Abstract
Before 2011, the prevalence rates of carbapenemase-producing Klebsiella pneumoniae (CPKP) among carbapenem nonsusceptible K. pneumoniae (CnSKP) isolates were below 10% in Taiwan. The study presents the dissemination and increased antimicrobial resistance of CPKP from January 2012 to August 2015, as shown by Taiwanese multicenter surveillance. Isolates with minimum inhibitory concentrations (MICs) of >1 μg/mL for imipenem or meropenem were collected, screened for various carbapenemase genes by PCR, and tested for antimicrobial susceptibility. Among 1,457 CnSKP isolates, 1,250 were collected from medical centers. The CnSKP prevalence in medical centers increased by 1.7-fold during the study. Among all CnSKP isolates, 457 were CPKP. The CPKP rate among CnSKP increased by 1.5-fold and reached 36.8% in 2015. The CPKP nonsusceptibility rate to aztreonam, fluoroquinolones, and aminoglycosides increased yearly. Six CPKP isolates carried dual carbapenemase genes. Three Ambler classes were identified in 451 isolates with a single carbapenemase: classes A (315 blaKPC-2, 2 blaKPC-3, 28 blaKPC-17, 2 blaKPC-34), B (26 blaIMP-8, 2 blaNDM-1, 36 blaVIM-1), and D (40 blaOXA-48). The blaOXA-48 rate among CPKP increased by 6-fold over three years. Most KPC and OXA-48 producers were ST11. CnSKP was increasingly prevalent, owing to CPKP dissemination. Additionally, CPKP became more resistant during the study period.
Similar content being viewed by others
Introduction
Klebsiella pneumoniae is a common cause of bacteremia, pneumonia, urinary tract infection, and liver abscess1. β-lactam antibiotics are often deemed the primary therapeutic option for these infections2. Among the β-lactams, carbapenems are considered the antibiotics of last resort3. Once K. pneumoniae isolates become nonsusceptible to carbapenems, they are often resistant to all currently available β-lactams and frequently resistant to non-β-lactam antibiotics4. In the clinical context, the emergence of carbapenem non-susceptible K. pneumoniae (CnSKP) poses a serious threat to patient survival because CnSKP infections have limited treatment options and are associated with high mortality5.
Among the many mechanisms conferring resistance to carbapenems, carbapenemases can efficiently hydrolyze carbapenems and have become an important cause of antimicrobial resistance6. Many carbapenemases have been identified in K. pneumoniae and are classified into Ambler class A (KPC, GES, IMI, NMC, SME), class B (IMP, VIM, NDM, GIM, SIM, SPM), and class D (OXA-48)7.
The molecular epidemiology of carbapenemase-producing K. pneumoniae (CPKP) varies by country. In the United States, K. pneumoniae carbapenemase-producing K. pneumoniae (KPC-KP) was first reported in 20018 and subsequently caused outbreaks in New York City9. KPC has become the predominant carbapenemase in the U.S.10 and China11. OXA-48 was first identified in K. pneumoniae in Turkey in 200312 and is the major carbapenemase in Turkey13 and Spain14. NDM-KP was first detected in 2008 in a patient returning to Sweden from India15 and is the most common CPKP in India16.
In Taiwan, IMP-8 was the first reported carbapenemase in K. pneumoniae in 200117. Our previous study revealed that IMP-8 was the only type of carbapenemase in CPKP prior to 200918. In a national surveillance study in 2010, we identified VIM-1 from K. pneumoniae isolates4. In the same year, K. pneumoniae isolates producing NDM-1 and KPC-2 were identified in Taiwanese patients returning from India and China, respectively19,20. Subsequently, outbreaks caused by KPC-KP were reported in northern Taiwan in 201121.
After the arrival of KPC-2 producers, the molecular epidemiology and antimicrobial resistance profile of CnSKP and CPKP in Taiwan has not been examined well. Herein, we analyzed these trends from 2012 to 2015 to better understand and possibly prevent their global spread.
Results
Prevalence of CnSKP and Specimen Sources
A total of 1,457 CnSKP isolates were collected during the study period. Among all CnSKP, 1,250 (85.8%) were collected from medical centers, and 207 (14.2%) from regional hospitals. In three major regional hospitals, 137 isolates nonsusceptible to carbapenem were identified from 6,446 clinical K. pneumoniae isolates during the study period, and the average prevalence of CnSKP was 2.13%.
In all medical centers, the average prevalence of CnSKP was 1.10%, with 114,065 clinical isolates screened during the study period. The temporal trends in prevalence were analyzed by geographic region (Fig. 1). In the North region, the average prevalence was 1.40%, with no significant increase during the study period (p = 0.380). In the West region, the prevalence significantly increased (p < 0.001) 6-fold from 0.50% (14/2778) in 2012 to 3.12% (91/2917) in 2015, which was the highest yearly prevalence observed by region in Taiwan. The prevalence also increased significantly in the South and East regions (p < 0.001 and p = 0.040, respectively). Overall, the prevalence of CnSKP in Taiwan significantly increased 1.7-fold (0.87% in 2012, 1.46% in 2015; p < 0.001).
Temporal trends in the prevalence of carbapenem nonsusceptible Klebsiella pneumoniae (CnSKP) among clinical isolates in major medical centers (line) and the rate of carbapenemase-producing isolates (CPKP) among CnSKP (bar) by geographic region in Taiwan from 2012 to 2015. The graphs in the figure are original graphs, and the map was created using CorelDRAW Graphics Suite X4 software.
CnSKP isolates were recovered primarily from urine (518, 35.6%), followed by sputum (including bronchoalveolar lavage; 461, 31.6%), abscess/drainage (158, 10.8%), blood (130, 8.9%), bile (38, 2.6%), ascites (30, 2.1%), and central vein catheters (28, 1.9%). The rates for blood and abscess/drainage increased significantly yearly (6.3%, 9.1%, 8.3%, 11.5%, p = 0.040; 8.7%, 8.0%, 12.5%, 13.2%, p = 0.016; respectively). The rate of bacteremia increased nearly 2-fold within the 4 years studied. The isolation rates for sputum and central vein catheters significantly decreased annually (36.4%, 35.1%, 26.7%, 30.8%, p = 0.024 and 3.1%, 2.3%, 1.5%, 1.1%, p = 0.043, respectively). The specimen sources of CnSKP are summarized in Table 1.
Temporal Trends of CPKP
Of the 1,457 CnSKP isolates, 457 (31.4%) were positive for genes encoding carbapenemase (CPKP), while no carbapenemase genes were detected in 1,000 isolates (non-CP-CnSKP). The temporal trends for the rate of CPKP among CnSKP were analyzed by geographic region (Fig. 1). In the West region, the yearly rate of CPKP significantly increased nearly 2-fold from 35.7% to 70.3% in four years (p = 0.002). The rates in the North, South and East regions also increased, but not significantly (p = 0.695, 0.447, 0.570; respectively). Overall, the rate of CPKP in Taiwan significantly increased 1.5-fold from 24.1% to 36.8% during the study period (p = 0.003). The rate of non-CP-CnSKP decreased from 75.9% to 63.2%.
Antimicrobial Nonsusceptibility Rates
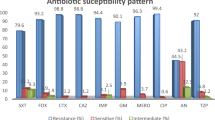
All CnSKP isolates were tested with β-lactam and non-β-lactam agents (Table 2). For the β-lactams, most (>99%) of the CnSKP isolates were nonsusceptible to cephalosporins, except for cefepime (nonsusceptible rate, 92.2%). The rate of nonsusceptibility to cefepime was lowest among non-carbapenem β-lactams, and the rates to meropenem and doripenem were the lowest among all β-lactams.
For the non-β-lactams, the nonsusceptibility rates of CnSKP to the fluoroquinolones (91.0% to ciprofloxacin and 88.3% to levofloxacin) were similar to that of cefepime. Among all tested agents, the rate of nonsusceptibility to tigecycline was the lowest, followed by colistin and the aminoglycosides.
The nonsusceptibility rates of CPKP and non-CP-CnSKP isolates were analyzed and compared, and different patterns were observed (Table 2). To β-lactams, CPKP were highly nonsusceptible, with rates >99% to all cephalosporins and >98% to piperacillin-tazobactam and all carbapenems. Aztreonam showed the lowest rate among all β-lactams. While non-CP-CnSKP isolates showed similar rates to most cephalosporins and aztreonam, they were significantly less nonsusceptible to piperacillin-tazobactam, cefepime, and carbapenems than their counterparts. The most obvious difference between these two groups were the rates of nonsusceptibility to doripenem and meropenem, which for non-CP-CnSKP were only 71.6% and 71.2%, respectively.
Among the non-β-lactams, CPKP isolates were significantly more nonsusceptible to fluoroquinolones than non-CP-CnSKP. No significant difference was seen between the two groups in terms of nonsusceptibility to colistin and tigecycline.
Temporal Trends of Nonsusceptibility Rates
The yearly trends for the nonsusceptibility rates of CPKP and non-CP-CnSKP isolates were analyzed (Fig. 2a,b, respectively). While being the least nonsusceptible β-lactam for CPKP, the rate of nonsusceptibility to aztreonam significantly increased during the study period (p = 0.040). A similar trend was also observed in non-β-lactams. The rates of nonsusceptibility to fluoroquinolones and trimethoprim-sulfamethoxazole also increased significantly (all p < 0.001). The rates of nonsusceptibility to gentamicin, amikacin, and colistin significantly increased 3- (21.7% to 66.4%), 4- (7.2% to 32.1%), and 5-fold (4.3% to 20.9%), respectively (all p ≤ 0.001).
Temporal antimicrobial nonsusceptibility rates of (a) carbapenemase-producing Klebsiella pneumoniae (CPKP) and (b) noncarbapenemase-producing carbapenem-nonsusceptible K. pneumoniae (non-CP-CnSKP) collected in Taiwan from 2012 to 2015. Abbreviations: AMK, amikacin; ATM, aztreonam; CAZ, ceftazidime; CIP, ciprofloxacin; CST, colistin; CTX, cefotaxime; DOR, doripenem; ETP, ertapenem; FEP, cefepime; FOX, cefoxitin; GEN, gentamicin; IPM, imipenem; LVX, levofloxacin; MEM, meropenem; SXT, trimethoprim-sulfamethoxazole; TGC, tigecycline; TZP, piperacillin-tazobactam. *p < 0.05, chi-square test for trend.
Non-CP-CnSKP isolates showed increasing nonsusceptibility to doripenem and meropenem, with rates that increased from 67.3% to 79.1% (p = 0.007) and from 66.4% to 78.7%, (p = 0.006), respectively; however, the rates of nonsusceptibility to levofloxacin and amikacin decreased significantly (from 91.7% to 84.8%, p = 0.007; and from 41.0% to 29.6%, p = 0.012; respectively).
Carbapenemase Types and Temporal Trends of CPKP
Among the 457 CPKP isolates, 6 (1.3%) were positive for dual carbapenemases, including 3 isolates for KPC-2/OXA-48, 2 for NDM-1/VIM-1, and 1 for KPC-2/IMP-8. Among the 451 CPKP isolates with a single carbapenemase, 347 (76.9%) were class A KPC [315 KPC-2, 2 KPC-3, 28 KPC-17, and 2 novel-variant KPC-34 (GenBank accession no. KU985429)], 64 (14.2%) were class B MBL (26 IMP-8, 2 NDM-1, and 36 VIM-1), and 40 (8.9%) were class D OXA-48. No matching hits were detected for GES, IMI, NMC, SME, GIM, SIM, or SPM.
The temporal trends of the rate of carbapenemase types among the 451 CPKP with a single carbapenemase were analyzed (Fig. 3). While KPC-2 was predominant throughout the study period, the rate of KPC-2 producers decreased significantly from 73.9% (51/69) in 2012 to 61.1% (80/131) in 2015 (p = 0.011). KPC-17 producers were first identified in 2012, and their rate increased significantly during the study period (p = 0.005). While only 4 (3.5%) isolates with the OXA-48 gene were found in 2013, this rate significantly increased nearly 6-fold to 20.6% (27/131) within three years (p < 0.001). The rates of IMP-8 and VIM-1 producers decreased significantly (p = 0.015 and p = 0.031, respectively), and KPC-3, KPC-34, and NDM-1 producers were identified sporadically.
Multilocus Sequence Types of CPKP
All CPKP were characterized with MLST. Among those with dual carbapenemases, all of the 3 KPC-2/OXA-48 producers were ST11, both of the NDM-1/VIM-1 producers were ST273, and the KPC-2/IMP-8 producer was ST11.
Among those with a single carbapenemase, most (340/347, 98.0%) of the KPC-KP were ST-11, including all KPC-17, both KPC-34, 309 (98.1%) KPC-2 and 1 (50%) KPC-3. ST11 was predominant among OXA-48 producers as well, with 87.5% (35/40) being ST11. MBL producers, however, were diverse in MLST; more than eight MLSTs (ST-11, -37, -45, -225, -309, -1087, -1192, -1355) were identified in IMP-8 producers, and only one IMP-8 was ST11. More than 11 MLSTs (ST-12, -29, -34, -268, -273, -307, -327, -656, -681, -741, -1665) were identified among VIM-1-producing isolates, but none was ST11. Of NDM-1 producers, one was ST11 and the other was ST313.
In summary, four CPKP among the six isolates with dual carbapenemases were ST11 (4/6, 66.7%) and 377 CPKP among the 451 isolates with single carbapenemase were ST11 (377/451, 83.6%).
Discussion
In this surveillance study, the prevalence of CnSKP in Taiwanese medical centers averaged 1.10% and increased 1.7-fold during the study period. This prevalence was much lower than that reported in the U.S. (6%), according to a report from the 2007–2009 SENTRY Antimicrobial Surveillance Program10. A similar trend of increased CnSKP prevalence during the same period in Taiwan was observed by the Taiwan Nosocomial Infections Surveillance (TNIS) which only included those isolates collected from patients in intensive care units. The prevalence from the TNIS was indeed much higher than that in this study, in which we collected isolates from all patients, regardless they were inpatients or outpatients. The TNIS showed that the prevalence of CnSKP in intensive care units in Taiwanese medical centers increased nearly 1.5-fold from 15.7% in 2012 to 22.5% in 201522. The geographic differences of CnSKP prevalence of medical centers found in this study were also reported by the TNIS, with the highest prevalence being 28.1% in the West region in 201522. Geographic differences in CnSKP prevalence were also reported in the U.S.23. While an increase of 1.7-fold over four years in this study is not so impressive, the prevalence of CnSKP in the TNIS increased 15.7-fold from 1.8% in 2005 to 28.2% in 201722. Additionally, this study showed that the rates of CPKP and non-CP-CnSKP among CnSKP increased and decreased annually, respectively. The increasing prevalence of CnSKP is regarded as a result of CPKP dissemination.
This study found that the majority of CnSKP in Taiwan were isolated from sputum and urine, which is compatible with other surveys24,25. Our study also showed that the incidence of bacteremia increased nearly 2-fold within the four years studied. A similar increase in the incidence of bacteremia caused by CnSKP was also observed in a large teaching hospital in northern Italy26. Although K. pneumoniae is the main cause of liver abscess in Taiwan, those isolates are usually community-acquired and rarely reported to be resistant1. However, liver abscess caused by carbapenem-resistant Klebsiella pneumoniae was reported in a liver transplant patient in Italy27. Moreover, 1.2% (2/165) of K. pneumoniae isolates causing liver abscess were resistant to carbapenem in a China hospital during 2010–201428. The barrier between community-acquired liver abscess caused by susceptible K. pneumoniae and hospital-acquired infection by resistant isolates is becoming blurred as resistance is becoming increasingly prevalent.
The rate of CPKP among CnSKP increased annually in Taiwan. A similar trend was also observed in other surveys, which showed a rate of 3.1% between 2010 and 201224 that increased to 20.0% in 201325. While the highest rate in Taiwan was 70.3% in the West region in 2015, rates as high as 89.9% (113/126) and 86.7% (65/75) of CnSKP isolates were identified as CPKP in the U.S.10 and Romania29, respectively.
Most of the CnSKP in this study were nonsusceptible to all β-lactams and fluoroquinolones; however, some non-β-lactams with low nonsusceptibility rates, such as tigecycline30, colistin31, and aminoglycosides32, may represent potential treatment choices. From an epidemiological study of carbapenem-resistant Enterobacteriaceae in U.S.23, low nonsusceptibility rates against tigecycline (11.2%), colistin (25.0%) and at least one aminoglycoside (18.3%) were similarly observed.
Different profiles of antimicrobial nonsusceptibility were found between CPKP and non-CP-CnSKP in this study, as CPKP was more nonsusceptible to β-lactams than non-CP-CnSKP. Few studies have compared the resistance profiles between these two groups of clinical isolates; however, a study on carbapenem-resistant Enterobacteriaceae demonstrated that carbapenemase-producing isolates were more resistant to meropenem than their non-carbapenemase-producing counterparts33.
Our study showed that CPKP was significantly increasingly nonsusceptible to fluoroquinolones and aminoglycosides, while non-CP-CnSKP showed greater nonsusceptibility to doripenem and meropenem, during the study period. Few studies have examined this trend specifically in these resistant bacteria; however, a similar trend for increasing resistance of general K. pneumoniae isolates was found in national surveillance studies from Taiwan34 and Korea35.
The coproduction of dual carbapenemases by K. pneumoniae has been reported sporadically36,37. Many combinations have been identified, including KPC/MLB and OXA/MLB7; however, the KPC-2/OXA-48 coproducing isolates found in this study have not been reported before to the best of our knowledge. Furthermore, these dual carbapenemase producers belonged to the epidemic ST11 clone. Because of their high resistance profile and transferability, these resistant organisms warrant intensive monitoring.
Since it was first reported in 20018, KPC has spread worldwide38. This study showed that KPC outnumbered previous endemic MBL and became the most common carbapenemase in Taiwan soon after its arrival, spreading to all regions of this island. Such rapid dissemination of KPC-KP was also observed in Greece39 and Italy40. We previously sequenced the complete Taiwanese plasmid encoding KPC-2 and analyzed its high transferability41. In this study, we identified one novel blaKPC variant, namely, blaKPC-34, and two that had not been reported in Taiwan before 2012. The first KPC-3 producer was isolated in 2013 and was geographically and genetically linked to those in the U.S.42, and the emergence of a KPC-17 producer in Taiwan was also previously observed25,43. Similar to the U.S.10 and China11, KPC was the predominant carbapenemase in Taiwan; however, most of the Taiwanese KPC-producers were ST11, which is similar to China44, but different from the U.S., where the dominant clone is ST25845. ST11 is a single-locus variant of ST258, indicating their close relationship46. However, our previous study showed that K. pneumoniae ST23 strains are strongly associated with liver abscess in Asian countries47. Our study results indicated that these hospital-acquired resistant isolates are quite different from their susceptible counterparts causing community-acquired liver abscess.
After its emergence in 2013, OXA-48 became the second most prevalent type of carbapenemase two years later. Four OXA-48 producers were isolated in 2013 from patients without a travel history abroad48. Most of the OXA-48 producers in this study were the endemic ST11 clone, while ST101 was the most prevalent clone in Europe and North Africa49. The OXA-48 ST11 clone caused an outbreak in Spain in 200947; however, the plasmids of the Taiwanese and Spanish clones belonged to different incompatibility groups48,50.
While this extensive surveillance study involved all regions in Taiwan, not all carbapenemase types were identified during the study period. In a previous study, an isolate producing KPC-16 was reported in the South region in 201443. Additionally, all isolates were screened by PCR in this study, which is consistent with many previous studies4,24,25. However, some emerging yet unidentified carbapenemases might be missed by this method. Thus, phenotypic methods, such as Carba NP, the carbapenem inactivation method (CIM), and the modified Hodge test (MHT), are mandatory to identify novel carbapenemases51. Moreover, these CnSKP will be assessed using virulence assay, as hypervirulent strains have been reported in China recently52.
In conclusion, the current CnSKP, and especially CPKP, scenario in Taiwan is ominous. New types of CPKP have emerged, and these resistant bacteria have become increasingly nonsusceptible to particular antimicrobials, especially aztreonam, fluoroquinolones, trimethoprim-sulfamethoxazole, gentamicin, amikacin, and colistin over time. This increase would make the treatment of infections caused by CnSKP more difficult. Close monitoring and enhanced infection control measures are mandatory to curb the further spread of these highly resistant organisms.
Materials and Methods
Study Area and Hospital Settings
Between January 2012 and August 2015, 21 hospitals in Taiwan were enrolled in this study, including 7 in the North, 4 in the West, 7 in the South, and 3 in the East region. Nine of these hospitals are regional hospitals, while 12 are medical centers that provide tertiary care; each of the included hospitals contains more than 1,000 beds. This study was approved by the Institutional Review Boards of participating hospitals, including Chang Gung Memorial Hospital (IRB No.: 1003399B), Taipei Veterans General Hospital (VGHIRB No.: 2011-11-001IC), National Taiwan University Hospital (IRB No.: 20111004 3RB), Tri-Service General Hospital (IRB No.: 100-05-205), Kaohsiung Medical University Chung-Ho Memorial Hospital (KMUH-IRB-20110328), Chi- Mei Medical Center (IRB No.: 10012-001), China Medical University Hospital (CMUH IRB No.: DMR100-IRB-214) and Kaohsiung Armed Forces General Hospital (IRB No.: 100-076). The IRBs waived the need for informed consents from source patients of the enrolled bacterial isolates because the isolates were obtained as part of routine hospital care procedures, and involved very minimal risk to the source patients; this waiver does not adversely affect the rights and welfare of the source patients.
Nonduplicate K. pneumoniae isolates collected from various sites in adult patients were tested for susceptibility to carbapenems at the participating hospitals, as part of routine laboratory procedures. Preliminary isolates that were nonsusceptible to imipenem or/and meropenem were sent to a reference laboratory at the National Health Research Institutes, Miaoli, Taiwan. After species identification was confirmed with a VITEK 2 automated system (bioMérieux, Marcy l’ Etoile, France), the isolates were stored at −70 °C until further testing. All experimental procedures were performed in accordance with specified guidelines for the use of studied isolates, and were approved by the Institutional Biosafety Committee of Chi Mei Medical Center.
Antimicrobial Susceptibility Testing
All preliminary isolates were tested for minimum inhibitory concentrations (MICs) for β-lactam agents [including penicillin (piperacillin-tazobactam), cephalosporins (cefazolin, cefoxitin, cefuroxime, cefotaxime, ceftazidime, and cefepime), carbapenems (doripenem, ertapenem, imipenem, and meropenem), and a monobactam (aztreonam)] and non-β-lactams [including fluoroquinolones (ciprofloxacin and levofloxacin), aminoglycosides (gentamicin and amikacin), trimethoprim-sulfamethoxazole, colistin, and tigecycline]. The MIC for tigecycline was determined with the E-test (AB Biodisk, Solna, Sweden) on Mueller-Hinton media, and the MICs for other agents were determined using the broth microdilution method (Sensititre, Trek Diagnostic Systems, Cleveland, OH, USA). Isolates with MICs of >1 μg/mL for imipenem or/and meropenem were defined as CnSKP. Susceptibilities to colistin and tigecycline were determined based on the European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines53, while susceptibility to other agents was based on updated guidelines from the Clinical & Laboratory Standards Institute (CLSI)54.
Detection of Carbapenemase Genes
All verified CnSKP isolates were subjected to polymerase chain reaction (PCR) detection of genes encoding carbapenemases, including class A (blaKPC, blaGES, blaIMI, blaNMC, blaSME), class B (blaIMP, blaVIM, blaNDM, blaGIM, blaSIM, blaSPM), and class D (blaOXA-48), using the primers described previously 4. The amplicons were further sequenced to identify the molecular type. Isolates positive for the carbapenemase gene were defined as CPKP, and those that were negative were defined as non-CP-CnSKP.
Multilocus Sequence Typing
Multilocus sequence typing (MLST) was performed on all isolates with a single carbapenemase gene according to the protocol described on the K. pneumoniae MLST website (http://www.pasteur.fr/recherche/genopole/PF8/mlst/Kpneumonia.html). MLST results were typed according to the international database created in 2005 at the Pasteur Institute in Paris, France.
Statistical Analyses
SPSS version 17.0 (SPSS, Chicago, IL, USA) was used to perform statistical analyses. The chi-square test was applied for categorical variables, and linear-by-linear association was used to analyze trends (chi-square for trend). A p value of < 0.05 was considered statistically significant.
References
Siu, L. K., Yeh, K. M., Lin, J. C., Fung, C. P. & Chang, F. Y. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect. Dis. 12, 881–887, https://doi.org/10.1016/s1473-3099(12)70205-0 (2012).
Kong, K. F., Schneper, L. & Mathee, K. Beta-lactam antibiotics: from antibiosis to resistance and bacteriology. APMIS. 118, 1–36, https://doi.org/10.1111/j.1600-0463.2009.02563.x (2010).
Papp-Wallace, K. M., Endimiani, A., Taracila, M. A. & Bonomo, R. A. Carbapenems: past, present, and future. Antimicrob. Agents Chemother. 55, 4943–4960, https://doi.org/10.1128/aac.00296-11 (2011).
Chiu, S. K. et al. National surveillance study on carbapenem non-susceptible Klebsiella pneumoniae in Taiwan: the emergence and rapid dissemination of KPC-2 carbapenemase. PloS. One 8, e69428, https://doi.org/10.1371/journal.pone.0069428 (2013).
Borer, A. et al. Attributable mortality rate for carbapenem-resistant Klebsiella pneumoniae bacteremia. Infect. Control Hosp. Epidemiol. 30, 972–976, https://doi.org/10.1086/605922 (2009).
Queenan, A. M. & Bush, K. Carbapenemases: the versatile beta-lactamases. Clin. Microbiol. Rev. 20, 440–458, https://doi.org/10.1128/cmr.00001-07 (2007).
Lee, C. R. et al. Global Dissemination of Carbapenemase-Producing Klebsiella pneumoniae: Epidemiology, Genetic Context, Treatment Options, and DetectionMethods. Front. Microbiol. 7, 895, https://doi.org/10.3389/fmicb.2016.00895 (2016).
Yigit, H. et al. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 45, 1151–1161, https://doi.org/10.1128/AAC.45.4.1151-1161.2001 (2001).
Bratu, S. et al. Rapid spread of carbapenem-resistant Klebsiella pneumoniae in New York City: a new threat to our antibiotic armamentarium. Arch. Intern. Med. 165, 1430–1435, https://doi.org/10.1001/archinte.165.12.1430 (2005).
Kaiser, R. M., Castanheira, M., Jones, R. N., Tenover, F. & Lynfield, R. Trends in Klebsiella pneumoniae carbapenemase-positive K. pneumoniae in US hospitals: report from the 2007-2009 SENTRY Antimicrobial Surveillance Program. Diagn. Microbiol. Infect. Dis. 76, 356–360, https://doi.org/10.1016/j.diagmicrobio.2013.03.032 (2013).
Li, H. et al. Molecular characteristics of carbapenemase-producing Enterobacteriaceae in China from 2008 to 2011: predominance of KPC-2 enzyme. Diagn. Microbiol. Infect. Dis. 78, 63–65, https://doi.org/10.1016/j.diagmicrobio.2013.10.002 (2014).
Poirel, L., Heritier, C., Tolun, V. & Nordmann, P. Emergence of oxacillinase-mediated resistance to imipenem in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 48, 15–22 (2004).
Nordmann, P. & Poirel, L. The difficult-to-control spread of carbapenemase producers among Enterobacteriaceae worldwide. Clin. Microbiol. Infect. 20, 821–830, https://doi.org/10.1111/1469-0691.12719 (2014).
Palacios-Baena, Z. R. et al. Comprehensive clinical and epidemiological assessment of colonisation and infection due to carbapenemase-producing Enterobacteriaceae in Spain. J. Infect. 72, 152–160, https://doi.org/10.1016/j.jinf.2015.10.008 (2016).
Yong, D. et al. Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob. Agents Chemother. 53, 5046–5054, https://doi.org/10.1128/aac.00774-09 (2009).
Kazi, M. et al. Molecular characterization of carbapenem-resistant Enterobacteriaceae at a tertiary care laboratory in Mumbai. Eur. J. Clin. Microbiol. Infect. Dis. 34, 467–472, https://doi.org/10.1007/s10096-014-2249-x (2015).
Yan, J. J., Ko, W. C. & Wu, J. J. Identification of a plasmid encoding SHV-12, TEM-1, and a variant of IMP-2 metallo-beta-lactamase, IMP-8, from a clinical isolate of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 45, 2368–2371, https://doi.org/10.1128/aac.45.8.2368-2371.2001 (2001).
Ma, L., Lu, P. L., Siu, L. K. & Hsieh, M. H. Molecular typing and resistance mechanisms of imipenem-non-susceptible Klebsiella pneumoniae in Taiwan: results from the Taiwan surveillance of antibiotic resistance (TSAR) study, 2002–2009. J. Med. Microbiol. 62, 101–107, https://doi.org/10.1099/jmm.0.050492-0 (2013).
Wu, H. S. et al. First identification of a patient colonized with Klebsiella pneumoniae carrying blaNDM-1 in Taiwan. J. Chin. Med. Assoc. 73, 596-598, doi:S1726-4901(10)70129-5 [pii]10.1016/S1726-4901(10)70129-5 (2010).
Chung, K. P. et al. Arrival of Klebsiella pneumoniae carbapenemase (KPC)-2 in Taiwan. J. Antimicrob. Chemother. 66, 1182–1184, https://doi.org/10.1093/jac/dkr025 (2011).
Lee, C. M. et al. Outbreak of Klebsiella pneumoniae carbapenemase-2-producing K. pneumoniae sequence type 11 in Taiwan in 2011. Antimicrob. Agents Chemother. 56, 5016–5022, https://doi.org/10.1128/AAC.00878-12 (2012).
Taiwan Centers for Disease Control. Annual report of Taiwan Nosocomial Infection Surveillance Systems. http://www.cdc.gov.tw/professional/list.aspx?treeid = beac9c103df952c4&nowtreeid = 4d41d91ffd50d559 (2015).
Guh, A. Y. et al. Epidemiology of Carbapenem-Resistant Enterobacteriaceae in 7 US Communities, 2012–2013. Jama. 314, 1479–1487, https://doi.org/10.1001/jama.2015.12480 (2015).
Wang, J. T. et al. Carbapenem-nonsusceptible Enterobacteriaceae in Taiwan. PloS. one 10, e0121668, https://doi.org/10.1371/journal.pone.0121668 (2015).
Tseng, I. L. et al. Emergence of carbapenemase producing Klebsiella pneumonia and spread of KPC-2 and KPC-17 in Taiwan: a nationwide study from 2011 to 2013. PloS. One 10, e0138471, https://doi.org/10.1371/journal.pone.0138471 (2015).
Alicino, C. et al. Trends in the annual incidence of carbapenem-resistant Klebsiella pneumoniae bloodstream infections: a 8-year retrospective study in a large teaching hospital in northern Italy. BMC. Infect. Dis. 15, 415, https://doi.org/10.1186/s12879-015-1152-0 (2015).
Arena, F. et al. Infections caused by carbapenem-resistant Klebsiella pneumoniae with hypermucoviscous phenotype: A case report and literature review. Virulence 8, 1900–1908, https://doi.org/10.1080/21505594.2017.1286439 (2017).
Kong, H., Yu, F., Zhang, W. & Li, X. Clinical and microbiological characteristics of pyogenic liver abscess in a tertiary hospital in East China. Medicine (Baltimore) 96, e8050, https://doi.org/10.1097/MD.0000000000008050 (2017).
Lixandru, B. E. et al. Carbapenemase-Producing Klebsiella pneumoniae in Romania: A Six-Month Survey. PloS. One 10, e0143214, https://doi.org/10.1371/journal.pone.0143214 (2015).
Tasina, E., Haidich, A. B., Kokkali, S. & Arvanitidou, M. Efficacy and safety of tigecycline for the treatment of infectious diseases: a meta-analysis. Lancet Infect. Dis. 11, 834–844, https://doi.org/10.1016/s1473-3099(11)70177-3 (2011).
Li, J. et al. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect. Dis. 6, 589–601, https://doi.org/10.1016/s1473-3099(06)70580-1 (2006).
Shields, R. K., Clancy, C. J., Press, E. G. & Nguyen, M. H. Aminoglycosides for Treatment of Bacteremia Due to Carbapenem-Resistant Klebsiella pneumoniae. Antimicrob. Agents Chemother. 60, 3187–3192, https://doi.org/10.1128/aac.02638-15 (2016).
Tamma, P. D. et al. Comparing the Outcomes of Patients With Carbapenemase-Producing and Non-Carbapenemase-Producing Carbapenem-Resistant Enterobacteriaceae Bacteremia. Clin. Infect. Dis. 64, 257–264, https://doi.org/10.1093/cid/ciw741 (2017).
Lin, W. P. et al. The Antimicrobial Susceptibility of Klebsiella pneumoniae from Community Settings in Taiwan, a Trend Analysis. Sci. Rep. 6, 36280, https://doi.org/10.1038/srep36280 (2016).
Kim, D. et al. Increasing Resistance to Extended-Spectrum Cephalosporins, Fluoroquinolone, and Carbapenem in Gram-Negative Bacilli and the Emergence of Carbapenem Non-Susceptibility in Klebsiella pneumoniae: Analysis of Korean Antimicrobial Resistance Monitoring System (KARMS) Data From 2013 to 2015. Ann. Lab. Med. 37, 231–239, https://doi.org/10.3343/alm.2017.37.3.231 (2017).
Doi, Y. et al. Co-production of NDM-1 and OXA-232 by Klebsiella pneumoniae. Emerg. Infect. Dis. 20, 163–165, https://doi.org/10.3201/eid2001.130904 (2014).
Cho, S. Y. et al. Klebsiella pneumoniae co-producing NDM-5 and OXA-181 carbapenemases, South Korea. Emerg. Infect. Dis. 21, 1088–1089, https://doi.org/10.3201/eid2106.150048 (2015).
Munoz-Price, L. S. et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect. Dis. 13, 785–796, https://doi.org/10.1016/s1473-3099(13)70190-7 (2013).
Spyropoulou, A. et al. A ten-year surveillance study of carbapenemase-producing Klebsiella pneumoniae in a tertiary care Greek university hospital: predominance of KPC- over VIM- or NDM-producing isolates. J. Med. Microbiol. 65, 240–246, https://doi.org/10.1099/jmm.0.000217 (2016).
Calia, C. et al. Carbapenemases-producing Klebsiella pneumoniae in hospitals of two regions of Southern Italy. APMIS. 125, 491–498, https://doi.org/10.1111/apm.12666 (2017).
Chen, Y. T. et al. KPC-2-encoding plasmids from Escherichia coli and Klebsiella pneumoniae in Taiwan. J. Antimicrob. Chemother. 69, 628–631, https://doi.org/10.1093/jac/dkt409 (2014).
Tang, H. J. et al. Identification of the first imported KPC-3 Klebsiella pneumoniae from the USA to Taiwan. Int. J. Antimicrob. Agents 44, 431–435, https://doi.org/10.1016/j.ijantimicag.2014.07.009 (2014).
Yu, W. L. et al. Emergence of KPC new variants (KPC-16 and KPC-17) and ongoing outbreak in southern Taiwan. Clin. Microbiol. Infect. 21(347), e345–348, https://doi.org/10.1016/j.cmi.2014.11.030 (2015).
Qi, Y. et al. ST11, the dominant clone of KPC-producing Klebsiella pneumoniae in China. J. Antimicrob. Chemother. 66, 307–312, https://doi.org/10.1093/jac/dkq431 (2011).
Kitchel, B. et al. Molecular epidemiology of KPC-producing Klebsiella pneumoniae isolates in the United States: clonal expansion of multilocus sequence type 258. Antimicrob. Agents Chemother. 53, 3365–3370, https://doi.org/10.1128/aac.00126-09 (2009).
Chen, L., Mathema, B., Pitout, J. D., DeLeo, F. R. & Kreiswirth, B. N. Epidemic Klebsiella pneumoniae ST258 is a hybrid strain. MBio. 5, e01355–01314, https://doi.org/10.1128/mBio.01355-14 (2014).
Siu, L. K. et al. Molecular typing and virulence analysis of serotype K1 Klebsiella pneumoniae strains isolated from liver abscess patients and stool samples from noninfectious subjects in Hong Kong, Singapore, and Taiwan. J Clin Microbiol 49, 3761–3765, https://doi.org/10.1128/JCM.00977-11 (2011).
Ma, L. et al. Emergence of OXA-48-Producing Klebsiella pneumoniae in Taiwan. PloS. one 10, e0139152, https://doi.org/10.1371/journal.pone.0139152 (2015).
Potron, A., Poirel, L., Rondinaud, E. & Nordmann, P. Intercontinental spread of OXA-48 beta-lactamase-producing Enterobacteriaceae over a 11-year period, 2001 to 2011. Euro. Surveill. 18 (2013).
Branas, P. et al. Molecular epidemiology of carbapenemase-producing Klebsiella pneumoniae in a hospital in Madrid: Successful establishment of an OXA-48 ST11 clone. Int. J. Antimicrob. Agents 46, 111–116, https://doi.org/10.1016/j.ijantimicag.2015.02.019 (2015).
Lutgring, J. D. & Limbago, B. M. The Problem of Carbapenemase-Producing-Carbapenem-Resistant-Enterobacteriaceae Detection. J. Clin. Microbiol. 54, 529–534, https://doi.org/10.1128/jcm.02771-15 (2016).
Gu, D. et al. A fatal outbreak of ST11 carbapenem-resistant hypervirulent Klebsiella pneumoniae in a Chinese hospital: a molecular epidemiological study. Lancet Infect Dis 18, 37–46, https://doi.org/10.1016/S1473-3099(17)30489-9 (2018).
European Committee on Antimicrobial Susceptibilities Testing. Breakpoint tables for interpretation of MICs and zone diameters. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_7.1_Breakpoint_Tables.pdf 2017.
Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing (M100-S26). (CLSI, 2016).
Acknowledgements
We thank the investigators at the participating hospitals for their assistance in this study. This study was supported by research grants from the Ministry of Science and Technology, R.O.C. (MOST 103-2314-B-016-020-MY3) and Taiwan Centers for Disease Control (MOHW 104-CDC-C-114-144406).
Author information
Authors and Affiliations
Contributions
S.K.C., C.P.F., T.L.W., Y.C.C., J.C.L., P.L.L., J.T.W. and K.M.Y. conceived and designed the study; L.M. and P.L.L. performed the susceptibility test of the isolates; L.M., T.L.W. and P.L.L. performed the detection of the carbapenemase genes by PCR. Y.C.C., Y.T.L., J.C.L., P.L.L. and C.P.F. planned and coordinated the activities of the Study Group of Carbapenem Resistance in Klebsiella pneumoniae; S.K.C., L.M. and Y.T.L. entered and analyzed the data; S.K.C., M.C.C. and K.M.Y. wrote the paper; all authors revised and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chiu, SK., Ma, L., Chan, MC. et al. Carbapenem Nonsusceptible Klebsiella pneumoniae in Taiwan: Dissemination and Increasing Resistance of Carbapenemase Producers During 2012–2015. Sci Rep 8, 8468 (2018). https://doi.org/10.1038/s41598-018-26691-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-26691-z
This article is cited by
-
Characterization of resistance mechanisms of Enterobacter cloacae Complex co-resistant to carbapenem and colistin
BMC Microbiology (2021)
-
Increasing New Delhi metallo-β-lactamase-positive Escherichia coli among carbapenem non-susceptible Enterobacteriaceae in Taiwan during 2016 to 2018
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.