Abstract
A broad panel of tens of thousands of microsatellite loci is unveiled for an endangered piracema (i.e. migratory) South American fish, Brycon orbignyanus. Once one of the main fisheries resources in the Platine Basin, it is now almost extinct in nature and focus of intense aquaculture activity. A total of 178.2 million paired-end reads (90 bases long) were obtained through the use of sequencing-by-synthesis (from a primary genomic library of 500 bp DNA fragments) and is made available through NCBI’s Sequence Read Archive, SRA accession SRX3350440. Short reads were assembled de novo and screening for perfect microsatellite motifs revealed more than 81 thousands unique microsatellite loci, for which primer pairs were proposed. A total of 29 polymorphic microsatellite markers were already previously validated for this panel. A partial genomic assembly is hereby presented and these genomic resources are publicly made available. These data will foster the rapid development of hundreds of new DNA markers for genetic diversity studies, conservation initiatives and management practices for this important and depleted species. The availability of such preliminary genomic data will also be of use in the areas of bioinformatics, ecology, genetics and evolution.
Similar content being viewed by others
Introduction
Piracema is the seasonal upstream reproductive migratory run undergone by millions of medium and large-sized Neotropical freshwater fish, from different species in South America1. These potamodromous species are heavily impacted by pollution, loosely regulated fisheries, species introduction, suppression of riverine vegetation and an intense hydroelectric exploitation regime in many parts of the Platine Basin, the second largest in the continent2, encompassing Argentina, Bolivia, Brazil, Paraguay and Uruguay. The successive impoundment of its major rivers lead to deleterious effects across the ichthyofauna: habitat loss (e.g. seasonal floodplain lagoons used as natural rearing environments for juveniles), altered landscapes, flood regimes and fish communities. Most conspicuously for migratory species, there is a potential for heavy fragmentation, impeding the long migrations characteristic from the piracema runs3.
Brycon orbignyanus (Valenciennes, 1850) is a threatened piracema fish from the Platine Basin. It is a medium-sized species with insectivorous-omnivorous dietary habits affected by pollution, deforestation of riparian vegetation, overfishing and damming4. Once one of the most prominent fisheries resources in the Platine Basin, it is now almost extinct in the wild, being listed as endangered in the Red book of Brazilian fauna threatened with extinction5. Extant populations have been presumably maintained mostly by means of broodstocking practices by environmental hatchery operations, which constitute one of the main mitigation efforts to counteract stocks decline, despite the traditional absence of proper planning and clear definition of goals6. These practices still lack adequate efficiency evaluations in the area, as demonstrated elsewhere7 and have historically ignored genetic guidelines to reduce inbreeding, random genetic drift, selection to captivity and other potential relevant genetic effects8. The shortfall of a deeper understanding of this species’ genetic structure (despite some first efforts9) hampers the goal-oriented planning of strategies for effective broodstoking initiatives in B. orbignyanus. Thus, the rapid development of molecular markers as tools for the investigation and management of these fish stocks is urgent.
The innovations in DNA sequencing methods during the first decade of the 21st Century have catapulted the de novo development of molecular markers for non-model species to new peaks10. The first microsatellite loci described for this species were recently unveiled11, with empirically validated data from the genomic resources first full and publicly presented herein. Therefore, we hereby unveil a broad panel of potentially amplifiable characterized microsatellite loci in the endangered piracema fish Brycon orbignyanus.
Results
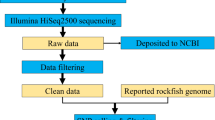
A total of 16.04 Gb (i.e. gigabases or 1 × 109 DNA bases) of filtered data was obtained, represented in 178,212,428 paired-end reads, 90 bases long, grouped in two parallel FASTQ files (Supplementary Data S1 stored at NCBI’s Sequence Read Archive - SRA - https://www.ncbi.nlm.nih.gov/sra/SRX3350440). The quality scoring system was the Illumina 1.5, which uses Phred +64 scheme, with characters ranging from “@” to “j”. A total of 97.73% of the data showed a quality value of Q ≥ 20, and the average quality per read was around Q = 38. The GC content of these reads was 41.11%.
A potential amplifiable microsatellite panel was produced for B. orbignyanus and made available through the Figshare online data repository (Supplementary Table S2 - https://doi.org/10.6084/m9.figshare.5661988). It consists of a genome-wide characterization table, with 81,241 unique perfect simple sequence repeat loci (di- through hexanucleotides) and shows, among other information, the locus ID (Borb#), microsatellite motif, candidate forward and reverse primers, expected PCR (Polymerase Chain Reaction) products and position over two alternative de novo assemblies. Given the lack of the original assembly A0 (Assembly 0), made by service provider BGI (see Methods), we resorted to build alternative assemblies with the filtered short reads and arbitrarily settled with A1 (Assembly 1), created with k-mer = 55 (Supplementary Data S3 - accessible through Figshare - https://doi.org/10.6084/m9.figshare.5661802). The known PCR products expected from the missing assembly A0 were mapped back to A1. The average estimated PCR product length was 146.2 (±21.5) bp. Motif abundance is described in detail in Table 1. Dinucleotides were almost three times more abundant than all the other motif classes together. This panel permitted the rapid empiric validation of the first 29 polymorphic microsatellite markers for B. orbignyanus, out of 50 assayed candidates (Borb01-Borb50) and these results alone were previously published11.
The final size of the genomic assembly A1 is 1,113,754,917 bp (including unknown base calls and gaps, N) and 1,039,212,289 bp (not counting Ns – Δ = 74,542,628). A total of 1,273,306 contigs or scaffolds were obtained, the shortest being 100 bp and the longest 172,138 bp (average = 874). Only 55 scaffolds were longer than 100 kbp; 1.97% longer than 10 kbp; 11% longer than 1 kbps and 18.09% of 500 bp or more. The A1 assembly had the CG content of 41.18% and N50 = 8,463.
We examined all expected PCR product sequences from the target microsatellite loci determined from the A0 assembly, using BLAST (and then using SWIPE, if it failed to retrieve a match) against the resulting A1 assembly. Still missing A0 microsatellite loci were then searched in A1, using forward and reverse primers as queries, with SWIPE. This procedure allowed us to retrieve and locate 97.51% of loci discovered in A0 back to A1. These results are detailed in Table 2, with exact, partial or missing hits. A total of 2,025 loci from A0 could not be accounted for in A1 according to our criteria, including three previous empirically validated loci (Borb13, Borb34 and Borb35)11.
Mapping the short reads onto the A1 assembly resulted in a paired-end BAM file with 108,435,142 alignments, all being mapped end-to-end and properly paired (i.e. within the same scaffold, showing the expected orientation and being separated 500 bp or less). This alignment has a mean coverage per contig of 11.37 (±5.77). A second BAM file containing exclusively singleton alignments, average depth of coverage 5.8 (±18), was also produced. Both files were combined into a single run at SRA (Supplementary Data S4 - https://www.ncbi.nlm.nih.gov/sra/SRX3427716).
Discussion
The results presented here provide the first genomic resources for B. orbignyanus and the Bryconidae family available to the scientific community. The sequencing data were considered of high quality (i.e. the average probability of a wrong base call is less than one in 16,000). It will possibly find applications in bioinformatics, where bioinformaticians need to test algorithms and pipelines with real-world data sets, in evolutionary comparative studies and in the description of protein coding genes from this species. It also provides a departing point for the complete genome characterization12 in this Neotropical fish. Our main goal achieved here, nevertheless, was to provide a broad resource for rapid microsatellite development, so hundreds of markers can be promptly made available to the conservation and aquaculture initiatives for this threatened migratory species. More thorough molecular diversity surveys will allow, for instance, the urgent assessment whether this species is critically impoverished genetically, for it seems to exhibit sudden population booms in adequate environmental conditions9, it has recently diminished in certain parts of the Platine Basin and it has likely experienced several bottlenecks due to broodstocking practices. All these scenarios favour strong action of random genetic drift and thus, given this species’ delicate conservation status, it inspires a strong need for focused and intensive studies and directed actions over its potential low genetic variability.
Since we lacked the original service provider’s version of the genomic assembly (A0), we were satisfied with a close but not exactly similar alternative de novo assembly (A1), because possible differences in program version used by BGI and our group, along any eventual undisclosed setting of parameters or pipelines would result in slightly divergent genomic assembly outcomes. We were able to successfully track down more than 95% of PCR products inferred from A0 back to genomic assembly A1, with sequence similarity of 90% or more. Unfortunately, around 2.5% of the loci found in the A0 assembly could not be accounted for in A1, according to our criteria, including three previous empirically validated loci11. We feel this justifies the salvaging of the unmapped loci from A0 in our final panel (as opposed to their elimination from it), since some of these missing potentially amplifiable loci can knowingly lead to valid PCR results.
The BAM files made available herein constitute a possible departure point for the description of new genes (including those associated with trinucleotide repeats), structural variants and molecular markers in this threatened species and can be used as reference for each individual microsatellite locus selected by other researchers for future validation and development, from this panel. Despite recent promising next-generation sequencing based approaches for surveying genetic diversity13,14,15,16, traditional microsatellite and other PCR-based marker analyses will still contribute as useful, quick and simple genetic tools to be easily applied17 at hatchery and conservation initiatives for B. orbignyanus.
This work produced genome-wide resources able to contribute to the rapid and cost-effective development of hundreds of new microsatellite markers, whilst accumulating the first partial genomic data for B. orbignyanus. It will certainly foster research, aquaculture and conservation for this species and will likely find application to other diverse areas of biology, evolution and comparative studies.
Methods
Specimen collection and DNA extraction
A single individual was used for whole-genome shotgun sequencing. A mature female specimen was captured in the Volta Grande Environmental Station Hatchery, Grande River, MG, Brazil (−20.026197, −48.220430). The fish was euthanized and frozen at around 0 °C, on ice. It was then transported to the laboratory and maintained under −20 °C refrigeration, wrapped in aluminium foil, until DNA extraction. The specimen was thawed at 4 °C overnight and a longitudinal abdominal incision was made with a sterile scalpel for the collection of muscle tissue and gonadal confirmation of the specimen’s sex. This individual is kept as Voucher 120457 from the DNA Bank at the Laboratório de Recursos Genéticos at Universidade Federal de São João Del Rei, LARGE-UFSJ, accessible through www.ufsj.edu.br/recgenlab. The specimen was collected with SISBIO license number 37222-2, following UFSJ’s ethical committee guidelines for animal research (CEUA-UFSJ: Comissão de Ética no Uso de Animais, Process 27/2011). No experiments were performed on live specimens.
Total genomic DNA was extracted from ≈2g of muscle tissue using Wizard Genomic DNA Purification Kit (Promega, Fitchburg, USA). A total of 6.06 μg of good quality DNA was obtained, verified in 1% agarose gel electrophoresis, as a single clear band around 15 kb, only slightly degraded, with a concentration of 233 μg/μl, quantified in a Qubit fluorometer. This material was shipped to service providers for downstream treatment.
Library preparation and sequencing
Library construction, sequencing and first bioinformatics analyses were conducted under the auspices of BGI, Hong Kong/Síntese Biotecnologia, Belo Horizonte, Brazil. Contracted services involved the construction of a single genomic library, sequencing, delivery of raw data and bioinformatics microsatellite search. The short fragments library was constructed according to BGI’s in-house protocols, with random DNA fragmentation and agarose gel purification for genomic fragments with nominal size of 500 bp, followed by adaptor ligation and linear amplification, for the enrichment of fragments with adaptors at both ends. Sequencing was performed over approximately 50% of a single lane from a flow-cell in a HiSeq 2000 equipment (Illumina, San Diego, USA). Sequencing run was January 01, 2014. The library was sequenced targeting an average of 10× coverage depth, for a genome estimated, a priori, to be 1.5 Gb. The sequencing assay layout was paired-end with reads 90 bases long.
Bioinformatics
Service providers performed a bioinformatics pipeline and produced a first table of microsatellite loci and the raw (filtered) short reads data, but delivered no genomic assembly (as per contract), here called A0 (not available): at BGI, short reads were demultiplexed, trimmed for adaptors and filtered by removing reads with quality rate values of Q ≤ 5 in 50% or more bases, and deleting duplicates. The resulting paired-end data were stored in two parallel FASTQ files and used by BGI for de novo assembly based on de Bruijn graph using SOAPdenovo 218, with k-mer = 47. The resulting assembly (A0) was screened for perfect simple sequence repeats (microsatellite loci), with repeat motifs ranging from di- through hexanucleotides, with a minimum of five repetitions, except for hexanucleotides which had the minimum number of repeats parameter set as four, with the aid of the SSRIT program19. The microsatellite loci sequences found were targeted for primer design with Primer320. Primers were aligned with A0 (using SOAPaligner - http://soap.genomics.org.cn/soapaligner.html), for the retaining of exclusively unique hits. From these results service providers produced a single comprehensive table with characterized microsatellite loci, proposed primers and expected PCR products.
Given the absence of BGI’s assembly (A0), we subsequently evaluated our own de novo genomic assembly from the same paired-end short reads, Assembly 1 (A1). We examined it for the presence of partial or exact matches for the microsatellite loci revealed from A0 (represented as expected PCR products retrieved from BGI’s table). We performed the assembly using SOAPdenovo 2 (version 2.04), with default parameters, using k-mer = 55, with the computer cluster at PPGF-UFSJ. We proceeded using BLAST21, querying expected PCR products from A0 onto the available A1 assembly. Missing hits were then further searched against A1, using SWIPE22. Finally, still unaccounted loci were searched with SWIPE, using forward and reverse primers as independent queries, withholding results where primer pairs were fully detected in the same contig/scaffold. Post-assembly short read alignment with A1 was conducted using SOAPaligner. The resulting SAM files were converted to BAM and analysed with SAMtools23 and Tablet24. With the joint results from BGI’s table and A1 we produced a final comprehensive panel of genome-wide potentially amplifiable microsatellite loci for B. orbignyanus.
Data availability
The genomic data revealed here are now publicly available from NCBI’s Sequence Read Archive (SRA - https://www.ncbi.nlm.nih.gov/sra) database. Short reads (Supplementary Data S1) can be fetched under Accession https://www.ncbi.nlm.nih.gov/sra/SRX3350440. Supplementary Data S4 with the BAM alignments (made using the genomic assembly A1 as reference) can be retrieved from https://www.ncbi.nlm.nih.gov/sra/SRX3427716.
The proposed genome-wide microsatellite panel for B. orbignyanus (Supplementary Table S2 - https://doi.org/10.6084/m9.figshare.5661988) in the “.xlsx” format for Microsoft Excel and the genomic assembly A1 (Supplementary Data S3 - https://doi.org/10.6084/m9.figshare.5661802) in “.fsa” (fasta) format are both available from the Figshare data repository.
Change history
30 April 2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Carolsfeld, J. Migratory fishes of South America: biology, fisheries and conservation status (IDRC, 2003).
Barletta, M. et al. Fish and aquatic habitat conservation in South America: a continental overview with emphasis on neotropical systems. Journal of Fish Biology 76, 2118–2176 (2010).
Reis, R. et al. Fish biodiversity and conservation in South America. Journal of Fish Biology 89, 12–47 (2016).
Oliveira, D. J., Ashikaga, F. Y., Foresti, F. & Senhorini, J. A. Conservation status of the “piracanjuba” Brycon orbignyanus (Valenciennes, 1850)(Characiformes, Bryconidae): Basis for management programs. Biodiversidade Brasileira 7, 18–33 (2017).
Machado, A. B. M., Drummond, G. M. & Paglia, A. P. Livro vermelho da fauna brasileira ameaçada de extinção (MMA; Fundação Biodiversitas, 2008).
Agostinho, A. A., Pelicice, F. M., Gomes, L. C. & Júlio, H. F. Jr. Reservoir fish stocking: when one plus one may be less than two. Natureza & Conservação 8, 103–111 (2010).
Attard, C. et al. A novel holistic framework for genetic-based captive-breeding and reintroduction programs. Conservation Biology 30, 1060–1069 (2016).
DeSalle, R. & Amato, G. The expansion of conservation genetics. Nature Reviews Genetics 5, 702–712 (2004).
Ashikaga, F. Y., Orsi, M. L., Oliveira, C., Senhorini, J. A. & Foresti, F. The endangered species Brycon orbignyanus: genetic analysis and definition of priority areas for conservation. Environmental Biology of Fishes 98, 1845–1855 (2015).
Ekblom, R. & Galindo, J. Applications of next generation sequencing in molecular ecology of non-model organisms. Heredity 107, 1–15 (2011).
Arias, M. C. et al. Microsatellite records for volume 8, issue 1. Conservation Genetics Resources 8, 43–81 (2016).
Ekblom, R. & Wolf, J. B. A field guide to whole-genome sequencing, assembly and annotation. Evolutionary applications 7, 1026–1042 (2014).
Davey, J. W. et al. Genome-wide genetic marker discovery and genotyping using next-generation sequencing. Nature Reviews Genetics 12, 499–510 (2011).
Peterson, B. K., Weber, J. N., Kay, E. H., Fisher, H. S. & Hoekstra, H. E. Double digest RADseq: an inexpensive method for de novo SNP discovery and genotyping in model and non-model species. PloS one 7, e37135 (2012).
Sonah, H. et al. An improved genotyping by sequencing (GBS) approach offering increased versatility and efficiency of SNP discovery and genotyping. PloS one 8, e54603 (2013).
Shin, G. et al. CRISPR–Cas9-targeted fragmentation and selective sequencing enable massively parallel microsatellite analysis. Nature Communications 8, 14291 (2017).
Hodel, R. G. et al. The report of my death was an exaggeration: A review for researchers using microsatellites in the 21st century. Applications in plant sciences 4, 1600025 (2016).
Luo, R. et al. SOAPdenovo2: an empirically improved memory-efficient short-read de novo assembler. Gigascience 1, 18 (2012).
Temnykh, S. et al. Computational and experimental analysis of microsatellites in rice (Oryza sativa l.): frequency, length variation, transposon associations, and genetic marker potential. Genome research 11, 1441–1452 (2001).
Untergasser, A. et al. Primer3—new capabilities and interfaces. Nucleic acids research 40, e115–e115 (2012).
Boratyn, G. M. et al. BLAST: a more efficient report with usability improvements. Nucleic acids research 41, W29–W33 (2013).
Rognes, T. Faster Smith-Waterman database searches with inter-sequence SIMD parallelisation. BMC bioinformatics 12, 221 (2011).
Li, H. et al. The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Milne, I. et al. Using Tablet for visual exploration of second-generation sequencing data. Briefings in bioinformatics 14, 193–202 (2012).
Acknowledgements
Thanks to João de Magalhães Lopes, PhD; Míriam Aparecida de Castro, MSc and everyone at Projeto Peixe-Vivo at CEMIG; Juliana Pimenta, BSc at Síntese Biotecnologia and Vicente Wen, PhD at BGI; Professors André Luiz Mota, PhD (PROPE); Hewerson Zansávio Teixeira, PhD (DEZOO); Milene Barbosa Carvalho, MSc (DCOMP); Daniel Cardoso Carvalho, PhD at PUC-MG; Carolina Ferreira Cardoso, PhD and PPGBiotec-UFSJ for their direct or indirect assistance to this study. We would also like to thank UFSJ, CNPq and CAPES. This work was supported by grants CEMIG-ANEEL (GT345) and FAPEMIG (APQ-04569-10).
Author information
Authors and Affiliations
Contributions
All authors analysed the data and the results. G.M.Y. designed/supervised the study and wrote the report. R.S.O. handled bioinformatics data and pipelines. Both authors have contributed equally for this work. J.M.R. executed the genomic assembly and performed results quality check. R.D.G. performed results quality check. R.P.S. helped in specimen collection and performed results quality check. F.M.S.C. helped in specimen collection, executed DNA extraction and preparation. D.L. supervised bioinformatics methods.
Corresponding author
Ethics declarations
Competing Interests
G.M.Y., J.M.R. and F.M.S.C. have applied for a patent in Brazil (BR10201700635 -pending) related to the combined use of groups among 16 microsatellite markers described within this genomewide panel and previously advanced in Arias, M. C. et al.(2016). The other authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yazbeck, G.M., Oliveira, R.S., Ribeiro, J.M. et al. A broad genomic panel of microsatellite loci from Brycon orbignyanus (Characiformes: Bryconidae) an endangered migratory Neotropical fish. Sci Rep 8, 8511 (2018). https://doi.org/10.1038/s41598-018-26623-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-26623-x
This article is cited by
-
Scientific knowledge on threatened species of the Brazilian Red List: freshwater fish as a case study
Environmental Biology of Fishes (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.