP, including brachial plexus-associated neuropathy.
NP is a major clinical problem since existing analgesics are often ineffective. First-line pharmacologic therapies include tricyclic antidepressants (eg, amitriptyline), serotonin-norepinephrine reuptake inhibitors (eg, duloxetine), and anticonvulsants (eg, gabapentin, pregabalin). However, these agents only have a modest effect. Second-line options include lidocaine patches, capsaicin high-concentration patches, and tramadol. Opiates and botulinum toxin A are typically reserved for refractory cases, but only with marginal therapeutic benefit. Studies continue to explore the mechanisms underlying NP, its treatment and management.
Genome wide association studies are an agnostic approach for exploring potential markers of disease susceptibility genes, uncovering underlying mechanisms of disease conditions and identifying novel targets for drug development. We have conducted a genome wide association (GWA) study in patients with head and neck cancer (HNSCC) to identify single nucleotide polymorphisms (SNPs) associated with neuropathy, a precursor to the development of NP.
Results
Our study population include 1,043 patients with HNSCC, the most common type of HNC, who were treated at the Head and Neck Center at MD Anderson Cancer Center. Cases include 130 HNSCC patients with neuropathy (104 male, 26 female; mean age = 58 years; standard deviation [sd] = 10), and controls include 913 HNSCC patients without neuropathy (689 male, 224 female; mean age = 59 years; sd = 11). All patients included in this study were self-reported Caucasians. No hidden population substructures were detected based on the analysis of identity-by-state distance4. With the application of the standard quality control procedure, including Hardy-Weinberg proportion test, minor allele frequency (MAF) etc, we analyzed 714,494 SNPs for these 1,043 patients with HNSCC.
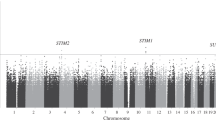
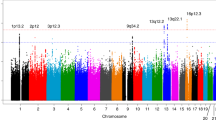
From the GWA analysis, we identified four SNPs representing four genomic regions that satisfied the genome-wide significance level, P values < 5 × 10−08 (Table 1). The Manhattan plot of GWA results is shown in Fig. 1 and the plots for the four genomic regions, including four significant SNPs, are shown in Fig. 2. In particular, the most significant SNP was rs10950641 (odds ratio [OR] = 2.88; 95% confidence interval [CI] = [2.19, 3.79]; P value = 3.39 × 10−14), which localizes within the gene SNX8 (Sorting Nexin 8) on the chromosome 7 (7q22.3; 2334386 bp; Fig. 2, panel (A)). The second was rs4804217 (OR = 0.58; 95% CI = [0.48, 0.69]; P value = 2.95 × 10−9), which localizes within the gene PCP2 (Purkinje Cell Protein 2) on the chromosome 19 (19p13.2; 7699347 bp; Fig. 2, panel (B)). Other SNPs identified included rs6796803 (OR = 0.51; 95% CI = [0.41, 0.64]; P value = 6.42 × 10−9), which localizes near gene KNG1 (Kininogen 1) on chromosome 3 (3q27.3; 186464107 bp; Fig. 2, panel (C)) and rs4775319 (OR = 1.59; 95% CI = [1.36, 1.87]; P value = 1.02 × 10−8), which localizes within gene RORA (RAR-related Orphan Receptor Alpha) on chromosome 15 (15q22.2; 61213564 bp; Fig. 2, panel (D)).
Linkage disequilibrium structure and association results for the four neuropathy-associated genomic regions. (A) SNX8; (B) PCP2; (C) KNG1; and (D) RORA. Base pair positions and genes were based on the build of the human genome GRCh37. Fisher’s exact test P values (as −log10 values; left y axis) are shown for SNPs analyzed in the GWA studies.
Discussion
In this study, we found that 12.5% of our patients with HNSCC received a neuropathy associated diagnosis. Using GWA study, we found four SNPs in four genes (SNX8; PCP2; KNG1; and RORA) to be statistically significantly associated with neuropathy.
Sortin Nexin 8, the most significant gene that we found to be associated with neuropathy, belongs to a family of proteins that are involved in endocytosis, endosomal sorting and signaling. Its yeast homologue (Mvp1) has been shown to be involved in endosome-to-Golgi retrieval5. SNX8’s role in endosomal content sorting has been implicated in the risk for neuropathologies, i.e., for late onset Alzheimer’s disease. While SNXs fundamental role is increasingly being investigated, SNXs has been recently implicated in pain. A study by Lin et al.6 showed the importance of SNXs as a critical element for glutamatergic-receptor-dependent neural plasticity and in endosomal sorting machinery in an animal model of NP. Specifically, they showed in an experimental neuropathic injury the upregulation of SNX27 and spinal vacuolar protein sorting associated protein (VPS26A); and VPSA’s recruitment and interaction with SNX27 (VPS26A-SNX27). They also showed association between VPS26A-SNX27 complex and spinal plasticity underlying NP.
Kininogen-1 (KNG1), identified as protective in this study, is a protein coding gene, which generates two different proteins, high and low molecular weight kininogens (HMWK and LMWK, respectively)7. Bradykinin, released from HMWK, and its related kinins are a family of small peptides which act as mediators of pain and inflammation7,8. KNG1 has been associated with diseases including high molecular weight kininogen deficiency and angioedema8,9. A study of mutant kininogen-deficient rats showed a low incidence of thermal and mechanical hyperalgesia induced by chronic nerve constriction injury suggesting that kinin were partly involved in nociceptor hypersensitivity10. Subsequent studies showed that kinin- receptor deficient mice have a significant reduction in paclitaxel-induced hyper-nociceptive responses compared to wild-type mice11,12.
Purkinje Cell Protein 2 (PCP2), identified as protective in this study, is also a protein coding gene that is expressed only in Purkinje cells of the cerebellum and in bipolar neurons of the retina, and may function as a neuron-specific modulator of intracellular signaling via G proteins13,14. The Gα, Gβ and Gγ form heterotrimers that transduce extracellular stimuli into intracellular responses, and the receptors coupled to G proteins constitute the largest superfamily of transmembrane receptors, ie, the G-protein-coupled receptors (GPCRs). PCP2 interacts with Gαo15, which is coupled to several neuronal receptors in the central nervous system that regulate some K+ channels and N-type Ca+2 channels16,17,18,19. Gαo can also be regulated by Growth Cone-Associated Protein 43 (GAP43), also known as neuromodulin, in neurites during development17. Knockout of Gap43 in mice causes derangement in neuronal pathfinding18; in contrast, Gnao1 (the gene for Gαo) knockout mice have anatomically normal appearing central nervous system but manifest motor and behavioral abnormalities and early death16,19. Given that μ opioid agonists act through GPCRs (e.g., OPRM1 gene)20, GPCRs are therapeutic targets in pain pathways. Interestingly, a transient expression study demonstrated that transcription of PCP2 is activated by RAR-related orphan receptor alpha (RORA), which binds to a half-site motif of a Retinoic Acid-Related Orphan Receptor Response Element (RORE) in the promoter region21.
Finally, RAR-related orphan receptor alpha (RORA) is also a protein coding gene. The protein encoded by this gene has been shown to interact with NM23-1, which is the product of a tumor metastasis suppressor candidate gene22,23. It has been associated with diseases such as atrial tachyarrhythmia with short PR interval and trait depression22,24,25,26. Li and colleagues27 conducted a molecular mapping of a developing dorsal horn by microarray. The dorsal horn of the spinal cord consists of distinct lamina serving as a region for relaying somatosensory signals of touch, temperature and pain. They found for the first time, that RORA transcription factors showed a unique expression pattern in lamina II, suggesting the potential importance of RORA in pain.
Ingenuity Pathway Analysis (Ingenuity® Systems, www.ingenuity.com) reveals that RORA, PCP2 and SNX8 can be connected in a regulatory network by microRNAs (Fig. 3). MiR-1229-39 regulates RORA and SNX8 and miR-6877-5p regulates PCP2 and SNX8. Although KNG1 belongs to a family of small peptides consisting of bradykinin and kinins which are mediators of pain and inflammation, the mechanism behind the link of this SNP near KNG1 gene with NP is unclear.
Ingenuity Pathway Analysis of SNX8, PCP2, KNG1, and RORA. Data were analyzed through the use of IPA (QIAGEN Inc., https://www.qiagenbioinformatics.com/products/ingenuitypathway-analysis).
To our knowledge, this is the first GWA study to identify the importance of SNPs in SNX8, PCP2, KNG1, and RORA for neuropathy in patients with head and neck cancer. In a recent GWA study, Hertz and colleagues28 found that a SNP in VAC14 (rs875858) was significant for docetaxel-induced neuropathy in patients with metastatic castration-resistant prostate cancer. Conversely, a GWA study of cisplatin-induced peripheral neuropathy in testicular cancer survivors did not show significance for any of the 5.1 million SNPs but showed significance for the expression level of RPRD1B with cisplatin-induced peripheral neuropathy29. Other GWA studies of neuropathic pain in patients with diabetes have shown the significance of Chr8p21.3 (GFRA2)30 and sex-specific involvement of Chr1p35.1 (ZSCAN20-TLR12P) for females and Chr8p23.1 (HMGB1P46) for males for diabetic neuropathic pain31. In a recent study, Zorina-Lichtenwalter and colleagues32 replicated an association for two variants in IL10, which underscores the importance of neuroimmune interactions for neuropathic pain. Studies continue to explore potential genetic mechanisms underlying the development of neuropathic pain.
There are limitations to our study. Using ICD-9 and ICD-10 diagnosis for identifying patients with neuropathy may have resulted to an underestimation of its prevalence. This study should be considered exploratory and preliminary in nature, and future validation using independent data sets as well as in other cancer sites is necessary. Moreover, our study population only involved self-reported Caucasians, therefore, future GWA studies that incorporate other races or ethnicities would be important. The sample size of our study (1,043 patients with head and neck cancer, with 130 patients having neuropathy) is relatively small, implying limited statistical power. Thus, we conducted a post hoc power analysis to investigate the statistical power using the software PS: Power and Sample Size Program33. With a total sample size of 1,043 patients, we found that we had 80% power to detect an OR of 3.03 for a SNP‒neuropathic pain association in patients with HNC, given a genome-wide significance level of 5 × 10−8 to account for multiple comparisons. We might also have missed additional SNPs that could be associated with neuropathic pain, due to the limitation from our sample size. Future studies exploring the physiological effects of the identified SNPs will provide a better understanding of the biological mechanisms underlying NP and may provide insights into novel therapeutic targets for treatment and management of NP.
Methods
Patients
The study was approved by the Institutional Review Board at The University of Texas MD Anderson Cancer Center, and all procedures adhered to its guidelines and regulations, in accordance with the Declaration of Helsinki. All participants provided written informed consent. This study included patients with newly diagnosed and histologically confirmed HNSCC. The details of patient recruitment (including inclusion and exclusion criteria), the demographic characteristics and clinical data collection have been described in our previous study34.
Specifically for this study, we used the International Classification of Diseases, ninth and tenth revisions (ICD-9 and ICD-10, respectively), which is the system of codes for diagnoses and procedures in the United States, to identify neuropathy associated diagnosis among the patient cohort. We specifically searched for the following ICD-9 codes (053.13; 337.0; 337.09; 337.1; 356.4; 356.8; 356.9; 357.2; 357.3; 357.9; 377.41) and ICD-10 codes (G58.8; G58.9; G62.0; G62.2; G62.9; G63.0). Epidemiological data, such as age and sex, were also collected.
Genotyping and Quality Controls
These procedures were previously described34. In brief, genomic DNA was genotyped using Illumina HumanOmniExpress-12v1 BeadChip (Illumina, San Diego, CA)35. Clustering and SNP calling were conducted using Illumina’s BeadStudio36. SNPs were considered as missing genotypes if no clusters were observed at a locus. Study genotypes were filtered and limited to autosomal SNPs with (1) call rate ≥90%; (2) MAF ≥0.05 and (3) Hardy-Weinberg proportion test P value ≥ 10−6 in control subjects37,38. We excluded individuals with: (1) call rates less than 95%; (2) discordant sex information and (3) duplicates. The genome-wide identity-by-state distances on SNPs39,40 for each pair of individuals were calculated to assess the cryptic relatedness among individuals. If two individuals have allele sharing of >80%, the one with lower call rate was excluded. We also investigate the non-Western European ancestry for all individuals by using the information from 2,502 reference samples from the 1000 Genomes Project data set (phase 3)41. All the quality control procedures were conducted using PLINK (v1.07)4. GRCh37 was the build of the human genome used for the study and the base pair (bp) locations will only match exactly with that build.
Statistical analyses
The primary variable of interest was the binary outcome of neuropathy. The chi-squared test or Fisher’s exact test (cell count <5) was used to assess the Hardy-Weinberg proportion for each SNP38. A nearest neighbor cluster analysis4,39 was conducted to obtain the information of cluster for each individual based on genetic similarity. Specifically, with at least one case and one control in each cluster, it was obtained using pairwise population concordance at P value < 0.005. Associations between the neuropathy and SNPs were assessed using multivariable unconditional logistic regression, where sex, age and information of clusters were included as covariates. Significance was assessed using a Fisher’s exact test. We assumed an additive genetic model for each SNP in the association analysis. The genome-wide significance P value threshold of 5 × 10−8 was employed to account for multiple testing issue. Statistical analyses were conducted using PLINK (v1.07)4. Manhattan plot (Fig. 1) was generated using R software42. Gene Ontology (GO) information was retrieved from the Gene Ontology Annotation database43,44.
Ingenuity Pathway Analysis
We used the Ingenuity Pathway Analysis (IPA, Ingenuity® Systems, www.ingenuity.com) to investigate the interactions among genes that harbor or near the SNPs (SNX8, PCP2, KNG1, and RORA) found to be significantly associated with neuropathy in our GWA study45. In brief, genes of interest were entered into the IPA software to build an interaction network, using the following steps: (1) The “Grow” tool was used to obtain both direct and indirect interactions of the genes of interest with other molecules. We added a maximum of 10 molecules at a time for ease of viewing and optimal performance46. Ingenuity Knowledge Base was used, with a limitation to only human species. (2) The “Connect” tool was used to obtain both direct and indirect connections among the genes. (3) Unconnected genes or pathways were trimmed with the “Trim” tool to improve the clarity of the network.
Data availability
For future meta-analysis, information on all p-values are available from authors. The datasets generated during and/or analyzed during the current study are available in the dbGaP repository, phs001173.v1.p1.
References
Epstein, J. B. et al. A systematic review of orofacial pain in patients receiving cancer therapy. Supportive care in cancer: official journal of the Multinational Association of Supportive Care in Cancer 18, 1023–1031, https://doi.org/10.1007/s00520-010-0897-7 (2010).
Reyes-Gibby, C. C., Morrow, P. K., Buzdar, A. & Shete, S. Chemotherapy-induced peripheral neuropathy as a predictor of neuropathic pain in breast cancer patients previously treated with paclitaxel. J. Pain 10, 1146–1150, https://doi.org/10.1016/j.jpain.2009.04.006 (2009).
Andersen, K. G., Duriaud, H. M., Kehlet, H. & Aasvang, E. K. The Relationship Between Sensory Loss and Persistent Pain 1 Year After Breast Cancer Surgery. The journal of pain: official journal of the American Pain Society 18, 1129–1138, https://doi.org/10.1016/j.jpain.2017.05.002 (2017).
Purcell, S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575, https://doi.org/10.1086/519795 (2007).
Cullen, P. J. Endosomal sorting and signalling: an emerging role for sorting nexins. Nat Rev Mol Cell Biol 9, 574–582, https://doi.org/10.1038/nrm2427 (2008).
Lin, T. B. et al. VPS26A-SNX27 Interaction-Dependent mGluR5 Recycling in Dorsal Horn Neurons Mediates Neuropathic Pain in Rats. The Journal of neuroscience: the official journal of the Society for Neuroscience 35, 14943–14955, https://doi.org/10.1523/JNEUROSCI.2587-15.2015 (2015).
NCBI. KNG1 kininogen 1, https://www.ncbi.nlm.nih.gov/gene/3827 (2017).
Gene Cards. KNG1 Gene, http://www.genecards.org/cgi-bin/carddisp.pl?gene=KNG1&keywords=KNG1 (2017).
Morange, P. E. et al. KNG1 Ile581Thr and susceptibility to venous thrombosis. Blood 117, 3692–3694, https://doi.org/10.1182/blood-2010-11-319053 (2011).
Yamaguchi-Sase, S. et al. Amelioration of hyperalgesia by kinin receptor antagonists or kininogen deficiency in chronic constriction nerve injury in rats. Inflamm Res 52, 164–169 (2003).
Costa, R. et al. Kinin Receptors Sensitize TRPV4 Channel and Induce Mechanical Hyperalgesia: Relevance to Paclitaxel-Induced Peripheral Neuropathy in Mice. Mol Neurobiol, https://doi.org/10.1007/s12035-017-0475-9 (2017).
Costa, R. et al. Anti-nociceptive effect of kinin B(1) and B(2) receptor antagonists on peripheral neuropathy induced by paclitaxel in mice. British journal of pharmacology 164, 681–693, https://doi.org/10.1111/j.1476-5381.2011.01408.x (2011).
Gene Cards. PCP2 Gene, http://www.genecards.org/cgi-bin/carddisp.pl?gene=PCP2 (2017).
NCBI. PCP2 Purkinje cell protein 2, https://www.ncbi.nlm.nih.gov/gene/126006 (2017).
Luo, Y. & Denker, B. M. Interaction of heterotrimeric G protein Galphao with Purkinje cell protein-2. Evidence for a novel nucleotide exchange factor. The Journal of biological chemistry 274, 10685–10688 (1999).
Valenzuela, D. et al. G alpha(o) is necessary for muscarinic regulation of Ca2+ channels in mouse heart. Proceedings of the National Academy of Sciences of the United States of America 94, 1727–1732 (1997).
Strittmatter, S. M., Valenzuela, D., Kennedy, T. E., Neer, E. J. & Fishman, M. C. G0 is a major growth cone protein subject to regulation by GAP-43. Nature 344, 836–841, https://doi.org/10.1038/344836a0 (1990).
Strittmatter, S. M., Fankhauser, C., Huang, P. L., Mashimo, H. & Fishman, M. C. Neuronal pathfinding is abnormal in mice lacking the neuronal growth cone protein GAP-43. Cell 80, 445–452 (1995).
Jiang, M. et al. Multiple neurological abnormalities in mice deficient in the G protein Go. Proceedings of the National Academy of Sciences of the United States of America 95, 3269–3274 (1998).
Lu, Z. et al. Mediation of opioid analgesia by a truncated 6-transmembrane GPCR. The Journal of clinical investigation 125, 2626–2630, https://doi.org/10.1172/JCI81070 (2015).
Matsui, T. Transcriptional regulation of a Purkinje cell-specific gene through a functional interaction between ROR alpha and RAR. Genes Cells 2, 263–272 (1997).
Gene Cards. RORA Gene, http://www.genecards.org/cgi-bin/carddisp.pl?gene=RORA&keywords=RORA (2017).
NCBI. RORA RAR related orphan receptor A, https://www.ncbi.nlm.nih.gov/gene/6095 (2017).
Ming, Q., Wang, X., Chai, Q., Yi, J. & Yao, S. Retinoid-related orphan receptor alpha (RORA) gene variation is associated with trait depression. Psychiatry research 229, 629–630, https://doi.org/10.1016/j.psychres.2015.07.014 (2015).
Mala Cards. Atrial Tachyarrhythmia with Short Pr Interval, http://www.malacards.org/card/atrial_tachyarrhythmia_with_short_pr_interval (2017).
Terracciano, A. et al. Genome-wide association scan of trait depression. Biological psychiatry 68, 811–817, https://doi.org/10.1016/j.biopsych.2010.06.030 (2010).
Li, M. Z. et al. Molecular mapping of developing dorsal horn-enriched genes by microarray and dorsal/ventral subtractive screening. Dev Biol 292, 555–564, https://doi.org/10.1016/j.ydbio.2006.01.033 (2006).
Hertz, D. L. et al. Pharmacogenetic Discovery in CALGB (Alliance) 90401 and Mechanistic Validation of a VAC14 Polymorphism that Increases Risk of Docetaxel-Induced Neuropathy. Clin Cancer Res 22, 4890–4900, https://doi.org/10.1158/1078-0432.CCR-15-2823 (2016).
Dolan, M. E. et al. Clinical and Genome-Wide Analysis of Cisplatin-Induced Peripheral Neuropathy in Survivors of Adult-Onset Cancer. Clin Cancer Res 23, 5757–5768, https://doi.org/10.1158/1078-0432.CCR-16-3224 (2017).
Meng, W. et al. A genome-wide association study suggests an association of Chr8p21.3 (GFRA2) with diabetic neuropathic pain. Eur J Pain 19, 392–399, https://doi.org/10.1002/ejp.560 (2015).
Meng, W. et al. A Genome-wide Association Study Provides Evidence of Sex-specific Involvement of Chr1p35.1 (ZSCAN20-TLR12P) and Chr8p23.1 (HMGB1P46) With Diabetic Neuropathic Pain. EBioMedicine 2, 1386–1393, https://doi.org/10.1016/j.ebiom.2015.08.001 (2015).
Zorina-Lichtenwalter, K., Parisien, M. & Diatchenko, L. Genetic studies of human neuropathic pain conditions: a review. Pain 159, 583–594, https://doi.org/10.1097/j.pain.0000000000001099 (2018).
Dupont, W. D. & Plummer, W. D. Jr. Power and sample size calculations for studies involving linear regression. Control. Clin. Trials 19, 589–601 (1998).
Reyes-Gibby, C. C. et al. Genome-wide association study suggests common variants within RP11-634B7.4 gene influencing severe pre-treatment pain in head and neck cancer patients. Sci. Rep. 6, 34206, https://doi.org/10.1038/srep34206 (2016).
Bentley, D. R. et al. Accurate whole human genome sequencing using reversible terminator chemistry. Nature 456, 53–59, https://doi.org/10.1038/nature07517 (2008).
Illumina. Illumina BeadStudio data analysis software modules, http://www.illumina.com/Documents/products/datasheets/datasheet_beadstudio.pdf (2016).
Shete, S. et al. Genome-wide association study identifies five susceptibility loci for glioma. Nat. Genet. 41, 899–904, https://doi.org/10.1038/ng.407 (2009).
Wang, J. & Shete, S. Testing Departure from Hardy-Weinberg Proportions. Methods in molecular biology (Clifton, N.J.) 1666, 83–115, https://doi.org/10.1007/978-1-4939-7274-6_6 (2017).
Lee, W. C. Testing the genetic relation between two individuals using a panel of frequency-unknown single nucleotide polymorphisms. Annals of human genetics 67, 618–619 (2003).
Stevens, E. L. et al. Inference of relationships in population data using identity-by-descent and identity-by-state. PLoS genetics 7, e1002287, https://doi.org/10.1371/journal.pgen.1002287 (2011).
Auton, A. et al. A global reference for human genetic variation. Nature 526, 68–74, https://doi.org/10.1038/nature15393 (2015).
R Development Core Team. R: A language and environment for statistical computing. (R Foundation for Statistical Computing, Vienna, Austria, 2014).
Ashburner, M. et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nature genetics 25, 25–29, https://doi.org/10.1038/75556 (2000).
The Gene Ontology, C. Expansion of the Gene Ontology knowledgebase and resources. Nucleic acids research 45, D331–D338, https://doi.org/10.1093/nar/gkw1108 (2017).
Kramer, A., Green, J., Pollard, J. Jr. & Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 30, 523–530, https://doi.org/10.1093/bioinformatics/btt703 (2014).
Ingenuity Pathway Analysis. Grow, http://qiagen.force.com/KnowledgeBase/articles/Basic_Technical_Q_A/Grow (2018).
Acknowledgements
This work was supported by the National Institutes of Health through grants R01 DE022891 (CCR and SS), R25 DA026120 (SS), R03 CA192197 (JW), and the Cancer Center Support Grant P30 CA016672 (JW and SS). This work was also supported by the Cancer Prevention Research Institute of Texas grants RP130123 (SS) and RP170259 (SS); the Barnhart Family Distinguished Professorship in Targeted Therapy (SS); and the Program in Oncologic Emergency Medicine (CCR). The authors are grateful to the patients and other individuals for their participation. They thank the clinicians and other hospital staff, cancer registries and study staff who contributed to the blood sample and data collection for this study.
Author information
Authors and Affiliations
Contributions
All authors contributed to the final paper. C.C.R. and S.S. conceptualized the study. R.Y. performed the genome-wide association analyses. C.C.R., J.W., S.J.Y., P.C., E.Y.H. and S.S. drafted and revised the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Reyes-Gibby, C.C., Wang, J., Yeung, SC.J. et al. Genome-wide association study identifies genes associated with neuropathy in patients with head and neck cancer. Sci Rep 8, 8789 (2018). https://doi.org/10.1038/s41598-018-27070-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-27070-4
This article is cited by
-
Painful physical symptoms and antidepressant treatment outcome in depression: a systematic review and meta-analysis
Molecular Psychiatry (2024)
-
Die EAN-NeuPSIG-Leitlinie zur Diagnostik bei neuropathischen Schmerzen – eine Kurzfassung
Der Schmerz (2024)
-
Leveraging GWAS data derived from a large cooperative group trial to assess the risk of taxane-induced peripheral neuropathy (TIPN) in patients being treated for breast cancer: Part 2—functional implications of a SNP cluster associated with TIPN risk in patients being treated for breast cancer
Supportive Care in Cancer (2023)
-
Identification of a SNP cluster associated with taxane-induced peripheral neuropathy risk in patients being treated for breast cancer using GWAS data derived from a large cooperative group trial
Supportive Care in Cancer (2023)
-
MicroRNA-7, synergizes with RORα, negatively controls the pathology of brain tissue inflammation
Journal of Neuroinflammation (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.