Abstract
Eliciting plant response protein (Epl) is a small Trichoderma secreted protein that acts as an elicitor to induce plant defense responses against pathogens. In the present study, the differential expression, promoter analysis, and phylogenetic tree analysis of Epl1-Tas (GenBank JN966996) from T. asperellum ACCC30536 were performed. The results showed Epl1-Tas could play an important role in the interaction between T. asperellum ACCC30536 and woody plant or woody plant pathogen. Furthermore, the effect of the Escherichia coli recombinant protein rEpl1-e and the Pichia pastoris recombinant protein rEpl1-p on Populus davidiana × P. alba var. pyramidalis (PdPap) was studied. In PdPap seedlings, rEpl1-e or rEpl1-p induction altered the expression levels of 11 genes in the salicylic acid (SA, three genes), jasmonic acid (JA, four genes) and auxin (four genes) signal transduction pathways, and five kinds of enzymes activities The induction level of rEpl1-p was significantly higher than that of rEpl1-e, indicating that rEpl1-p could be used for further induction experiment. Under 3 mg/mL rEpl1-p induction, the mean height of the PdPap seedlings increased by 57.65% and the mean lesion area on the PdPap seedlings leaves challenged with Alternaria alternata decreased by 91.22% compared with those of the control. Thus, elicitor Epl1-Tas could induce the woody plant resistance to pathogen.
Similar content being viewed by others
Introduction
A plant-mediated response has been confirmed as a component of bio-control using Trichoderma spp.1,2,3, for example, T. virens on cotton and maize4, T. asperellum on soybean5, and T. harzianum on beans6. Some small molecular weight proteins secreted by Trichoderma spp. can induce plant defense responses. One of the largest groups of proteins secreted by Trichoderma is the small secreted cysteine-rich proteins (SSCPs), which are identified as being 100 aa long and contain four or more cysteine residues7. SSCPs are subdivided into four groups: (i) Hydrophobins and hydrophobin-like proteins; (ii) elicitor-like proteins; (iii) proteins with similarity to MAP kinase repressed secreted protein 1 (MRSP1); and (iv) SSCPs with no attribution to any functional category7,8.
Eliciting plant response protein (Epl) secreted by Trichoderma is an elicitor-like SSCP belonging to the cerato-platanin family, and has four cysteines that form two disulfide bonds9. Recently, Epl was confirmed to act as an elicitor to induce plant defense responses5, which provided insights into the mechanisms underlying the processes of Trichoderma-plant recognition, defense elicitation, and induction of resistance. Small molecular protein Sm1 (also known as Epl1) from T. virens Gv29-8 is non-toxic to plants and microbes. Instead, native, purified Sm1 from T. virens Gv29-8 triggered the production of reactive oxygen species in rice and cotton seedlings, and induced the expression of defense-related genes, both locally and systemically, in cotton10. Overexpression of Sm1 in T. virens Gv29-8 significantly enhanced levels of disease protection of maize seedlings challenged with the pathogen Colletotrichum graminicola, and this protection was associated with notable induction of jasmonic acid and green leaf volatile-biosynthetic genes, which demonstrated that the activity of a functional elicitor was required for T. virens-mediated induced systemic resistance (ISR) in maize4. In addition, glycosylation of T. virens Sm1 maintained the protein in a monomeric form, which elicited ISR, and deglycosylation led to the formation of an Sm1 dimer, which did not elicit ISR11. Similarly, overexpression of Epl1 in T. atroviride IMI206040 promoted disease resistance against many pathogens, and induced the expression of the peroxidase and the α-dioxygenase encoding genes in tomato12. Moreover, the absence of Epl-1 in T. harzianum ALL42 affected not only the recognition of T. harzianum ALL42 as a symbiotic fungus by beans6, but also the transcription of tomato defense-related genes during the T. harzianum-tomato interaction13, which indicated that Epl-1 could be important for plant protection in Trichoderma. Besides, EplT4 from T. asperellum T4 was transformed into Pichia pastoris, and soybean leaves were protected by the recombinant EplT4 monomer against the pathogen Cercosporidium sofinum5. Epls have been studied in T. virens, T. atroviride, T. harzianum, and T. asperellum, only in the presence of herbage plant, including dicot (cotton, tomato, bean and soybean) and monocot (rice and maize) plants. However, there are no reports about the effect of Epl as an elicitor on woody plants.
In our previous study, the eliciting plant response protein gene Epl1-Tas from T. asperellum ACCC30536 was cloned. It encodes a protein comprising 138 aa with a molecular weight of 12.6 kDa. And the Escherichia coli recombinant protein rEpl1 (rEpl1-e) was obtained and could promote poplar tissue-culture seedling growth. In the present study, the transcription of Epl1-Tas in T. asperellum ACCC30536 was studied using quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR) under eight inducing conditions. Similar sequences to Epl1-Tas and their promoters were analyzed in four Trichoderma genomes to further certify the genome wide biocontrol function of Epl1-Tas. Epl1-Tas was also recombinantly expressed in P. pastoris (rEpl1-p). Under rEpl1-e or rEpl1-p induction, the transcription of 11 genes related to hormone signaling and 5 physiological enzymes activities in Populus davidiana × P. alba var. pyramidalis (Pdpap) seedlings were detected, and the disease resistance against phytopathogen Alternaria alternata and the growth of PdPap transplanted seedlings were also investigated. The results could provide theoretical support and a practical reference for the development of biological small molecular protein elicitors from T. asperellum in forests.
Results
Promoter analysis and multiple sequence alignment of Epl1-Tas
The promoter of Epl1-Tas is 1,476 bp in length. Many cis-elements known to be involved in stress responses such as MYB, MYC, and WRKY motifs were found in the Epl1-Tas promoter (see Supplementary Fig. 1). The disease resistance element ‘ASF1MOTIFCAMV’ (S000024) appears three times and the disease resistance response element ‘BIHD1OS’ (S000498) appears once in promoter region of Epl1-Tas (Supplementary Fig. 1), suggesting that Epl1-Tas might be regulated by the transcription factors that bind to MYB motifs.
Multiple sequence alignment of similar sequences from 25 fungal strains revealed that the amino acid sequences of these proteins were conserved, with identities ranging from 50 to 92%. Epl1-Tas from T. asperellum ACCC30536 shared the highest similarity (92%, E value = 7e-95) with a known elicitor Epl1 (ABF73692) from T. atroviride ATCC740589,14. Furthermore, four cysteine residues that form two disulfide bonds were highly conserved among the 25 sequences (Fig. 1).
Multiple sequence alignment of sequence similar to Epl1-Tas from 25 fungal strains. Four highly conserved cysteine residues, C, are boxed, forming two disulfide bonds. Asterisk (*): identity. Colon (:): high similarity. Period (.): low similarity. CAL80754 and CAL80753: T. asperellum; ABE73692: T. atroviride; CAL80755 and CAL80756: T. viride; ADB82652: T. reesei; ABE97920 and AAZ80388: T. viren; XP_002999871 and XP_003007509: Verticillium dahliae; EFW99471: Ophisma penicillatum; XP_359969: Magnaporthe oryzae; XP_958708: Neurospora crassa; EFQ31446: Glomerella graminicola; CBI56510: Sordaria humana; XP_001911154: Podospora anserina; AAQ87930: Cochliobolus lunatus; XP_001559499: Botrytis cinerea; EFZ01862: Metarhizium anisopliae; XP_003300798: Rhynchosporium secalis; ADB23420: Marssonina brunnea; AAM33130: Pectobacterium atrosepticum; AAT11911: Antrodia camphorate.
Differential expression of Epl1-Tas under eight inducing conditions
The response to eight biotic stresses of the eliciting plant response protein gene Epl1-Tas in T. asperellum ACCC30536 was investigated using qRT-PCR (Fig. 2). Epl1-Tas transcription was upregulated strongly at 72 h in response to both 1% A. alternate cell walls and 5% A. alternate fermentation liquid, by 76.64 (26.26)-fold and 150.12 (27.23)-fold, respectively (Fig. 2D,E). Epl1-Tas transcription was also upregulated in response to the 1% stem powder and 1% leaf powder of PdPap seedlings, by 362.04 (28.5)-fold and 44.94 (25.49)-fold at 12 and 8 h, respectively (Fig. 2G,H). Interestingly, the transcription of Epl1-Tas was mainly upregulated under carbon source starvation (Fig. 2B). However, Epl1-Tas transcription was inhibited by minimal medium (MM) (0.5% glucose and 0.5% ammonium sulfate), nitrogen source starvation, and 1% root powder. The lowest transcription levels were −1.7 (2−3.70), −1.43 (2−3.43) and −3.01 (2−5.01)-fold at 4, 8, and 4 h, respectively (Fig. 2A,C,F). These results suggested that the Epl1-Tas gene is closely associated with the response of T. asperellum ACCC30536 against biotic stresses.
Differential expression of Epl1-Tas in T. asperellum ACCC30536 under eight inducing conditions. X-axis: time points, Y-axis: expression level = Log2(fold change in expression), namely expression of Epl1-Tas under minimal medium (MM) conditions (A), C starvation conditions (B), N starvation conditions (C), 1% mycelia powder of A. alternata grown in 1/4PD for 10 d (D), 5% fermentation liquid of A. alternata grown in 1/4PD for 10 d (E), 1% root powder of Populus davidiana × P. alba var. pyramidalis (PdPap) seedlings (F), 1% stem powder of PdPap seedlings (G), and 1% leaf powder of PdPap seedlings (H). All experiments were performed three times. The quantitative data are presented in Supplementary Table 1.
Sequences similar to Epl1-Tas and their promoters from four Trichoderma species genomes
We screened four Trichoderma spp. genomes and identified twelve putative open reading frames (ORFs) (Table 1) that were predicted to be members of the small molecular cerato-platanin protein family. There were three Epls in each Trichoderma spp. genome. All the Epls were predicted to be acidic proteins, had the same number of amino acids (138 aa), and contained one intron and two exons (Table 1). They were divided into four groups by phylogenetic analysis (Fig. 3). Epl1s (Epl1-Tas, Sm1-Tvi, Epl1-Tat, and Epl1-Tha) from the four Trichoderma species were divided into two groups (Group1 and Group 4 in the phylogenetic tree), in which two Epl1s (Epl1-Tat and Epl1-Tha) were reported to be elicitors. The other eight Epl2s and Epl3s from the four Trichoderma species were placed in Group 2 and Group 3 in the phylogenetic tree (Fig. 3), which were not reported as elicitors.
Promoter analysis showed that the promoter region of Epl1-Tas and its similar sequences contained many stress response elements; for example, disease resistance (S000024), disease resistance responses (S000498), and drought and low temperature responsive (S000418, S000135) (Fig. 4). Moreover, many hormone response elements, such as gibberellin (S000030), ethylene (S000037), and salicylic acid (S000142), were also identified in the promoters (Fig. 4). In particular, dehydration protein (S000409) with 24.22% and disease resistance (S000024) with 19.53% appeared most frequently in the promoters, and thus might play the most important roles in the bio-control function of Epl protein. The promoter of Epl1-Tas contains 14 elements and thus could have more functions than the promoter of Sm1-Tvi (reported), which only contains five elements (Fig. 4). Moreover, the disease resistance element ‘ASF1MOTIFCAMV’ (S000024) appeared three times and the disease resistance response element ‘BIHD1OS’ (S000498) appeared once in promoter region of Epl1-Tas, which indicated that Epl1-Tas could participate in the disease resistance response.
Motif analysis of promoters of Epl1-Tas and its similarity sequences.  : putative transcription start site;
: putative transcription start site;  : WRKY transcript factor binding site, disease resistance (ASF1MOTIFCAMV, S000024);
: WRKY transcript factor binding site, disease resistance (ASF1MOTIFCAMV, S000024);  : disease resistance responses (BIHD1OS, S000498);
: disease resistance responses (BIHD1OS, S000498);  : low temperature responsive element (LTRECOREATCOR15, S000153);
: low temperature responsive element (LTRECOREATCOR15, S000153);  : disease mechanism, (WBOXATNPR1, S000390);
: disease mechanism, (WBOXATNPR1, S000390);  : MYB transcript factor binding sit, dehydration protein (MYB2CONSENSUSAT, S000409);
: MYB transcript factor binding sit, dehydration protein (MYB2CONSENSUSAT, S000409);  : Gibberellin (CCAATBOX 1, S000030);
: Gibberellin (CCAATBOX 1, S000030);  : Ethylene responsive element (ERELEE4, S000037);
: Ethylene responsive element (ERELEE4, S000037);  : Salicylic acid responsive element (GAREAT, S000142);
: Salicylic acid responsive element (GAREAT, S000142);  : drought response and low temperature responsive element (DRECRTCOREAT, S000418).
: drought response and low temperature responsive element (DRECRTCOREAT, S000418).
Among the three homologous Epls (Epl1-Tas, Epl2-Tas and Epl3-Tas) from T. asperellum ACCC30536 genome, Epl1-Tas has the most elements in its promoter (Fig. 4). Furthermore, only Epl1-Tas showed a high expression level in four inducing conditions, while Epl2-Tas had a lower expression level and the expression of Epl3-Tas barely detectable under the four inducing conditions (see Supplementary Table 2). Taken together, these results suggested that Epl1-Tas has multiple functions in T. asperellum ACCC30536.
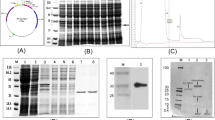
SDS-PAGE analysis of the recombinant Epl1-Tas expressed in P. pastoris (rEpl1-p)
Compared with the control transformant GS115-pPIC9K, the transformant GS115-Epl1 showed a clear protein band with a molecular mass of approximately 12.6 kDa (Fig. 5). This result indicated that the recombinant protein rEpl1-p was successfully synthesized in P. pastoris.
SDS-PAGE analysis of the recombinant protein rEpl1-p from GS115-Epl1. Lanes 1,2,6. The supernatant of control transformant GS115-pPIC9K induced for 6 h; lanes 3,4,5. The supernatant of transformant GS115-Epl1 induced for 2, 4, 6 h; lane 7,8. The supernatant of transformant GS115-Epl1 induced for 1 h; the concentration of methanol was 0.5% (v/v). M. Protein Marker. Full-length gels are presented in Supplementary Figure 2.
Effect of recombinant rEpl1-e or rEpl1-p on 11 hormone signal related genes of PdPap seedlings
To determine whether the recombinant rEpl1-e (Epl1-Tas expressed by E. coli obtained in our previous study) or rEpl1-p could stimulate a response in PdPap seedlings, the transcription levels of 11 genes related to plant hormone signaling were studied using qRT-PCR (Figs 6 and 7). The transcription levels of three genes in the SA signal transduction pathway (Fig. 6L) in PdPap seedlings were significantly induced by rEpl1-e (Fig. 6A–C) and rEpl1-p (Fig. 7A–C). The expression of NPR1 (encoding nonexpresser of PR genes 1) was downregulated from 6–12 h under rEpl1-e induction, and then upregulated to the same level as the control (Fig. 6A). The expression of NPR1 showed a transient decrease at 6 h under rEpl1-p induction, and then also increased to the same level as the control (Fig. 7A). The expression of TGA (encoding TGACGTCA cis-element-binding protein) was mainly downregulated under rEpl1-e and was lower than that of the control (Fig. 6B); however, the expression of TGA was mainly upregulated under rEpl1-e with a peak at 57.68 (25.85)-fold compared with the control (Fig. 7B). Compared with the downregulated control, the expression of PR1 (encoding pathogenesis-related 1) was upregulated under rEpl1-e (Fig. 6C) and rEpl1-p (Fig. 7C), by 2210.26 (211.11) and 9607.86 (213.23)-fold compared with the control at 2 d and 12 h, respectively.
Transcription of 11 genes related to hormone signaling in Populus davidiana × P. alba var. pyramidalis (PdPap) seedlings under rEpl1-e induction. X-axis: time points, Y-axis: expression level = Log2 (fold change in expression). (A–C) The expression levels of NPR1, TGA, and PR1 in the SA signal transduction pathway (L); (D–G). The expression levels of COI, JAZ6, MYC2, and ORCA3 in the JA signal transduction pathway (M); (H–K). The expression levels of TIR1, IAA8/AUX, MP/ARF, and GH3 in the auxin signal transduction pathway (N). (L–N) The plant hormone signal transduction of the KEGG software from the Kanehisa laboratory (http://www.genome.jp/kegg-bin/show_pathway?map04075). Different capital letters represent significant differences among different time points of treatment or the control group; different lowercase letter represent significant differences between the treatment and the control at the same time point; *significant difference between rEpl1-e and rEpl1-p treatments at the same time point. All significances were at p < 0.5. All experiments were performed three times. The quantitative data are presented in Supplementary Table 3.
Transcription of 11 genes related to hormone signaling in Populus davidiana × P. alba var. pyramidalis (PdPap) seedlings under rEpl1-p induction. X-axis: time points, Y-axis: expression level = Log2 (fold change in expression). (A–C) The expression levels of NPR1, TGA, and PR1 in the SA signal transduction pathway; (D–G) The expression levels of COI, JAZ6, MYC2, and ORCA3 in the JA signal transduction pathway; (H–K) The expression levels of TIR1, IAA8, MP/ARF, and GH3 in the auxin signal transduction pathway. Different capital letters represent significant differences among different time points of treatment or the control group; different lowercase letter represent significant differences between the treatment and the control at the same time point; *significant difference between rEpl1-e and rEpl1-p treatments at the same time point. All significances were at p < 0.5. All experiments were performed three times. The quantitative data are presented in Supplementary Table 4.
The transcription levels of four genes in JA signal transduction pathway (Fig. 6M) in PdPap seedlings were significantly induced by rEpl1-e (Fig. 6D–G) or rEpl1-p (Fig. 7D–G). The expression levels of COI, JAZ6 and MYC2 (encoding cytochrome oxidase I, jasmonate-zim-domain protein 6, and MYC-related transcriptional activator, respectively) were mainly upregulated under rEpl1-e (Fig. 6D–G) or rEpl1-p (Fig. 7D–F) induction. The peaks were 11.88 (23.57), 53.08 (25.73) and 8.63 (23.11)-fold higher, respectively, than those of the control at 6 h, 6 h, and 1 d under rEpl1-e induction (Fig. 6D–F), and the peaks respectively were 18.90 (24.24), 67.65 (26.08) and 5.43 (22.44)-fold higher, respectively, than the control at 6 h, 6 h, and 1 d under rEpl1-p induction (Fig. 7D–F). Compared with the downregulated control, the expression level of ORCA3 (encoding octadecanoid-derivative responsive catharanthus AP2-domain 3) was upregulated under both rEpl1-e (Fig. 6G) and rEpl1-p (Fig. 7G) induction, and the peaks were 349.71 (28.45) and 5007.93 (212.29)-fold higher than those of the control at 2 d and 12 h, respectively.
The transcription levels of four genes in the auxin signal transduction pathway (Fig. 6N) in PdPap seedlings were also significantly induced by rEpl1-e (Fig. 6H–K) and rEpl1-p (Fig. 7H–K). The expression of IAA8/AUX (encoding auxin/indole-3-acetic acid 8) was mainly upregulated under both rEpl1-e (Fig. 6J) and rEpl1-p (Fig. 7I) induction, and the peaks were 63.56 (25.99) and 188.71 (27.56)-fold higher than those of the control at 2 d and 2 d, respectively. Compared with the upregulated control, the expression levels of TIR1 (encoding transport inhibitor response 1 and MP/ARF (encoding monopteros/auxin respnse factor) were downregulated under both rEpl1-e (Fig. 6H,J) and rEpl1-p (Fig. 7H,J) induction. Compared with the downregulated control, the expression level of GH3 (encoding Gretchen Hagen 3) was upregulated under both rEpl1-e (Fig. 6K) and rEpl1-p (Fig. 7K) induction and the peaks were 3040.30 (211.57) (Fig. 6K) and 46663.28 (215.51) (Fig. 7K)-fold higher than those of the control at 6 h, respectively. In summary, the recombinant rEpl1-e and rEpl1-p had an important influence on PdPap seedlings’ hormone signaling. The inducing effect of rEpl1-p was significantly higher than that of rEpl1-e, based on significance analysis.
Effect of recombinant rEpl1-e or rEpl1-p on enzymes activities in PdPap seedlings
To gain a further insight into the PdPap seedlings response to rEpl1-e or rEpl1-p, the activities of five kinds of enzymes in the PdPap seedlings were analyzed under rEpl1-e or rEpl1-p induction (Fig. 8). The enzyme activities showed obvious differences under rEpl1-e or rEpl1-p induction. Compared with the control, the activities of the growth-related indole acetic acid oxidase (IAAO) in PdPap seedlings slowly decreased under rEpl1-e (Fig. 8A) or rEpl1-p (Fig. 8B) induction. In addition, the activities of the defense-related peroxidase (POD), superoxide dismutase (SOD), polyphenol oxidase (PPO) and phenylalanine ammonia lyase (PAL) in PdPap seedlings were all higher than those of the control under rEpl1-e or rEpl1-p induction (Fig. 8C–J). The peaks under rEpl1-e induction were 77.01, 34.08, 110.42, and 66.37-fold higher than the control at 7, 7, 1 and 3 d, respectively (Fig. 8C,E,G,I). The peaks under rEpl1-p induction were 108.53, 104.06, 122.42, and 41.43-fold higher than the control at 5, 3, 1, and 7 d, respectively (Fig. 8D,F,H,J). In summary, recombinant rEpl1-e and rEpl1-p had important effects on growth-related and defense-related enzymes activities in PdPap seedlings. The induction effect of rEpl1-p was also significantly higher than that of rEpl1-e, based on significance analysis.
The activities of five kinds of enzymes in Populus davidiana × P. alba var. pyramidalis (PdPap) seedlings leaves under rEpl1-e or rEpl1-p induction. X-axis: time points, Y-axis: enzyme activities, namely IAAO (A,B), POD (C,D), SOD (E,F), PPO (G,H), and PAL (I,J) activities in PdPap seedlings leaves under rEpl1-e or rEpl1-p induction, respectively. Solid lines: the treatment group; dotted line: the control group. *Significant difference between rEpl1-e and rEpl1-p treatments at the same time point (p < 0.5). All experiments were performed three times.
Effect of rEpl1-p on growth and disease resistance of PdPap transplanted seedlings
To determine the ability of Epl1-Tas to promote the growth and induce disease resistance of PdPap, different concentrations rEpl1-p were used to induce PdPap transplanted seedlings (Fig. 9). The growth rate of the PdPap transplanted seedlings varied under the different concentrations of rEpl1-p (Fig. 9A). Compared with the control, the mean heights of the PdPap transplanted seedlings increased significantly under induction by 3 and 5 mg/mL rEpl1-p, by 57.65% and 51.18%, respectively (Fig. 9A’).
Growth and disease resistance of the Populus davidiana × P. alba var. pyramidalis (PdPap) transplanted seedlings under recombinant rEpl1-p induction. (A,A′): Differential growth rate and mean plant height of PdPap transplanted seedlings under different concentrations rEpl1-p induction; (B,B′): Disease-resistance ability and mean lesion area on leaves of PdPap transplanted seedlings challenged with A. alternata under different concentrations rEpl1-p induction. Different letters represent significant differences under different protein content inductions (P < 0.05). Each treatment contained five repetitions, and the experiment was repeated twice.
Moreover, the disease-resistance ability of the PdPap seedlings against A. alternate also varied under the different concentrations rEpl1-p (Fig. 9B). Compared with the control, the mean lesion areas of leaves decreased significantly under 0.5, 1, 3, or 5 mg/mL rEpl1-p induction (Fig. 9A’). Especially, under induction by 3 and 5 mg/mL rEpl1-p, the mean lesion areas of leaves decreased by 91.22% and 93.17%, respectively (Fig. 9B’). Thus, rEpl1-p promoted PdPap transplanted seedlings growth and induced resistance to the pathogen A. alternata in PdPap seedlings, especially at 3 mg/mL.
Discussion
Previously, differential expression of Epl1 in Trichoderma was studied only in the presence of dicot and monocot herbage plants4,9,10. Sm1 (Epl1) from T. virens Gv29-8 was upregulated in the presence of the cotton and maize plants early in the interaction4,10, and Sm1 from different Trichoderma showed increased expression in presence of bean seeds15. In the present study, we also showed that Epl1-Tas from T. asperellum ACCC30536 was upregulated in the presence of the woody plant PdPap seedlings, stems, and leaves (Fig. 2G,H), which, combined with the results of previous studies, suggested that upregulated expression of Epl1 participates in the interaction between Trichoderma and plants ubiquitously. However, this increase in the expression of Epl1-Tas was not observed in the presence of plant roots, as it was for Sm1 from T. harzianum T3715 (Fig. 2F), Furthermore, the transcription of Epl1 from T. atroviridis ATCC74058 was induced cell walls from the plant pathogen Rhizoctonia solani9. Similarly, in this study, a high transcription level of Epl1-Tas was induced by cell walls and fermentation liquid from the PdPap pathogen A. alternata. Thus, Epl1-Tas of T. asperellum ACCC30536 might not only play an important role during the interaction with herbage plants or their pathogenic fungi, but also in the interaction with woody plants and their pathogenic fungi, indicating the enriched functions of Epls.
T. jecorina, T. virens, and T. atroviride each have three paralogs of Epls (sm1)7,14,16. In our analysis, T. asperellum and T. harzianum also have three Epls (Table 1), which indicated that Epls as elicitor are ubiquitous in the Trichoderma genus and could be important for the bio-control of Trichoderma. In addition, the elicitor Sm1 from T. virens Gv29-8 (Sm1-Tvi) could induce local and systemic defenses in plants, including rice, cotton and maize4,10,11,12,15,17. Our analysis showed that compared with the promoter of Sm1-Tvi, the Epl1-Tas promoter has more types and numbers of regulatory motifs (6 types; 14 numbers vs. 4 types; 5 numbers), and the disease resistance element ‘ASF1MOTIFCAMV’ (S000024) appears three times and the disease resistance response element ‘BIHD1OS’ (S000498) appears once in promoter region of Epl1-Tas (Fig. 4). This indicated that Epl1-Tas might have more functions than Sm1-Tvi, and might play an important role in bio-control of T. asperellum ACCC30536, as suggested by Pozo et al.18. Furthermore, compared with its homologs in T. asperellum ACCC30536 (Epl2-Tas and Epl3-Tas), the Epl1-Tas promoter has more types and numbers of regulatory motifs (6 type; 14 numbers vs. 6 type, 13 numbers (Epl2-Tas) vs. 4 types; 9 numbers (Epl3-Tas)) (Fig. 4). This might explain why the expression of Epl1-Tas was higher than that of Epl2-Tas and Epl3-Tas under four inducing conditions (see Supplementary Table 2), which agreed with and enriched the conclusion of Frischmann et al.14.
In the phylogenetic tree, Epl1s (Epl1-Tas, Sm1-Tvi, Epl1-Tat and Epl1-Tha) from four species of Trichoderma were divided into two groups (Group1 and Group 4), in which two Epl1s (Epl1-Tat and Epl1-Tha) were reported to be elicitors (Fig. 3). However, the other eight Epl2s and Epl3s from the four species were placed in Group 2 and Group 3 (Fig. 3), which were not reported as elicitors. The highest expression of Epl1-Tas among Group 4 Epl1s under four inducing conditions (see Supplementary Table 2) further indicated that Epl1 proteins play an important role in different Trichoderma, while Epl2 and Epl3 had no obvious effect. Thus, the promoter (Fig. 4), differential expression (see Supplementary Table 2), and phylogenetic tree (Fig. 3) analyses further suggested that Epl1-Tas functions in stimulating plant defense responses as an elicitor.
Furthermore, to study the function of Epl1-Tas from T. asperellum ACCC30536 to the woody plant PdPap seedlings, we examined the transcription of 11 genes related to hormone signal in PdPap seedlings under E. coli recombinant rEpl1-e or yeast recombinant rEpl1-p (Fig. 5) induction, respectively. Trichoderma spp. colonization could induce a transient increase in expression of defense-related genes of plant19. That observation was in agreement with our results that 11 hormone signaling-related genes in PdPap seedlings showed transiently upregulated expression under rEpl1-e or rEpl1-p induction (Figs 6 and 7). In the SA signal transduction pathway, NPR1 plays an important role in both innate immunity and systemic acquired resistance (SAR), and PR1 is often associated with plant host resistance. Although NPR1 is a key component in the induction of SAR-related PR1 expression, its overexpression did not lead to PR1 expression in Arabidopsis20. Similarly, although the transcription of NPR1 in PdPap seedlings was downregulated under rEpl1-e or rEpl1-p induction (Figs 6A and 7A), PR1 was upregulated compared with the control group, which was consistent with the results of Mou et al.20. The transient peak of PR1 was 2210.26 (211.11)-fold higher at 2 d under rEpl1-e induction (Fig. 6C), and the transient peak of PR1 was 9607.86 (213.23)-fold at 12 h under rEpl1-p induction (Fig. 7C), which showed rEpl1-e and rEpl1-p could trigger SAR in PdPap seedlings. In addition, in the JA signal transduction pathway, Octadecaniod-derivative responsive catharanthus AP2-domain protein gene ORCA3 is a jasmonic acid responsive gene and regulates jasmonate-responsive expression, which could induce ISR in the host plant21,22. In this study, ORCA3 showed higher expression in PdPap under rEpl1-e or rEpl1-p induction (Figs 6G and 7G). The highest peaks of ORCA3 were 349.71 (28.45)-fold at 2 d under rEpl1-e induction (Fig. 6G) and 5007.93 (212.29)-fold at 2 h under rEpl1-p induction (Fig. 7G), respectively, which showed that rEpl1-e and rEpl1-p, similar to jasmonic acid, could also trigger ISR in PdPap seedlings. These results showed that rEpl1-e and rEpl1-p affected SA and JA signal transduction pathways in PdPap seedlings; however, the transcription level under rEpl1-p induction was significantly higher than under rEpl1-e induction.
Alterations to defense-related enzyme activities in the leaves of PdPap seedlings were detected under rEpl1-e or rEpl1-p induction. When plants suffer damage, more O·2− is produced, in which case, SOD dismutates O·2−, and the subsequent excessive H2O2 induces the upregulation of POD, which showed that POD and SOD activities would increase to avoid injury to plant cells23,24,25. In this study, the overall trend of the POD and SOD activities in leaves of PdPap seedlings were similar under rEpl1-e or rEpl1-p induction, and their activities were obviously higher than those of the control (Fig. 8C–F). POD and PPO act synergistically during enzymatic browning because PPO can promote POD activity by generating H2O2 from the oxidation of phenolic compounds25. In addition, expression of PAL has been reported to be activated by the JA Signaling pathway4,19,26. These enzymes play an important role in plant defense, increasing the toxicity to invading pathogens and pests25. In this study, the activities of defense-related enzymes PPO and PAL in PdPap seedlings increased under rEpl1-e or rEpl1-p induction compared with those in the control group (Fig. 8G,I,H,J). Epl1-Tas as elicitor triggered ISR and SAR in PdPap seedlings resulting in the physiological response of PdPap seedlings. Moreover, the defense-related enzymes activities under rEpl1-p induction were also significantly higher than those under rEpl1-e induction. Perhaps the E. coli system expresses the target protein in inclusion bodies27, which can cause functional decreases after denaturation and renaturation, while the P. pastoris system has been engineered to yield high amounts of extracellularly secreted proteins with very few contaminating native proteins17, which explain why the yeast recombinant rEpl1-p had a better induction effect on PdPap seedlings. Therefore, we chose rEpl1-p further to induce PdPap transplanted seedlings challenged with pathogen A. alternata.
In induction test, the mean heights of the PdPap transplanted seedlings increased significantly under induction by 3 and 5 mg/mL rEpl1-p, by 57.65% and 51.18%, respectively (Fig. 9A,A’). This was consistent with the changes of auxin signal related genes (Fig. 7H–K) and IAAO activity (Fig. 8B) in PdPap seedlings leaves under rEpl1-p induction. Auxin acts by directly binding to the TIR1/AFB proteins, and promoting the degradation of transcriptional repressors called IAA/AUX proteins28. The original auxin response gene, GH3, could enhance sensitivity to IAA in root inhibition assays29. The relationship between the levels of IAA and IAAO activity is negatively correlated30. Auxin can regulate auxin-responsive genes31. Epl1-Tas, like auxin, could promote PdPap transplanted seedling growth. Moreover, the stronger transplanted seedlings had the stronger disease resistance. Under 3 and 5 mg/mL rEpl1-p induction, the mean leaf lesion areas decreased significantly, by 91.22% and 93.17% compared with that of the control, respectively (Fig. 9B,B’), which provided further evidence that the elicitor of defense response, Epl1-Tas, could induce protection of PdPap seedlings against attack by A. alternata.
In summary, the transcription of Epl1-Tas could be induced by woody plant PdPap seedlings stems and leaves and by the cell wall and fermentation liquid of its pathogen A. alternata. Disease resistance element ‘ASF1MOTIFCAMV’ (S000024) and disease resistance response element ‘BIHD1OS’ (S000498) were found in the promoter region of Epl1-Tas. Epl1-Tas was also successfully expressed in P. pastoris GS115. The recombinant proteins rEpl1-e and rEpl1-p both affected the transcription of 11 genes related to JA, SA, and auxin signal transduction pathways and the activities of five enzymes in seedlings of the woody plant PdPap. However, rEpl1-p had a better induction effect than rEpl1-e, making it more suitable for woody plant stimulation. Furthermore, rEpl1-p not only promoted PdPap transplanted seedling growth, but also protected PdPap transplanted seedling against attack by the pathogen A. alternata. This study increased our understanding of the role of Epl1-Tas in biocontrol and provides a practical reference for applications of Epl1-Tas in forests.
Materials and Methods
Strains, vectors and plant materials
T. asperellum ACCC30536 was obtained from the Agricultural Culture Collection of China (ACCC). P. pastoris GS115 and vector pPIC9K (Invitrogen, USA) were used for eukaryotic expression. Aseptic Populus davidiana × P. alba var. pyramidalis (PdPap) tissue-culture seedlings were used for the induction experiment. The stems, roots, and leaves of aseptic PdPap seedlings were used as inducing substrates for T. asperellum ACCC30536. Poplar pathogenic fungus A. alternata (the causative agent of poplar leaf wither) was used to prepare a carbon source in the inducing media for T. asperellum.
Promoter analysis and multiple sequence alignment of Epl1-Tas
The 1,500 bp upstream fragments of the coding regions were obtained as predicted promoter regions. Regulatory motifs in these regions were predicted using the Promoter Database of Saccharomyces cerevisiae (http://rulai.cshl.edu/SCPD/) and the STRING tool32.
Proteins similar to Epl1-Tas were obtained using BlastP at NCBI (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The multiple sequence alignment was conducted using the ClustalX program (http://www.ebi.ac.uk/Tools/ clustalw2/)32.
Differential expression of Epl1-Tas in T. asperellum under eight inducing conditions
The transcription of Epl1-Tas in T. asperellum ACCC30536 under eight inducing conditions was studied, namely minimal medium (MM) with 0.5% (w/v) glucose and 0.5% (w/v) ammonium sulfate33, carbon starvation medium, nitrogen starvation medium, and variable carbon source in MM as follows: 1% (w/v) root powder, 1% (w/v) stem powder, or 1% (w/v) leaf powder from PdPap seedlings, 1% (w/v) powdered cell walls; and 5% (v/v) fermentation supernatant of A. alternata for 10 d. Root, stem and leaf powders, fungal phytopathogen cell walls, and fungal phytopathogen fermentation supernatants were prepared according to a previous study34. All experiments were performed in triplicate.
Total RNA was extracted from the mycelia using the Trizol reagent (Invitrogen, Carlsbad, CA, USA) and then subjected to qRT-PCR. The qRT-PCR products were analyzed using the IQ5 real-time PCR detection system (Bio-Rad Laboratories Co., Ltd., Shanghai, China). The expression level of Epl1-Tas was calculated from the threshold cycle according to 2−ΔΔCT method32. The qRT-PCR primers are shown in Supplementary Table 5.
Sequences similar to Epl1-Tas and their promoters from four Trichoderma species genomes
The Epl1-Tas amino acid sequence was used as a query to search for similar coding sequences using tBLASTN in the genome databases of T. asperellum ACCC30536, T. virens Gv29-8, T. atroviride ATCC74058, and T. harzianum CBS 226.95 genomes (http://genome.jgi-psf.org/). Bioinformatic analysis was performed according to the methods of Fan32. The phylogenetic tree was constructed using the neighbor-joining method in the MEGA 5.10 program32. The promoter analysis method is detailed in the “Promoter analysis and multiple sequence alignment of Epl1-Tas” section.
Differential expression of three Epls in T. asperellum ACCC30536 genome under four inducing conditions
The transcription of Epl1-Tas and its two homologous genes in the T. asperellum ACCC30536 genome was analyzed in C-starvation (MM with 0.5% (w/v) ammonium sulfate), N-starvation (MM with 0.5% (w/v) glucose) and SXY (variable carbon source in MM as follows: 1% (w/v) root powder, 1% (w/v) stem powder, or 1% (w/v) leaf powder from PdPap seedlings)35. All experiments were performed in triplicate.
P. pastoris transformations and SDS-PAGE analysis
The ORF of Epl1-Tas was amplified using the primers Epl1p1: 5′-ATCGG AATTCGATACGGTCTCCTACGACAC-3′ (EcoRI site underlined) and Epl1p2: 5′-CGATGCGGCCGCGAGGCCGCAGTTGCTCACGGC-3′ (NotI site underlined), digested with the indicated enzymes, ligated into the expression vector of pPIC9K, and transferred into E. coli Top10 competent cells to select positive clones. The recombinant vector pPIC9K-Epl1 was transferred into P. pastoris GS115 to induce expression according to the instructions of the Pichia expression kit (Invitrogen, Cat. No. K1710-01, USA). P. pastoris transformant GS115-Epl1 and the control transformant GS115-pPIC9K (P. pastoris GS115 transformed with empty vector pPIC9K), were induced with 0.5% (v/v) methanol per 24 h at 30 °C and 200 rpm, respectively. The supernatant of the transformant cultures was harvested at 1, 2, 4 and 6 h. The supernatants were collected by centrifugation at 10,000 × g at 4 °C for 10 min. After the addition of 1× loading buffer, the supernatant or cells were boiled for 5 min, centrifuged for 10 min at 8,000 rpm and loaded into a 12% SDS-PAGE gel36. Recombinant E. coli proteins were purified according to a pervious study37.
Differential expression of hormone signal related genes of PdPap seedlings under recombinant rEpl1-e or rEpl1-p induction
The rEpl1-e obtained in our previous study38 and the rEpl1-p obtained in present study were used for the induction experiment. PdPap tissue-culture seedlings were cultured in WPM medium containing 10% (v/v) recombinant rEpl1-e or rEpl1-p. The recombinant protein was heat inactivated, and the PdPap tissue-culture seedlings were cultured in WPM medium containing 10% (v/v) heat-denatured protein as the control35. The leaves were harvested at 0 h, 6 h, 12 h, 1 d, 2 d, 5 d, and 7 d for RNA extraction. The elongation factor (Ef), β-tubulin, and actin genes were used as internal references to normalize the amount of total RNA present in each reaction. The qRT-PCR primers was listed in Supplementary Table 6. All experiments were performed three times.
Extraction and detection for enzymes of PdPap under rEpl1-e or rEpl1-p induction
The treatment of the PdPap tissue-culture seedlings with rEpl1-e or rEpl1-p is detailed in the “Differential expression of hormone signal related genes of PdPap seedlings under recombinant rEpl1-e or rEpl1-p induction” section and the leaves were harvested after induction for 0, 0.5, 1, 2, 3, 5, and 7 d. Fresh tissue (0.5 g) was weighed from the above samples in each treatment with three replicates. The sample was frozen in liquid nitrogen and ground on ice with 10 mL of 100 mM pre-cold phosphate buffer (pH 6.0) containing 1% (w/v) dithiothreitol for enzyme extraction. IAAO, POD, SOD, polyphenol oxidase30, and PAL were extracted and their activities determined according to previous studies25,30.
A. alternata resistance assay of PdPap seedlings leaves under rEpl1-p induction
The PdPap tissue-culture seedlings were transplanted into aseptic soil for 10 d. The rhizosphere of PdPap transplant seedlings were watered using the supernatant of a GS115-Epl1 culture containing final concentrations of 0, 0.5, 1, 3 and 5 mg/mL rEpl1-p, respectively. The growth of PdPap transplanted seedlings were observed after treatment for 30 d, and the plant height of PdPap transplant seedlings was measured. Meanwhile, the same position of leaves selected randomly per plant was inoculated with A. alternata of 1 × 103 spores/mL (the optimum infection spore concentration). The disease spots were observed and the lesion areas were determined using Image Pro Plus 6.0 software after 10 d5. Each treatment contained five repetitions, and the experiment was repeated twice.
References
Yedidia, I. et al. Concomitant induction of systemic resistance to Pseudomonas syringae pv. Lachrymans in cucumber by Trichoderma asperellum (T-203) and accumulation of phytoalexins. Appl Environ Microbiol. 69, 7343–7353 (2003).
Yishay, M. et al. Differential pathogenicity and genetic diversity among Pectobacterium carotovorum ssp. Carotovorum isolates from monocot and dicot hosts support early genomic divergence within this taxon. Environ Microbio. 10, 2746–2759 (2008).
Shoresh, M. et al. Induced systemic resistance and plant responses to fungal biocontrol agents. Annu Rev Phytopathol. 48, 21–43 (2010).
Djonovic, S. et al. A proteinaceous elicitor Sm1 from the beneficial fungus Trichoderma virens is required for induced systemic resistance in maize. Plant Physiol. 145, 875–889 (2007).
Wang, Y. et al. Eplt4 proteinaceous elicitor produced in Pichia pastoris has a protective effect against Cercosporidium sofinum infections of soybean leaves. Appl Biochem Biotechnol. 169, 722–737 (2013).
Gomes, E. V. et al. Gutierrez Sand Silva RN, The Cerato-Platanin protein Epl-1 from Trichoderma harzianum is involved in mycoparasitism, plant resistance induction and self cell wall protection. Sci Rep. 5, 17998 (2015).
Kubicek, C. P. et al. Comparative genome sequence analysis underscores mycoparasitism as the ancestral life style of Trichoderma. Genome Biol. 12, R40 (2011).
Druzhinina, I. S. et al. Novel traits of Trichoderma predicted through the analysis of its secretome. Fems Microbiol Lett. 337, 1–9 (2012).
Seidl, V. et al. Allmaier Gand Kubicek CP, Epl1, the major secreted protein of Hypocrea atroviridis on glucose, is a member of a strongly conserved protein family comprising plant defense response elicitors. Febs J. 273, 4346–4359 (2006).
Djonovic, S. et al. Howell CRand Kenerley CM, Sm1, a proteinaceous elicitor secreted by the biocontrol fungus Trichoderma virens induces plant defense responses and systemic resistance. Mol Plant Microbe Interact. 19, 838–853 (2006).
Vargas, W. A. et al. Dimerization controls the activity of fungal elicitors that trigger systemic resistance in plants. J Biol Chem. 283, 19804–19815 (2008).
Salas-Marina, M. A. et al. The Epl1 and Sm1 proteins from Trichoderma atroviride and Trichoderma virens differentially modulate systemic disease resistance against different life style pathogens in Solanum lycopersicum. Front Plant Sci. 6, 77 (2015).
Gomes, E. V. et al. Involvement of Trichoderma harzianum epl-1 protein in the regulation of botrytis virulence and tomato defense-related genes. Front Plant Sci. 8, 880 (2017).
Frischmann, A. et al. Self-assembly at air/water interfaces and carbohydrate binding properties of the small secreted protein EPL1 from the fungus Trichoderma atroviride. J Biol Chem. 288, 4278–4287 (2013).
Freitas, R. S. et al. Cloning and characterization of a protein elicitor Sm1 gene from Trichoderma harzianum. Biotechnol Lett. 36, 783–788 (2014).
Druzhinina, I. S. et al. Trichoderma: The genomics of opportunistic success. Nat Rev Microbiol. 9, 749–759 (2011).
Buensanteai, N. et al. Expression and purification of biologically active Trichoderma virens proteinaceous elicitor Sm1 in Pichia pastoris. Protein Expr Purif. 72, 131–138 (2010).
Pozo, M. J. et al. Functional analysis oftvsp1, a serine protease-encoding gene in the biocontrol agent Trichoderma virens. Fungal Genet Biol. 41, 336–348 (2004).
Shoresh, M. et al. Involvement of jasmonic Acid/Ethylene signaling pathway in the systemic resistance induced in cucumber by Trichoderma asperellum t203. Phytopathology. 95, 76–84 (2005).
Mou, Z. et al. Inducers of plant systemic acquired resistance regulate NPR1 function through redox changes. Cell. 113, 935–944 (2003).
Choudhary, D. K. et al. Induced systemic resistance (ISR) in plants: mechanism of action. Indian J Microbiol. 47, 289–297 (2007).
Vom, E. D. et al. Identification of a bipartite jasmonate-responsive promoter element in the Catharanthus roseus ORCA3 transcription factor gene that interacts specifically with AT-Hook DNA-binding proteins. Plant Physiol. 144, 1680–1689 (2007).
Passardi, F. et al. Peroxidases have more functions than a Swiss army knife. Plant Cell Rep. 24, 255–265 (2005).
Li, Y. et al. Cadmium accumulation, activities of antioxidant enzymes, and malondialdehyde (MDA) content in Pistia stratiotes L. Environ Sci Pollut Res Int. 20, 1117–1123 (2013).
Wang, M. et al. Growth and physiological changes in continuously cropped eggplant (Solanum melongena L.) upon relay intercropping with garlic (Allium sativum L.). Front Plant Sci. 6, 262 (2015).
Kato, M. et al. Wound-induced ethylene synthesis and expression and formation of 1-aminocyclopropane-1-carboxylate (ACC) synthase, ACC oxidase, phenylalanine ammonia-lyase, and peroxidase in wounded mesocarp tissue of Cucurbita maxima. Plant Cell Physiol. 41, 440–447 (2000).
Liu, R. et al. An easy way to purify the inclusion body protein with high purity from prokaryotic expression cells. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 29, 399–401 (2011).
Wang, R. et al. HSP90 regulates temperature-dependent seedling growth in Arabidopsis by stabilizing the auxin co-receptor F-box protein TIR1. Nat Commun. 7, 10269 (2016).
Staswick, P. E. et al. Characterization of an Arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid. Plant Cell. 17, 616–627 (2005).
Yan, Y. H. et al. Effect of naphthalene acetic acid on adventitious root development and associated physiological changes in stem cutting of Hemarthria compressa. Plos One. 9, e90700 (2014).
Tian, Q. et al. Arabidopsis SHY2/IAA3 inhibits auxin-regulated gene expression. Plant Cell. 14, 301–319 (2002).
Fan, H. et al. Functional analysis of a subtilisin-like serine protease gene from biocontrol fungus Trichoderma harzianum. J Microbiol. 52, 129–138 (2014).
Penttila, M. et al. A versatile transformation system for the cellulolytic filamentous fungus Trichoderma reesei. Gene. 61, 155–164 (1987).
Huang, Y. et al. Functional analysis of the class II hydrophobin gene HFB2-6 from the biocontrol agent Trichoderma asperellum ACCC30536. Microbiol Res. 171, 8–20 (2015).
Dou, K. et al. Cloning and characteristic analysis of a novel aspartic protease gene Asp55 from Trichoderma asperellum ACCC30536. Microbiol Res. 169, 915–923 (2014).
Sambrook, J. et al. Molecular cloning. Cold Spring Harbor Laboratory Press (1989).
Sun, H. et al. Construction of a prokaryotic expression vector of human tau multi-epitope peptide and immunogenicity of the expressed product. Nan Fang Yi Ke Da Xue Xue Bao. 32, 185–188 (2012).
Yu, W. J. et al. Cloning, prokaryotic expression and function of the eliciting plant response protein of Trichoderma asperellum. Journal of Beijing Forestry University. 40(1), 26–35 (2018).
Acknowledgements
This work was supported by grants from the National High Technology Research and Development Program (The 13th Five-Year Plan Program) (2016YFC0501505), and the Science and Technology Innovation Talents Foundation of Harbin City of China (2016RQQXJ235).
Author information
Authors and Affiliations
Contributions
Zhihua Liu and Wenjing Yu conceived the experiment, Gulijimila Mijiti, Ying Huang and Wenjing Yu conducted the experiment, Zhihua Liu, Haijuan Fan and Yucheng Wang analyzed the result. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yu, W., Mijiti, G., Huang, Y. et al. Functional analysis of eliciting plant response protein Epl1-Tas from Trichoderma asperellum ACCC30536. Sci Rep 8, 7974 (2018). https://doi.org/10.1038/s41598-018-26328-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-26328-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.