Abstract
Broad coverage of mink enteritis virus (MEV) vaccination program in northeast of China has provided effective protection from mink viral enteritis. Nevertheless, MEV vaccine failures were reported due to continually evolving and changing virulence of field variants or wild-type MEV. In this study, a combined loop-mediated isothermal amplification (LAMP) and single nucleotide polymorphism (SNP) method, named LAMP-SNP assay, was developed for detection and differentiation of wild-type and vaccine strains of MEV. Four primers in MEV-VP2-LAMP were used to detect both wild-type and vaccine strains of MEV in our previous publication, and other four primers in LAMP-SNP were designed to amplify the NS1 gene in wild-type MEV and only used to detect wild-type viruses. The LAMP-SNP assay was performed in a water bath held at a constant temperature of 65 °C for 60 min. LAMP-SNP amplification can be judged by both electrophoresis and visual assessment with the unaided eyes. In comparison with virus isolation as the gold standard in testing 171 mink samples, the percentage of agreement and relative sensitivity and specificity of the LAMP-SNP assay were 97.1, 100%, and 94.0%, respectively. There were no cross-reactions with other mink viruses. The LAMP-SNP assay was found to be a rapid, reliable and low-cost method to differentiate MEV vaccine and field variant strains.
Similar content being viewed by others
Introduction
Mink viral enteritis (MVE) is an acute hemorrhagic enteritis that affects Mustela lutreola and Neovison vison of different ages, was first discovered in 1949, and mink enteritis virus (MEV) is a member of the Parvoviridae family1,2. Newborn and juvenile minks are particularly susceptible to MEV, and MVE affects the mink industry worldwide3,4,5,6. The main symptoms of MVE are severe diarrhea, feces containing sloughed-off gray intestinal mucosal cells, and severe leukopenia7. MEV is highly transmissible and can cause a fatality rate up to 80%1, which is highly detrimental to the mink economy8,9. Currently, vaccination of all farmed minks with the MEV vaccine has well controlled the MEV infection, however, the high rate of mutation of MEV (~10−4 substitutions per site per year10) can lead to new-variant strains and MVE epidemics. Although widespread vaccination against MVE has been executed for six decades, sometimes there were vaccine failure in minks and vaccinated animals showed clinical symptoms of MVE infections. Rapid and accurate diagnostic methods for the detection and differentiation of MEV wild-type and vaccine strains are required for effective control of this devastating mink disease.
MEV is a non-enveloped virus containing a ~5 kb ssDNA genome within an icosahedral capsid of 18–24 nm. The genome contains two large open reading frames (ORFs), which give rise to smaller structural ORFs by alternative splicing5. The most common methods currently in use for MEV detection include PCR, virus isolation, electron microscopy, and serological methods (Hemagglutination and Hemagglutination inhibition assays), which are effective in MEV diagnostics but are unable to differentiate between MEV wild-type and vaccine strains. To address the MEV strain differentiation need, we combined loop-mediated isothermal amplification (LAMP) and single nucleotide polymorphism (SNP) technologies in a LAMP-SNP assay. LAMP is a new isothermal amplification technology that performs auto-cycling strand-displacement DNA synthesis with Bacillus stearothermophilus (Bst) DNA polymerase at constant temperature11,12. Four or six primers for specific target genes are used to produce amplification products with stem-loop structures13 in approximately one hour without the denaturation step. In addition, specific LAMP assay could be highly sensitive and specific14. LAMP is an easy and time-saving test15, and the cost is low because LAMP does not require the expensive PCR reagents or sophisticated equipment. LAMP has great potential to be used widely to detect pathogens and biological markers15,16,17.
In this study, we aimed to detect and differentiate wild-type and vaccine MEV strains from immunized minks. The forward inner primer (FIP) and the backward inner primer (BIP) were designed to contain an SNP at nucleotide 1846 of the NS1 gene (G for wild-type strains and A for vaccine strain). When using primers for the wild-type strains, DNA synthesis from a stem-loop structure and LAMP amplification cycling continues with the wild-type strains, but not for the vaccine strain. Therefore, the LAMP-SNP method can effectively distinguish between wild-type and vaccine strains without complicated DNA sequencing. This system provides a relatively quick and easy method for an on-site diagnostic test of MEV pathogens.
Results
Development of the LAMP-SNP assay
Total DNA was extracted from each of suspected MEV samples, non-MEV strains, wild-type MEV strains, and the vaccine strain MEVB. The MEV VP2 gene LAMP assay (MEV-VP2-LAMP) detected MEV as observed by the presence of typical ladder-like bands representing several inverted repeat LAMP products upon 2.0% agarose gel electrophoresis (data not shown). The MEV-NS1-LAMP (LAMP-SNP) assay detected wild-type MEV strains, including MEV SD, MEV-Z6, and MEV LN10 strains, but not the vaccine strain MEVB or the non-MEV viruses, mink pseudorabies virus (MPRV) J strain, Aleutian mink disease virus (AMDV) G strain, and canine distemper virus (CDV) 3 strain, because they did not contain the inverted repeat structures required for amplification (Fig. 1).
Detection MEV and non-MEV viruses by the LAMP-SNP assay. Lane M: DNA Marker DL2000 (TaKaRa, Dalian, China). Lane 1: LAMP amplification products with MEVB DNA as the template; Lanes 2–4: LAMP amplification products with MEV SD, MEV-Z6, and MEV-LN10 DNAs as templates, respectively; Lanes 5–7: LAMP amplification products with DNA/cDNAs of MPRV-J, AMDV-G, and CDV3 as templates, respectively. Full-length gel is presented in Supplementary Fig. 1.
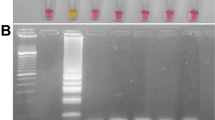
Moreover, LAMP-SNP products can be detected not only using agarose gel electrophoresis but also by visual observation of color change with intercalator SYBR Green I. In the MEV-VP2-LAMP assay, upon addition of the SYBR green I dye to tubes after the LAMP reaction, the color changed to yellowish green both vaccine and wild-type MEV virus and positive control, while that remained reddish orange in the negative control. And in the LAMP-SNP assay, the color of tube of wild-type MEV virus was changed to yellowish green and that of vaccine virus remained reddish orange (Fig. 2). With the two assays, we can simultaneously detect and differentiate wild-type and vaccine strains of MEV.
Visual inspection LAMP products by using SYBR Green I. (A) P: positive control (MEVB), N: negative control (double distilled water), V: vaccine MEVB strain, W: wild-type MEV SD isolate. (B) P: positive control (MEV SD), N: negative control (double distilled water), V: vaccine MEVB strain, W: wild-type MEV SD isolate. For visual inspection of the LAMP products, 4 μL diluted SYBR Green I dye 10,000× concentration in double distilled water was added to the reaction tube after the reaction termination. Yellowish green and reddish orange represent positive and negative reactions, respectively. Full-length tubes are presented in Supplementary Fig. 2A,B.
Detection limit of the LAMP-SNP assay
To determine the detection limit of the LAMP-SNP assay, the plasmid pT-MEV-NS1 was 10-fold serially diluted and used as the template. The lower limit of detection of LAMP-SNP assay was 101 copies of pT-MEV-NS1 per reaction. The detection was determined not only using agarose gel electrophoresis but also by visual judgment by the unaided eye with intercalator SYBR Green I (Fig. 3).
Detection limit of LAMP-SNP assay. (A) Electrophoresis. Lane M: DNA Marker DL2000 (TaKaRa, Dalian, China). Lanes 1–8 represent 2 × 106–2 × 10−1 copies/mL of pT-MEV-NS1 plasmid, respectively. (B) Visual inspection. Tubes 1–8 represent 2 × 106–2 × 10−1 copies/mL of pT-MEV-NS1 plasmid, respectively. Both methods exhibited the same results, the detection limit was 101 copies/mL, with no differences between electrophoresis and visual inspection. Full-length gels and tubes are presented in Supplementary Fig. 3A,B.
Specificity and Sensitivity of the LAMP-SNP assay
Of the 171 mink specimens tested by both virus isolation and LAMP-SNP assay, 88 were positive for MEV and 78 were negative for MEV in both tests, the remaining 5 samples were negative by virus isolation but positive by LAMP-SNP assay. Therefore, this new LAMP-SNP assay for MEV achieved 100% (88/88) of sensitivity and 94.0% (78/83) of specificity (Table 1). The 88 positive samples were identified as wild-type by LAMP-SNP assay and they were also identified as wild-type virus by DNA sequencing. Figure 4 showed detection of clinical specimens by LAMP-SNP assay and indirect immunofluorescence assay. This new LAMP-SNP assay for MEV showed no cross-reactions with the other mink viruses tested, including MPRV, AMDV, and CDV (Fig. 1).
Evaluation of clinical specimens by (A) LAMP-SNP and (B) virus isolation. (A) P: positive control (MEV SD), N: negative control (double distilled water), tubes 1–10 represent different clinical fecal samples. (B) Virus isolation (MEV-Z6) was identified by an indirect immunofluorescence assay using a monoclonal antibody against the VP2 protein of MEV and goat anti-mouse IgG-FITC conjugated secondary antibody. Specific immunofluorescent signals were detected in cells exposed to the respective tissue filtrates but not in mock-infected control cells. Full-length tubes and immunofluorescence pictures are presented in Supplementary Fig. 4A, B.
Discussion
MVE, which is caused by MEV, is an acute infectious disease that threatens mink farming industry18. Currently, vaccination with attenuated and inactivated vaccines is the primary method to prevent MVE. Inoculation with an attenuated vaccine provides sustained immune responses, but the vaccine lacks stability and the attenuated virus can recover pathogenicity. Inactivated vaccines are easy to mass produce, but they may be less effective than attenuated vaccines and requires repeated inoculations. MVE prevention in China was primarily carried out by immunizations with the inactivated MEV vaccine, however, clinical symptoms, including diarrhea, marasmus, and death, occasionally occurred in minks after inoculation. Therefore, it was important to determine whether the symptoms resulted from the vaccine preparation or whether the minks were infected with wild-type MEV prior to inoculation.
The LAMP assay, which amplifies nucleic acids in vitro, detects antigens simply and rapidly and does not require sophisticated equipment19. In this study, we found that SNP typing using the LAMP assay is highly specific and allows for amplification of specific alleles. In this method, both strands of DNA containing a specific SNP are amplified simultaneously with calibration. If the SNP is present on both strands, amplification occurs, thus SNPs polymorphic typing is achieved by detecting the presence or absence of amplification reactions. We successfully developed a diagnostic system that combines LAMP and SNP technologies to detect wild-type MEV in mink that were vaccinated against MEV. This diagnostic system has potential use for laboratory and clinical diagnosis practices.
There are many methods for the detection and diagnosis of MEV, including virus isolation, the hemagglutination assay, electron microscopy, ELISA, and PCR3, but they have disadvantages, and cannot differentiate between wild-type and vaccine strains of MEV around the world. A disadvantage of all of these diagnostic methods is that they require expensive instruments and special material, which limits their clinical applications, especially for field-testing. In contrast, LAMP is a simple and efficient method that only requires a constant temperature instrument for incubation under isothermal conditions, thus this method can be used for quick diagnosis of MVE and is suitable for field-testing.
We found that our LAMP-SNP assay diagnosed specifically different types of MEV. In addition, the LAMP-SNP assay was more sensitive than virus isolation and did not require an additional gene-sequencing step. This novel detection technique is useful for timely clinical diagnosis of MEV so that the infection can be controlled. In conclusion, the LAMP-SNP assay is rapid, simple, sensitive, and specific and can rapidly detect and differentiate between wild-type and vaccine MEV strains.
Methods
Viruses
The MEVB20 was a vaccine strain from the Jilin Teyan Biological Technology Company, Changchun, China; the wild-type viruses, MEV SD21, MEV-Z622, and MEV-LN1023, were isolated from the Shandong, Jilin, and Liaoning provinces in China. The non-MEV viruses, including MPRV-J, AMDV-G24, and CDV325, were maintained at the Key Laboratory of Special Animal Epidemic Disease, Ministry of Agriculture, PR China, Institute of Special Animal and Plant Sciences, Chinese Academy of Agricultural Sciences. All the viruses were prepared as described in some previous studies20,21,22,23,24,25.
Primer design
The primers used in the MEV-VP2-LAMP assay to detect MEV were designed in a previous study15. The primers F3-NS1, B3-NS1, FIP-NS1, and BIP-NS1 were designed using online LAMP primer software PrimerExplorer (http://primerexplorer.jp/e/), and the primer sequences are shown in Table 2. Primers to NS1 gene from wild-type MEV strains and the vaccine strain MEVB were aligned using BioEdit software (Fig. 5). The nucleotide at position 1846 of the NS1 gene of wild-type strains is G, while that is A for vaccine strain. The alignment showed that the primers for the wild-type MEV strains were conserved, but that the primers for the vaccine strain MEVB were not. The primer design principle is shown in Fig. 6.
Partial sequence alignment of NS1. NS1 sequences from wild-type strains and vaccine strain MEVB were analyzed. In wild-type strains, there is a G at position 1846 of NS1, whereas there is an A at that position in the vaccine strain. This nucleotide difference was used to design the differentiation primers used in the LAMP-SNP assay.
Schematic of single nucleotide polymorphism typing using the LAMP method. The BIP and FIP primers were designed to contain the wild-type SNP allele. With wild-type DNA template and these primers, a dumbbell-like structure is formed and amplification occurs. If the template DNA is from the vaccine strain MEVB or from a non-MEV virus, a dumbbell-like structure will not form and amplification will not occur.
Collection of mink samples
A total of 171 mink samples, including 67 mink tissue specimen and 104 mink fecal samples, were collected in our lab. These tissues were the homogenates of mink intestines and the fecal samples (Supplementary Table 1) were collected with sterile swabs, diluted in PBS, and stored at −20 °C until further use. All the samples were from Hebei, Shandong, Jilin, Liaoning and Heilongjiang in China, and all the animals show clinical symptoms of MVE include anorexia, vomiting, and diarrhea. MEVB, MEV SD, MEV-Z6, and MEV-LN10 strains were cultured in F81 cells26.
DNA extraction
Total DNA was extracted from fecal samples, cultured cells infected with MEV, and cultured cell that were not infected with MEV using the Takara MiniBEST Viral RNA/DNA Extraction Kit Ver. 5.0 (Takara Biotechnology Co., Ltd., Dalian, China) according to the manufacturer’s instructions. The extracted DNAs were used as the templates in the LAMP assays.
pT-MEV-NS1 plasmid construction
MEV-NS1 forward and reverse primers (NS1 F and NS1 R, see Table 2) were used to amplify NS1 from MEV-SD. The NS1 PCR product was cloned into the pMD18-T vector (Takara Biotechnology Co., Ltd., Dalian, China) and the resulting plasmid was named pT-MEV-NS1 and used as the positive control template.
Detection and differentiation of wild-type and vaccine MEV strains by LAMP-SNP assay
Both wild-type and vaccine viruses were detected by MEV-VP2-LAMP assay15. LAMP-SNP reactions were performed in a total volume of 25 μL and contained 0.4 μL (10 pM) each of the F3 and B3 primers, 2.5 μL (10 pM) each of the FIP and BIP primers, 3.5 μL (10 mM) of dNTPs, 2.5 μL of 10 × Bst DNA polymerase buffer, 0.5 μL (8 U) of Bst DNA polymerase (New England BioLabs, Herts, UK), 2 μL of template DNA, 4 μL (100 mM) of MgSO4, and 6 μL (20 μM) of betaine. The amplifications were performed at 65 °C in water bath for 60 min and were terminated by incubating the reactions at 80 °C in water bath for 10 min. The LAMP amplification products were analyzed by 2.0% (w/v) agarose gel electrophoresis. Besides agarose gel electrophoresis, the products of LAMP-SNP were judged by naked eyes with the addition of SYBR Green I (Solarbio, Beijing, China) dye into the tubes. Upon addition of the SYBR green I dye to tubes after the LAMP-SNP reaction, the color changed to yellowish green in the positive reactions and remained reddish orange in a negative reaction.
Detection limit of the LAMP-SNP assay
The detection limit of the LAMP-SNP assay was assessed by using a panel of 10-fold serial dilutions of pT-MEV-NS1 (2 × 106–2 × 10−1 copies/mL). The plasmid dilution series was used in the LAMP-SNP assay to determine the minimum concentrations required for detection by the assay.
Specificity and Sensitivity of the LAMP-SNP assay
A total of 171 mink tissue specimen, consisting of 88 positives and 83 negatives for MEV, were diagnosed by virus isolation for the “gold standard” and used for the LAMP-SNP assay’s specificity and sensitivity evaluation test to detect MEV from clinical mink specimen. All the specimens were collected from healthy minks or wild-type strains MEV SD, MEV Z6, and MEV-LN10. The cross-reaction of the LAMP-SNP assay was evaluated with other mink viruses including MPRV, AMDV, and CDV.
Virus isolation was performed using F81 cells as described previously15. And the virus isolation was identified by indirect immunofluorescence assay as follows: F81 cells were infected with MEV isolates at an input MOI of 0.01 TCID50 per cell. Following 48 h of incubation, cells were immunostained using a monoclonal antibody specific for the VP2 protein of MEV27 and FITC-labeled goat anti-mouse IgG. Nuclei were stained with 40,6-diamidino-2-phenylindole dihydrochloride (DAPI) (Sigma).
Ethics in publishing
The methods were carried out in accordance with Animal Epidemic Prevention Law of the People’s Republic of China28. Animal experiments were performed in accordance with animal ethics guidelines28 and approved by the Institute of Special Animal and Plant Sciences of the Chinese Academy of Agricultural Sciences.
References
Schofield, F. W. Virus enteritis in mink. North Am Vet 30, 651–654 (1949).
CG, W. Notes on infectious enteritis of mink and its relationship to feline enteritis. Canadian journal of comparative medicine and veterinary science 16, 419–420 (1952).
Wang, J. et al. Development of a nanoparticle-assisted PCR (nanoPCR) assay for detection of mink enteritis virus (MEV) and genetic characterization of the NS1 gene in four Chinese MEV strains. BMC Vet Res 11, 1, https://doi.org/10.1186/s12917-014-0312-6 (2015).
Tkachev, S. E., Sigitova, E. V., Mitrofanova, E. E., Durymanov, A. G. & Shestopalov, A. M. Newly isolated mink parvovirus strain. Voprosy virusologii 47, 35–39 (2002).
Storgaard, T., Oleksiewicz, M., Bloom, M. E., Ching, B. & Alexandersen, S. Two parvoviruses that cause different diseases in mink have different transcription patterns: transcription analysis of mink enteritis virus and Aleutian mink disease parvovirus in the same cell line. J Virol 71, 4990–4996 (1997).
Uttenthal, A. Apparent lack of effect of vaccination against mink enteritis virus (MEV). A challenge study. Arch Virol 99, 153–161 (1988).
Zhang, C. et al. Development of a PCR-RFLP assay for the detection and differentiation of canine parvovirus and mink enteritis virus. J Virol Methods 210, 1–6, https://doi.org/10.1016/j.jviromet.2014.09.014 (2014).
Hundt, B. et al. Establishment of a mink enteritis vaccine production process in stirred-tank reactor and Wave Bioreactor microcarrier culture in 1-10 L scale. Vaccine 25, 3987–3995, https://doi.org/10.1016/j.vaccine.2007.02.061 (2007).
Zhang, Q. et al. Selection of antiviral peptides against mink enteritis virus using a phage display Peptide library. Curr Microbiol 66, 379–384, https://doi.org/10.1007/s00284-012-0284-3 (2013).
Shackelton, L. A., Parrish, C. R., Truyen, U. & Holmes, E. C. High rate of viral evolution associated with the emergence of carnivore parvovirus. Proceedings of the National Academy of Sciences of the United States of America 102, 379–384, https://doi.org/10.1073/pnas.0406765102 (2005).
de Franchis, R., Cross, N. C., Foulkes, N. S. & Cox, T. M. A potent inhibitor of Taq polymerase copurifies with human genomic DNA. Nucleic acids research 16, 10355 (1988).
Song, T., Toma, C., Nakasone, N. & Iwanaga, M. Sensitive and rapid detection of Shigella and enteroinvasive Escherichia coli by a loop-mediated isothermal amplification method. FEMS microbiology letters 243, 259–263, https://doi.org/10.1016/j.femsle.2004.12.014 (2005).
Sahoo, P. R., Sethy, K., Mohapatra, S. & Panda, D. Loop mediated isothermal amplification: An innovative gene amplification technique for animal diseases. Veterinary world 9, 465–469, https://doi.org/10.14202/vetworld.2016.465-469 (2016).
Wenhui, L., Bingjie, Z., Qinxin, S. & Guohua, Z. Progress in multiplex loop-mediated isothermal amplification technology. Yi Chuan 37, 899–910, https://doi.org/10.16288/j.yczz.15-141 (2015).
Wang, J. et al. Detection of mink enteritis virus by loop-mediated isothermal amplification (LAMP). Journal of virological methods 187, 401–405, https://doi.org/10.1016/j.jviromet.2012.11.012 (2013).
Fujita, H., Kataoka, Y., Tobita, S., Kuwahara, M. & Sugimoto, N. Novel One-Tube-One-Step Real-Time Methodology for Rapid Transcriptomic Biomarker Detection: Signal Amplification by Ternary Initiation Complexes. Analytical chemistry 88, 7137–7144, https://doi.org/10.1021/acs.analchem.6b01192 (2016).
Yang, Q., Domesle, K. J., Wang, F. & Ge, B. Rapid detection of Salmonella in food and feed by coupling loop-mediated isothermal amplification with bioluminescent assay in real-time. BMC microbiology 16, 112, https://doi.org/10.1186/s12866-016-0730-7 (2016).
Hou, Q. et al. The phosphorylation of Ser221 in VP2 of mink enteritis virus and its roles in virus amplification. Virus Res 217, 76–84, https://doi.org/10.1016/j.virusres.2016.03.004 (2016).
Tang, Y., Chen, H. & Diao, Y. Advanced uracil DNA glycosylase-supplemented real-time reverse transcription loop-mediated isothermal amplification (UDG-rRT-LAMP) method for universal and specific detection of Tembusu virus. Scientific reports 6, 27605, https://doi.org/10.1038/srep27605 (2016).
Wu, W., Nie, J. Z., Wang, K. J. & Cheng, S. P. Studies on mink enteritis parvovirus main epizootic types in China. Chinese Journal of Veterinary Science 16, 359–361 (1996).
Wang, J., Zhao, H., Cheng, Y., Yi, L. & Cheng, S. Genome Sequence of Mink Enteritis Virus Strain SD 12/01, Isolated from a Mink with Severe Diarrhea in China. Genome announcements 1, https://doi.org/10.1128/genomeA.00306-13 (2013).
Yang, S. et al. Genetic Characteristic Analysis of Non-structural NS1 and Structural Protein VP2 of FPLV, CPV and MEV. Progress in Veterinary Medicine 32, 31–37 (2011).
Wang, J. et al. Evidence for natural recombination between mink enteritis virus and canine parvovirus. Virology journal 9, 252, https://doi.org/10.1186/1743-422x-9-252 (2012).
Wu, W. et al. Preparation of cell antigen of Aleutian mink disease virus for counterimmunoelectrophoresis. Chinese Agricultural Science Bulletin 6, 23–26 (1990).
Yi, L. et al. Development of a combined canine distemper virus specific RT-PCR protocol for the differentiation of infected and vaccinated animals (DIVA) and genetic characterization of the hemagglutinin gene of seven Chinese strains demonstrated in dogs. Journal of virological methods 179, 281–287, https://doi.org/10.1016/j.jviromet.2011.11.011 (2012).
Wang, J. et al. Continuing evolution of canine parvovirus in China: Isolation of novel variants with an Ala5Gly mutation in the VP2 protein. Infect Genet Evol 38, 73–78, https://doi.org/10.1016/j.meegid.2015.12.009 (2016).
Wang, J. et al. Preparation and biological characteristic of monoclonal antibodies against mink enteritis virus. Chinese Veterinary Science, 227–232 (2009).
Yang, H. et al. Prevalence, genetics, and transmissibility in ferrets of Eurasian avian-like H1N1 swine influenza viruses. Proceedings of the National Academy of Sciences of the United States of America 113, 392–397, https://doi.org/10.1073/pnas.1522643113 (2016).
Acknowledgements
We are indebted to Eiken Chemical Co., Ltd. for their technical and design primers assistance. The study was supported by National Natural Science Foundation of China (No. 31602056), Jilin Province Scientific and Technological Program (No. 20160520034 JH), and Jilin Provincial Key Science and Technology Project Fund (No. 20150204021 NY).
Author information
Authors and Affiliations
Contributions
P.L. and J.-K.W. conceived and designed the study and wrote the manuscript. N.-Y.C. and S.-S.S. conducted the experiments and analyzed the data. H.-L.W., Y.-R.S., M.Z., L.G., L.Y., M.-W.T., Z.-G.C., S.L. and S.-P.C. contributed to the interpretation of the results and participated in the critical revision of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lin, P., Wang, H., Cheng, Y. et al. Loop-mediated Isothermal Amplification-Single Nucleotide Polymorphism Analysis for Detection and Differentiation of Wild-type and Vaccine Strains of Mink Enteritis Virus. Sci Rep 8, 8393 (2018). https://doi.org/10.1038/s41598-018-26717-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-26717-6
This article is cited by
-
A duplex tetra-primer ARMS-PCR assay to discriminate three species of the Schistosoma haematobium group: Schistosoma curassoni, S. bovis, S. haematobium and their hybrids
Parasites & Vectors (2023)
-
Generation and immunogenicity of virus-like particles based on mink enteritis virus capsid protein VP2 expressed in Sf9 cells
Archives of Virology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.