Abstract
DEMETER-like DNA glycosylases (DMLs) initiate the base excision repair-dependent DNA demethylation to regulate a wide range of biological processes in plants. Six putative SmDML genes, termed SmDML1–SmDML6, were identified from the genome of S. miltiorrhiza, an emerging model plant for Traditional Chinese Medicine (TCM) studies. Integrated analysis of gene structures, sequence features, conserved domains and motifs, phylogenetic analysis and differential expression showed the conservation and divergence of SmDMLs. SmDML1, SmDML2 and SmDML4 were significantly down-regulated by the treatment of 5Aza-dC, a general DNA methylation inhibitor, suggesting involvement of SmDMLs in genome DNA methylation change. SmDML1 was predicted and experimentally validated to be target of Smi-miR7972. Computational analysis of forty whole genome sequences and almost all of RNA-seq data from Lamiids revealed that MIR7972s were only distributed in some plants of the three orders, including Lamiales, Solanales and Boraginales, and the number of MIR7972 genes varied among species. It suggests that MIR7972 genes underwent expansion and loss during the evolution of some Lamiids species. Phylogenetic analysis of MIR7972s showed closer evolutionary relationships between MIR7972s in Boraginales and Solanales in comparison with Lamiales. These results provide a valuable resource for elucidating DNA demethylation mechanism in S. miltiorrhiza.
Similar content being viewed by others
Introduction
Salvia, widely distributed in the world, is the largest genus in the Labiatae family. It includes about 900 species, of which many have significant economical and medicinal value. S. miltiorrhiza Bunge is a well-known Salvia species widely used in traditional Chinese medicine (TCM) for the treatment of dysmenorrhoea, amenorrhoea and cardiovascular diseases1,2,3,4. It is also one of the best selling TCM materials with long usage history and the first Chinese herb entering international market. Recently, S. miltiorrhiza is emerging as a model system for medicinal plant biology5. The whole genomes of two S. miltiorrhiza lines have been decoded6,7. A huge amount of RNA-seq-based transcriptome data have been obtained and are available for comparative analysis (https://www.ncbi.nlm.nih.gov/sra). It provides useful information for further elucidating the genetic and epigenetic regulatory mechanisms of S. miltiorrhiza development and bioactive compound production.
Epigenetic regulation is an important regulatory mechanism affecting many plant cellular processes. It is known that epigenetic phenotypes are caused by changes in a chromosome without alterations in the DNA sequence, including individual or combined changes in DNA methylation and histone modification, as well as the action of chromatin-remodeling factors and noncoding RNAs8. DNA methylation is an important epigenetic modification. It is involved in multiple biological processes, such as transposon silencing, genomic imprinting, and X-chromosome inactivation9,10,11,12,13. In mammals, DNA methylation predominantly occurs in CG sequence contexts14; however, in plants, three sequence contexts, including CG, CHG and CHH (H represents either A, T or G), are major DNA methylation targets15,16. DNA methylation levels and patterns in plants are dynamically regulated by DNA methyltransferases and demethylases. DNA METHYLTRANSFERASE 1 (MET1) and CHROMOMETHYLASE 3 (CMT3) maintain symmetric CG17 and CHG methylation18, respectively. Methylation of asymmetric CHH contexts must be de novo created by DOMAINS REARRANGED METHYLTRANSFERASE (DRM) during cell replication19. DNA demethylation can be regulated through passive or active processes. Passive demethylation occurs in newly synthesized DNA strand and is caused by dysfunction of DNA methyltransferase, whereas active demethylation is an outcome of replacement of methylated cytosine with non-methylated cytosine under the catalysis of DEMETER-like DNA glyscosylases (DMLs)20,21.
The DMLs act as bifunctional glycosylase/AP-lyase, which removes 5-methylcytosine (5 mC) followed by cleaving the abasic site8. They contain three essential domains, including a DNA glycosylase domain and two additional conserved domains, termed domain A and domain B22. The DNA glycosylase domain has a helix-hairpin-helix (HhH) motif, a glycine/proline-rich region followed by a conserved aspartic acid (GPD), and a [4Fe-4S] cluster motif22. The [4Fe-4S] cluster motif consists of four cysteine residues functioning to hold a [4Fe-4S] cluster and is required for 5 mC excision22. Amino acids in domain A are required for nonspecific DNA binding and all three domains are necessary and sufficient for 5 mC excision22.
DMLs play significant roles in many developmental and biological processes, such as reproduction, seed development, and plant response to biotic and abiotic stresses20,23,24,25,26,27. Arabidopsis contains four DML gene family members, known as AtDME, AtROS1, AtDML2 and AtDML3, respectively. AtDME is necessary for seed viability and is a core regulator of imprinted genes28. It is preferentially expressed in the central cell before fertilization, ensuring maternal expression of the imprinted genes MEA and FWA29,30. AtROS1, AtDML2 and AtDML3 are broadly expressed in vegetative tissues to maintain a proper genome methylation pattern and to regulate relevant genes and transposons31. AtROS1 counteracts RNA-directed DNA Methylation (RdDM) pathway for dynamic transcriptional regulation32, whereas AtDML2 and AtDML3 are involved in removing improper methylated cytosines and maintaining methylation levels of certain targeted sites to make sure an appropriate distribution of genome methylation31. A total of six DML genes exist in the rice genome. It includes OsROS1a, OsROS1b, OsROS1c, OsROS1d, OsDML3a and OsDML3b, of which four are AtROS1 orthologs, whereas the other two are AtDML3 orthologs24. The function of OsROS1a is analogous, to certain extent, to that of AtDME in both male and female gametophytes33. OsROS1c is responsible for the demethylation of retrotransposon Tos17 and plays a critical role in promoting the transposition of Tos1721. Little is known about the function of other rice DMLs and DMLs in other plants.
Based on current knowledge of epigenetics, we presume that epigenetic regulation is involved in secondary metabolism and Dao-di herb formation, two important issues in medicinal plant biology. With the long-term goal to test this hypothesis and to elucidate the underlying mechanisms, we analyze and report here the DML gene family in S. miltiorrhiza. Comparative analysis showed that SmDMLs were conserved with Arabidopsis and rice DMLs. They differentially expressed in various S. miltiorrhiza tissues and were responsive to DNA methylation inhibitor (5-aza-2′-deoxycytidine, 5Aza-dC) treatments. The expression of SmDML1 is posttranscriptionally regulated by Smi-miR7972. The existence of MIR7972s in plants was systematically investigated. MIR7972 genes were identified in some species belonging to three orders, including Lamiales, Solanales, and Boraginales. The distribution patterns were scattered and the number of gene members was varied among species. The results provide the first hand of information for elucidating the role of epigenetic regulation in medicinal plants.
Results
Identification and comparative analysis of SmDMLs
Since S. miltiorrhiza has significant medicinal value and is being developed rapidly as a model system for medicinal plant biology, two research groups sequenced the whole genomes of two different S. miltiorrhiza lines, one of which is known as 99-37, whereas the name of the other one is unknown6. The draft genome assemblies of line 99-3 and the name-unknown line are about 559 and 641 Mb, respectively, although the estimated genome sizes are 615 and 645.78 Mb, respectively6,7. It suggests that both of the current S. miltiorrhiza genome assemblies are incomplete. In order to identify S. miltiorrhiza DEMETER-like DNA glycosylase gene family (SmDMLs), we searched the two genome assemblies using four Arabidopsis AtDML proteins as queries. The retrieved genomic DNA sequences were first computationally predicted for gene models on the GENSCAN web server34 and then manually examined and corrected by comparison with DML genes identified from other plants (www.ncbi.nlm.nih.gov/blast/) and by alignment with RNA-seq data of S. miltiorrhiza transcriptome (http://www.ncbi.nlm.nih.gov/sra). After correcting various genomic DNA sequence errors, we obtained six full-length SmDML genes from the genome assembly of the name-unknown S. miltiorrhiza line and four full-length SmDML genes and several SmDML gene fragments from the genome assembly of line 99-3. Amino acid sequence alignment showed that each of the four SmDML proteins from line 99-3 was identical to a SmDML protein from the name-unknown S. miltiorrhiza line. Sequence comparison of line 99-3 SmDML gene fragments, transcriptome of line 99-3 and the other two SmDML genes from the name-unknown S. miltiorrhiza line showed that line 99-3 SmDML gene fragments encoded partial SmDML proteins identical to the other two SmDML proteins from the name-unknown S. miltiorrhiza line. Taken together, we conclude that the SmDML gene family includes six members, which are designated as SmDML1–SmDML6, respectively (Table 1). The number of SmDMLs is more than AtDMLs, whereas it is same as OsDMLs24.
SmDML genes contain 18–20 introns (Table 1, Fig. 1). Although similarities of gene structures exist among the six SmDMLs, it is particularly high among SmDML1, SmDML2 and SmDML3 and between SmDML5 and SmDML6. In addition, the structures of SmDML1, SmDML2 and SmDML3 are highly similar to four AtDMLs, OsROS1a–OsROS1d and OsDML3a (Fig. 1). The deduced SmDML proteins have amino acid number varying from 720 (SmDML4) to 2030 (SmDML3), isoelectric point (pI) from 6.76 (SmDML5) to 9.27 (SmDML4), and molecular weight (Mw) from 80.5 kDa (SmDML4) to 226.5 (SmDML3) (Table 1). Wide ranges of gene length, pI and Mw also exist among AtDMLs and OsDMLs (Table 1). It suggests the conservation and divergence of sequence features of plant DMLs.
Conserved domains and motifs of DMLs
It has been shown that DML proteins contain a DNA glycosylase domain and two additional conserved domains A and B22. Multiple sequence alignment of deduced amino acid sequences showed that all of the six SmDMLs also contain the three domains (Fig. 2, see Supplementary Figs S1–3). The DNA glycosylase domain is highly conserved among 15 analyzed DMLs, including six SmDMLs, four AtDMLs and five OsDMLs, and contains HhH, GPD and [4Fe-4S] cluster motifs (Fig. 2). Compared with other DMLs analyzed, OsDML3b has less conserved DNA glycosylase domain. Differing from the DNA glycosylase domain, the sequences of domains A and B are highly conserved among all 16S. miltiorrhiza, Arabidopsis and rice DMLs (see Supplementary Figs S2,3). In addition to the amino acid sequence, the location of domains is conserved among all DMLs from S. miltiorrhiza, Arabidopsis and rice (see Supplementary Fig. S1). These results suggest that the majority of domains in DMLs from different plant species are deeply conserved. It is consistent with the critical function of these domains in 5 mC excision.
In order to further determine the conservation and divergence of plant DMLs, we searched conserved motifs in SmDMLs, AtDMLs and OsDMLs using the MEME suite (http://meme.sdsc.edu/meme/meme.html). A total of 15 conserved motifs were identified (Fig. 3). The length of motifs ranges from 18 to 150 amino acids (Table 2). The number of motifs in each DML varies between 7 and 15. Motif 1 is actually the DNA glycosylase domain. It exists in 15 of the 16 DMLs analyzed (Fig. 3). No motif 1 was detected in OsDML3b. It is consistent with the fact that the DNA glycosylase domain is less conserved in OsDML3b compared with that in other DMLs analyzed. Motifs 2 and 9 are located in domain A. Motif 2 exists in all 16 DMLs analyzed, whereas motif 9 was found in 13 of the 16 DMLs. No motif 9 was detected in SmDML4, OsDML3a and OsDML3b. It suggests that motif 2 is more conserved than motif 9, although both of them are located in the conserved domain A. Motifs 3, 4, 5, 7, 8 and 11 are located in domain B. Among them, motifs 3, 4, 5, 7 and 8 existing in 14 or 15 DMLs show relatively high conservation, whereas motif 11, which was detected in 11 of 16 DMLs, is the least conserved. In addition to the nine motifs found in conserved domains, other six, including motifs 6, 10, 12, 13, 14 and 15, are located in less conserved regions. Among them, motifs 6, 10 and 13 are highly conserved, whereas motifs 12, 14 and 15 are specific to OsROS1c and OsROS1d. Motifs commonly existing in DMLs are probably associated with the conserved functions of DMLs, but those specific to a few DMLs seem to be related to gene-specific functions.
Phylogenetic analysis of DML proteins
In order to determine the evolutionary relationship among DMLs, an unrooted neighbor-joining tree was constructed using 66 full length protein sequences from 16 plant species. DML proteins were clustered into three orthology groups, including the DME group, the ROS1 group, and the DML3 group (Fig. 4). The ROS1 group is the largest group. It contains 33 DML proteins. Based on the phylogenetic tree constructed, the ROS1 group may be further divided into the monocot subgroup and the dicot subgroup. Similar to the ROS1 group, the DML3 group may also be divided into the monocot subgroup and the dicot subgroup. DME group is only restricted to dicots. It indicates that DME may be phylogenetically monophyletic in dicots. These results are consistent with previous phylogenetic tree constructed using conserved DNA glycosylase domains of DMLs from flowering plants24. It suggests the conservation and divergence of DML proteins in monocots and dicots.
All of SmDMLs showed close phylogenetic relationships with DMLs from monkey flower (Erythranthe guttatus) (Fig. 4). It is consistent with the fact that both S. miltiorrhiza and E. guttatus are members of Lamiales. Based on gene structures, conserved motifs and phylogenetic relationships, SmDML2 and SmDML5 are closely related with SmDML3 and SmDML6, respectively. SmDML2 and SmDML3 share 62.88% protein sequence identity and 66.16% cDNA similarity. Protein sequence identity and cDNA similarity between SmDML5 and SmDML6 are 89.58% and 93.17%, respectively. It implies duplication events occurred during SmDMLs evolution. To test the phylogenetic selection pressure on these genes after duplication, we analyzed the substitution rate ratios of nonsynonymous (Ka) versus synonymous (Ks) mutations (Ka/Ks)35. Generally, Ka/Ks = 1 suggests that the genes are pseudogenes with neutral selection, whereas less than 1 implies purifying or stabilizing selection35. The calculated Ka/Ks values between SmDML2 and SmDML3 and between SmDML5 and SmDML6 are 0.3833 and 0.9799, respectively. It indicated that SmDML2 and SmDML3 paralogous pairs experienced strong purifying selection, whereas SmDML5 and SmDML6 paralogous pairs could be pseudogenes with neutral selection. These results suggested that specific SmDMLs experienced distinct phylogenetic selection pressure.
Differential expression of SmDMLs in S. miltiorrhiza
As the main participants of DNA demethylation, DMLs play important roles in plant growth and development36,37. To preliminarily explore the biological function of SmDMLs, the expression of six SmDMLs in flowers, leaves, roots and stems of 2-year-old, field nursery-grown S. miltiorrhiza plants was analyzed using quantitative RT-PCR technology. Although the transcripts of all six SmDMLs were detected in the tissues analyzed, significant differential expression patterns were observed (Fig. 5). SmDML1 showed the highest expression in leaves and flowers. Its expression in roots was the lowest among the four tissues analyzed. The expression patterns of SmDML1 are different from its Arabidopsis counterpart, AtROS1, which showed high expression in stems and roots and low in flowers31. SmDML2 and SmDML3 are paralogs closely related (Fig. 4). Consistently, they showed similar expression patterns with the highest in flowers, followed by stems, leaves and roots. SmDML4 was predominantly expressed in flowers. Although SmDML5 and SmDML6 are probably pseudogenes based on Ka/Ks, they were expressed. SmDML5 showed the highest expression in flowers and stems, followed by leaves, and the lowest in roots, whereas SmDML6 was expressed mainly in flowers and leaves (Fig. 5). Expressed pseudogenes have been reported in other plants, such as rice35 and barley38.
Expression patterns of SmDMLs in roots (Rt), stems (St), leaves (Le) and flowers (Fl) of S. miltiorrhiza. The expression levels were analyzed using the quantitative RT-PCR method. Fold changes of SmDML expression are shown. Expression level in leaves was arbitrarily set to 1 and the levels in other organs were given relative to this. One-way ANOVA was calculated using IBM SPSS 20 software. P < 0.05 was considered statistically significant and was represented by different letters. Error bars was indicated by the standard deviations of three biological replicates.
To further analyze the expression patterns of SmDMLs, we analyzed RNA-seq data generated for periderm, phloem and xylem of S. miltiorrhiza roots. SmDML1 exhibited the highest expression in xylem, followed by phloem and periderm. The expression of SmDML2 and SmDML3 paralogs showed similar patterns with the highest in phloem, followed by xylem and periderm. However, the differential expression for each of them in the three tissues of S. miltiorrhiza roots was not significant as analyzed using TopHat2.0.12 and Cufflinks2.2.139 (Table S3). The FPKM (Fragments Per Kilobase of transcript per Million mapped reads) values of SmDML4–SmDML6 were very low, which are consistent with the low expression levels in roots analyzed using the quantitative RT-PCR technology (Fig. 5).
Expression patterns of SmDML genes in response to 5Aza-dC
It has been shown that DNA methylation-related genes are responsive to DNA methylation inhibitor treatment and methylation-sensitive expression of ROS1 in plants is conserved and adaptive40. To elucidate the expression patterns of SmDML genes in response to DNA methylation inhibitor treatment, we treated S. miltiorrhiza plantlets with different concentrations of the DNA methyltransferase inhibitor, 5Aza-dC. Gene expression analysis showed that SmDML1, SmDML2 and SmDML4 were significantly methylation-responsive (Fig. 6). SmDML1 transcripts were significantly reduced regardless of the concentration of 5Aza-dC treated (Fig. 6). The expression of SmDML2 was significantly decreased at 5 µM and 10 µM 5Aza-dC treatments; however, reduced expression of SmDML2 was not significant at higher 5Aza-dC concentrations (Fig. 6). The expression of SmDML4 was significantly decreased at 10 µM, 30 µM and 50 µM 5Aza-dC treatments. SmDML5 and SmDML6 transcript levels were not influenced by 5Aza-dC treatments, indicating they could be not involved in DNA demethylation. Interestingly, the expression of SmDML3 was not detected in S. miltiorrhiza plantlets (Fig. 6); however, it was expressed in all mature tissues analyzed (Fig. 6), implying its important roles in S. miltiorrhiza plant maturation. Taken together, these data suggest that DNA methylation changes can influence SmDML expression in S. miltiorrhiza.
Responses of SmDML genes to 5Aza-C treatment. Fold changes of SmDMLs in leaves of S. miltiorrhiza plantlets treated with 1, 5, 10, 30 or 50 µM of 5Aza-C for 15 days are shown. The expression levels were analyzed using the quantitative RT-PCR method. Expression level in leaves without treatment (0 µM) was arbitrarily set to 1 and the levels in leaves of 5Aza-C-treated plantlets were given relative to this. One-way ANOVA was calculated using IBM SPSS 20 software. P < 0.05 was considered statistically significant and was represented by asterisks.
miRNA-mediated posttranscriptional regulation of SmDML1
In Arabidopsis, AtDML3 transcripts are cleaved by miR40241,42. In Nicotiana benthamiana, NbROS1 is targeted by Nb_miRC1_3p43. In order to know whether SmDMLs are regulated by miRNAs, we searched high-throughput S. miltiorrhiza small RNA database for miRNAs potentially targeting SmDMLs using psRNATarget44,45. A total of 44 small RNAs with sequence reads greater than four were identified under the maximum expectation of 3.0. The retrieved small RNA sequences were aligned with S. miltiorrhiza 99-3 genome and then secondary structures of genomic DNA sequences surrounding the small RNAs were predicted and manually checked. It resulted in the identification of Smi-MIR7972. This miRNA gene generates two 21nt-miRNAs, termed Smi-miR7972a and Smi-miR7972b, respectively. Smi-miR7972a and Smi-miR7972b are overlapped, and Smi-miR7972a starts 1nt upstream relative to Smi-miR7972b (Fig. 7a). Quantitative qRT-PCR analysis using Smi-miR7972a- and Smi-miR7972b-specific primers showed that Smi-miR7972a was highly expressed in leaves of 2-year-old, field nursery-grown S. miltiorrhiza plants (Fig. 7b). Its expression in roots, stems and flowers is similar and relatively low compared with its expression in leaves. Smi-miR7972b showed the highest expression in stems. Its expression in roots, leaves and flowers is similar. The expression of Smi-miR7972a was higher than Smi-miR7972b in all tissues analyzed (Fig. 7b). Blast analysis of Smi-miR7972a and Smi-miR7972b against miRBase (http://www.mirbase.org/) showed that Smi-miR7972a was identical to the functionally unknown Rgl-miR7972 from Rehmannia glutinosa46. Examination of Smi-miR7972a and Nb_miRC1_3p sequences revealed that they were highly similar with only a mismatched nucleotide. It suggests that Nb_miRC1_3p is actually a member of the MIR7972 family. Thus, Nb_miRC1_3p was renamed to Nbe-miR7972 in this report (Fig. 7c).
Smi-miR7972 in S. miltiorrhiza. (a) The hairpin structure of Smi-miR7972. Smi-miR7972a and Smi-miR7972b are indicated by red and green lines. (b) Expression patterns of Smi-miR7972a and Smi-miR7972b in roots (Rt), stems (St), leaves (Le) and flowers (Fl) of S. miltiorrhiza. Fold changes of Smi-miR7972a and Smi-miR7972b are shown. Expression level of Smi-miR7972a in roots was arbitrarily set to 1 and the levels of Smi-miR7972a and Smi-miR7972b were given relative to this. Error bars was indicated by the standard deviations of three biological replicates. (c) Sequence alignment of miR7972s from S. miltiorrhiza, R. glutinosa and N. benthamiana. (d) Validation of Smi-miR7972a- and Smi-miR7972b-mediated cleavage using the modified 5′ RLM RACE method. Heavy black line represents open reading frame of SmDML1. The complementary sites of Smi-miR7972 in SmDML1 are represented by A and B and shown in red. The nucleotide sequences of Smi-miR7972a and Smi-miR7972b from 3′ to 5′ and the complementary sites of SmDML1 from 5′ to 3′ are shown in the expanded regions. Vertical dashes indicate Watson-Crick pairing. Circles indicate G:U wobble pairing. Vertical arrows indicate the 5′ termini of Smi-miR7972-mediated cleavage products, as obtained by 5′ RLM-RACE, with the frequency of clones shown.
Computational target prediction showed that SmDML1 contained two regions near-perfectly complementary to Smi-miR7972a and Smi-miR7972b (Fig. 7d). The regions were named region A and region B, respectively. The expectations are 1.0 and 3.0 for region A and region B, respectively, as calculated using psRNATarget44. To analyze the cleavage site of Smi-miR7972a and Smi-miR7972b in SmDML1, the modified 5′RLM-RACE experiments were carried out as previously described47. The results confirmed that SmDML1 was indeed cleaved at region A in vivo (Fig. 7d). However, the cleavage at region B was not validated. It indicates that region B is not cleaved due to the greater expectation. It is also possible that region B is cleaved only in certain tissues or plant developmental stages. Plant miRNAs usually cleave target mRNAs at the tenth complementary nucleotide from the 5′ end of the miRNA48,49. Investigation of cleavage site at region A showed that there were two cleavage sites. One corresponds to the tenth complementary nucleotide from the 5′ end of Smi-miR7972b, confirming the cleavage by Smi-miR7972b. The other one corresponds to the twelfth and thirteenth complementary nucleotides from the 5′ end of Smi-miR7972b and Smi-miR7972a, respectively. It implied that this site was cleaved neither Smi-miR7972b nor Smi-miR7972a, indicating the complexity of mRNA cleavage mechanism.
Phylogeny of MIR7972 genes
So far, miR7972 has been identified only in three plant species, including S. miltiorrhiza, N. benthamiana and R. glutinosa, all of which are placed in Lamiids. In order to know the phylogeny of MIR7972 genes in plants, systematic and comprehensive investigation was carried out on the whole genome sequences of 40 Lamiids species (Table S4) and almost all RNA-seq data available for Lamiids species in SRA database. MIR7972 genes exist only in Solanales, Boraginales and Lamiales. It was not found in other orders of the Lamiids clade. In total, 62 MIR7972 genes were identified (Table S5), of which 34 were from 29 Lamiales species, 26 from 8 Solanales species, whereas the other two were from two Boraginales species. Interestingly, in Solanales, MIR7972s were identified only in Nicotianeae of Solanaceae and Ipomoea of Convolvulaceae. In Boraginales, MIR7972s were identified only in two plant species of Lithospermum. However, Lamiales MIR7972s widely exist in various families, including Oleaceae, Gesneriaceae, Plantaginaceae, Pedaliaceae, Verbenaceae, Lamiaceae, Phrymaceae, Paulowniaceae, and Orobanchaceae (Fig. 8). The majority of these families are not evolutionarily close to each other (http://www.mobot.org/MOBOT/Research/APweb/welcome.html). It indicates the complexity of MIR7972 origin and evolution. The underlying mechanism remains to be elucidated. Most of the Lamiales species contain one MIR7972 gene, whereas multiple copies were found in all Nicotianeae species, suggesting the occurrence of MIR7972 duplication in Nicotianeae.
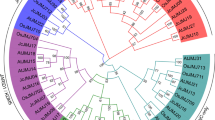
Phylogenetic relationships of MIR7972 precursors in various Lamiids species. It includes Ruellia speciosa (rsp), Mentha longifolia (mlo), Ocimum tenuiflorum (ote), Fraxinus excelsior (fex), Dorcoceras hygrometricum (dhy), Sesamum indicum (sin), Erythranthe guttata (egu), Nicotiana obtusifolia (nob), Ipomoea nil (ini), Ipomoea trifida (itr), Nicotiana attenuata (nat), Nicotiana sylvestris (nsy), Nicotiana tomentosiformis (nto), Nicotiana tabacum (nta), Nicotiana benthamiana (nbe), Andrographis paniculata (apa), Jasminum sambac (jsa), Syringa oblata (sob), Fraxinus pennsylvanica (fpe), Olea europaea (oeu), Osmanthus fragrans (ofr), Alectra vogelii (avo), Rehmannia glutinosa (rgl), Phtheirospermum japonicum (pja), Pedicularis keiskei (pke), Conopholis americana (cam), Paulownia fortunei (pfo), Paulownia tomentosa (pto), Plantago ovata (pov), Plantago lagopus (pla), Lippia dulcis (ldu), Tectona grandis (tgr), Ocimum basilicum (oba), Perilla frutescens (pfr), Rosmarinus officinalis (rof), Mentha spicata (msp), Lithospermum erythrorhizon (ler), Arnebia euchroma (aeu). Species from Lamiales, Solanales and Boraginales are shown red, green and purple, respectively. MIR7972s could be divided into two groups, including group I and group II. The branch length is shown.
In order to reveal the phylogenetic relationship of MIR7972s, a neighbor-joining (NJ) phylogenetic tree was constructed for precursor sequences of the identified MIR7972s using MEGA7.0 (Fig. 8). MIR7972s could be divided into two groups. Group I contains Lamiales MIR7972s, whereas group II consists of MIR7972s from Solanales and Boraginales. It suggests closer evolutionary relationships between MIR7972s in Boraginales and Solanales in comparison with Lamiales. The results are consistent with previous studies showing that Lithospermeae species exhibit closer evolutionary relationships with Solanales species50.
Disscussion
S. miltiorrhiza is a widely cultivated medicinal crop in East Asia and a model medicinal plant for TCM studies. It has been used as medicinal materials to treat cerebrovascular diseases and cardiovascular diseases for hundreds of years51,52. Many genes associated with the production of bioactive compounds, including lipid-soluble tanshinones and water-soluble phenolic acids, have been characterized2,53,54. However, little is known about epigenetic factors regulating gene expression in S. miltiorrhiza. Elucidation of regulatory process of DNA methylation is important for understanding gene expression regulatory mechanism associated with secondary metabolism and Dao-di herb formation in S. miltiorrhiza. In this study, six S. miltiorrhiza SmDML genes involved in DNA demethylation were identified and characterized. The number of SmDML genes is similar to those from castor bean, tomato, Arabidopsis and rice, ranging from three to six24,55,56.
Phylogenetic analysis of 66 DMLs from 16 plant species showed that SmDML1 was a member of the ROS1 group, SmDML2–SmDML4 belonged to the DME group, and SmDML5 and SmDML6 were included in the DML3 group (Fig. 4). The results from Arabidopsis and rice showed that DMLs in a group may have different functions. For instance, both rice OsROS1a and OsROS1c are members of the ROS1 group. OsROS1a is indispensable in both male and female gametophytes and critical to gametophytes33, whereas OsROS1c promotes expression and transposition of Tos1721. Among the three SmDMLs of the DME group, SmDML4 is the smallest with only 720 amino acids. It lacks conserved motifs 9, 10, 11, and 13 compared with SmDML2 and SmDML3 (Fig. 3). SmDML2–SmDML4 showed differential expression in mature plants (Fig. 5) and SmDML3 was not expressed in plantlets. Similarly, differential expression was observed for SmDML5 and SmDML6 (Fig. 5), two SmDMLs of the DML3 group. It indicates that SmDMLs from a group may also be involved in distinct biological processes.
Generally, DNA methylation suppresses gene expression, whereas DNA demethylation promotes gene expression. In this study, we found that the expression of SmDMLs was down-regulated after 5Aza-dC treatment. It is consistent with significant down-regulation of DMLs in DNA methylation mutants in Arabidopsis57,58. Low expression of SmDMLs may contribute to maintain the certain DNA methylation level under the presence of 5Aza-dC. Balance between DNA methylation and demethylation is important for plant growth and development59,60. In Arabidopsis, the balance is monitored by a DNA methylation monitoring sequence (MEMS) in the ROS1 promoter region40,60. It is unknown whether this mechanism also exists in S. miltiorrhiza. Alternatively, SmDMLs were not directly regulated by DNA methylation. Down-regulation of SmDMLs under 5Aza-dC treatment was mediated by a complex network with various mediators.
Plant miRNAs are a class of small non-coding RNAs with about 21–22 nt in length. They play vital roles in many biological processes through RNA cleavage61. Arabidopsis AtDML3 is regulated by miR402. The regulation is important for seed germination under stress conditions42. In N. benthamiana, miRNA-mediated repression of ROS1 may strength transcriptional gene silencing43. Although S. miltiorrhiza miRNAs have been reported3,45,62,63,64,65, their functions are largely unknown. Analysis of high-throughput sRNA data allowed us to identify Smi-miR7972a and Smi-miR7972b targeting SmDML1 for cleavage. It indicates that miRNAs play significant roles in the regulation network of DNA methylation in S. miltiorrhiza. Smi-miR7972b accumulated at lower levels than Smi-miR7972a in the tissues analyzed (Fig. 7b). The variance between the levels of Smi-miR7972a and Smi-miR7972b could be caused by differential sequence preference of DICER-LIKE 1 (DCL1) responsible for miRNA biogenesis66,67. Experimental evidence showed that the cleavage of SmDML1 was mediated by Smi-miR7972b rather than Smi-miR7972a in the tissues analyzed (Fig. 7d). The possibility that Smi-miR7972a cuts SmDML1 in other tissues cannot be ruled out. Alternatively, Smi-miR7972b is easier to be recruited by AGO proteins for the formation of RNA induced silencing complex (RISC)68.
MIR7972 genes were only identified in some species of the three Lamiids orders, including Solanales, Lamiales and Boraginales, and the number of MIR7972 genes varied among species. The actual origin and evolution mechanism of MIR7972 is unknown. One of the possibilities is that the MIR7972 genes in different plant species were originated from a common ancestor of Solanales, Lamiales and Boraginales. Loss and duplication of MIR7972 occurred in some plant species during evolution. This possibility is consistent with frequent birth and death of some MIRNA genes69. Independent origin and evolution of MIR7972s in different lineage is the other possibility. Evidence to support this possibility is that some miRNAs evolved are rarely lost and highly conserved across taxa70. The gain and loss of MIR7972 could be important for plants of some lineages to survive in the stressful environments. Further investigating the biological function of MIR7972 will help to elucidate the evolution mechanism of MIR7972.
Materials and Methods
Plant materials
Salvia miltiorrhiza Bunge (line 99-3) plants were cultivated in a field nursery at the Institute of Medicinal Plant Development, Beijing, China. Roots, stem, leaves and flowers of two-year-old plants were collected and stored immediately in liquid nitrogen until use. For 5-aza-2′-deoxycytidine (5Aza-dC) treatment, plantlets were grown on Murashige and Skoog (MS) agar medium71 supplemented with 0, 5, 10, 30 or 50 µM 5Aza-dC (Sigma) for 15 days at 25 °C under a photoperiod of 16 h light and 8 h dark. Newly generated leaves were collected and immediately stored in liquid nitrogen until use. Three independent biological replicates were carried out for each treatment.
SmDML gene identification
The deduced amino acid sequences of four Arabidopsis DML proteins were downloaded from the TAIR database (http://www.arabidopsis.org). To predict SmDML genes, AtDMLs were used as queries to search the two databases of S. miltiorrhiza whole genome sequence6,7. The searches were carried out using the tBLASTN program72. An e-value cut off of 1e−10 was applied. Gene models were predicted on the GENSCAN web server (http://genes.mit.edu/GENSCAN.html) for retrieved genomic DNA sequences34. The predicted gene models were then manually examined and corrected by comparison with DML genes identified from other plants using the BLASTx algorithm (www.ncbi.nlm.nih.gov/blast/) and by alignment with RNA-seq data of S. miltiorrhiza transcriptome (http://www.ncbi.nlm.nih.gov/sra). The INTERPRO database (http://www.ebi.ac.uk/interpro/) was finally used to confirm each predicted protein sequence to be a DML.
Gene structure and protein sequence analysis
Gene structures of SmDMLs, AtDMLs and OsDMLs were determined on the Gene Structure Display Server (GSDS 2.0; http://gsds.cbi.pku.edu.cn/index.php). Coding sequences and corresponding genomic sequences were used as inputs. The deduced protein sequences of SmDMLs, AtDMLs and OsDMLs were analyzed for amino acid number, molecular weight (Mw), theoretical isoelectric point (pI) using the EXPASY PROTOPARAM tool (http://www.expasy.org/tools/protparam.html). Multiple sequence alignment was performed for SmDML, AtDML and OsDML amino acid sequences using ClustalW. Conserved motifs in SmDML, AtDML and OsDML proteins were detected using the MEME suite (http://meme.sdsc.edu/meme/meme.html).
Phylogenetic analysis
Unrooted neighbor-joining (NJ) trees were constructed using MEGA (version 7.0) with 1000 bootstrap replicates73. Protein sequences of DMLs from 16 plant species were downloaded from Phytozome (http://phytozome.jgi.doe.gov/pz/portal.html) (Table S1). Ka and Ks values were calculated for two gene pairs, SmDML2/SmDML3 and SmDML5/SmDML6, using DNASP5 software74.
RNA extraction and qRT-PCR analysis
Total RNA was isolated from S. miltiorrhiza tissues using the plant total RNA extraction kit (Aidlab, China). The isolation was carried out following the manufacturer’s instructions. RNA integrity was analyzed on an agarose gel. RNA quantity was determined using a NanoDrop 2000C spectrophotometer (Thermo Scientific, USA). Reverse transcription was conducted using PrimeScript™ RT reagent kit (TaKaRa, Japan). Gene specific primers were designed using Primer Premier 6 (PREMIER Biosoft Int, USA) based on SmDML coding sequences. SmUBQ10 was used as an internal control as described previously2. The expression of Smi-miR7972a and Smi-miR7972b was analyzed using Mir-X miRNA qRT-PCR SYBR Kit (TaKaRa, Japan). The primers were listed in Table S2. qRT-PCR was performed in triplicate for each tissue sample using the SYBR premix Ex Taq™ kit (TaKaRa, China) on a CFX96 Touch™ real-time PCR system (Bio-Rad, USA). Three independent biological replicates were performed. Gene relative expression levels were calculated for Ct values using the 2−ΔΔCq method75. Differential expression among tissues and treatments was determined by one-way ANOVA using IBM SPSS 20 software (IBM Corporation, USA).
RAN-Seq data and bioinformatic analysis
Transcriptome sequencing data generated for periderm, phloem and xylem of S. miltiorrhiza roots was downloaded from SRA database of NCBI (SRX751296)54. Differential expression of SmDML genes was analyzed using TopHat2.0.12 and Cufflinks2.2.139.
Identification of S. miltiorrhiza miRNAs potentially targeting SmDMLs
S. miltiorrhiza small RNAs potentially targeting SmDMLs for cleavage were predicted using psRNATarget44. Small RNAs from roots, stems, leaves and flowers of S. miltiorrhiza were downloaded from SRA database (SRX686651, SRX686652, SRX686653 and SRX686654)45. The maximum expectations of 3.0 and the target accessibility-allowed maximum energy to unpair the target site of 25 were applied. The predicted small RNAs were mapped to S. miltiorrhiza 99-3 genome using Bowtie76. No mismatch was allowed. Secondary structures of genomic sequences surrounding small RNA-aligned regions were predicted on the mfold web server77. The structures were manually checked and miRNAs were annotated under the criteria described70.
5′ RLM-RACE validation of miR7972-directed cleavage
Roots, stems, leaves and flowers of two-year-old S. miltiorrhiza were used for validation of miR7972-directed cleavage. The modified RNA ligase-mediated rapid amplification of 5′ cDNAs (5′ RLM-RACE) was carried out using the FirstChoice® RLM-RACE Kit (Invitrogen, Carlsbad, CA). The nesting and nested primers were 5′-GGGGCAACCTGGTGAGATTCCATCT-3′ and 5′-ACCGGTTAACACCATTTTTCCGA-3′, respectively. Nesting PCR was carried out under the touchdown conditions: 94 °C for 3 min, 5 cycles of 94 °C for 30 s and 72 °C for 90 s, 5 cycles of 94 °C for 30 s, 70 °C for 30 s and 72 °C for 50 s, 25 cycles of 94 °C for 30 s, 60 °C for 30 s and 72 °C for 1 min, followed by a final extension at 72 °C for 10 min. Nested PCR amplification was performed under following conditions: 94 °C for 3 min, 35 cycles of 94 °C for 30 s, 60 °C for 30 s and 72 °C for 30 s, followed by a final extension at 72 °C for 10 min.
Identification of MIR7972 genes in Lamiids
MIR7972 precursors from S. miltiorrhiza, Rehmannia glutinosa and Nicotiana benthamian were used to blast genomes of 40 Lamiids plant species listed in Table S3 using BLASTn72. Transcriptome-wide identification of MIR7972 was performed through BLAST analysis of Smi-MIR7972 or Nbe-MIR7972 against RNA-seq reads (https://www.ncbi.nlm.nih.gov/sra) from Lamiids plants using BLASTn72.
References
Cheng, T. O. Danshen a popular chinese cardiac herbal drug. J. Am. Coll. Cardiol. 47, 1487–1501 (2006).
Ma, Y. et al. Genome-wide identification and characterization of novel genes involved in terpenoid biosynthesis in Salvia miltiorrhiza. J. Exp. Bot. 63, 2809–2823 (2012).
Shao, F., Qiu, D. & Lu, S. Comparative analysis of the Dicer-like gene family reveals loss of miR162 target site in SmDCL1 from Salvia miltiorrhiza. Scientific Reports 5, 9891 (2015).
Ji, A. J. et al. Genome-wide identification of the AP2/ERF gene family involved in active constituent biosynthesis in Salvia miltiorrhiza. Plant. Genome 9, 1–11 (2016).
Song, J. Y. et al. Salvia miltiorrhiza as medicinal model plant. Yao Xue Xue Bao 48, 1099–1106 (2013).
Zhang, G. et al. Hybrid de novo genome assembly of the Chinese herbal plant danshen (Salvia miltiorrhiza Bunge). GigaScience 4, 62 (2015).
Xu, H. et al. Analysis of the genome sequence of the medicinal plant Salvia miltiorrhiza. Molecular Plant 6, 949–952 (2016).
Zhang, H. & Zhu, J. K. Active DNA demethylation in plants and animals. Cold Spring Harb Symp. Quant. Biol. 77, 161–173 (2012).
Bala Tannan, N. et al. DNA methylation profiling in X;autosome translocations supports a role for L1 repeats in the spread of X chromosome inactivation. Hum. Mol. Genet. 23, 1224–1236 (2014).
Fu, Y. et al. Mobilization of a plant transposon by expression of the transposon-encoded anti-silencing factor. EMBO J. 32, 2407–2417 (2013).
Chan, S. W., Henderson, I. R. & Jacobsen, S. E. Gardening the genome: DNA methylation in Arabidopsis thaliana. Nat. Rev. Genet. 6, 351–360 (2005).
Bird, A. P. & Wolffe, A. P. Methylation-induced repression— belts, braces, and chromatin. Cell 99, 451–454 (1999).
Macdonald, W. A. Epigenetic mechanisms of genomic imprinting: common themes in the regulation of imprinted regions in mammals, plants, and insects. Genet Res Int. 2012, 585024 (2012).
Cedar, H. & Bergman, Y. Linking DNA methylation and histone modification: patterns and paradigms. Nat Rev Genet 10, 295–304 (2009).
Dunoyer, P. et al. An endogenous, systemic RNAi pathway in plants. EMBO J. 29, 1699–1712 (2010).
Law, J. A. & Jacobsen, S. E. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat Rev Genet. 11, 204–220 (2010).
Finnegan, E. J. & Kovac, K. A. Plant DNA methyltransferases. Plant Mol. Biol. 43, 189–201 (2000).
Lindroth, A. M. et al. Requirement of CHROMOMETHYLASE3 for maintenance of CpXpG methylation. Science 292, 2077–2080 (2001).
Wassenegger, M., Matzke, M. A. & Matzke, A. J. M. RNA-directed DNA methylation. Plant Mol. Biol. 43, 203–220 (2000).
Hsieh, T. F. et al. Genome-wide demethylation of Arabidopsis endosperm. Science 324, 1451–1454 (2009).
La, H. et al. A 5-methylcytosine DNA glycosylase/lyase demethylates the retrotransposon Tos17 and promotes its transposition in rice. Proc. Natl. Acad. Sci. USA 108, 15498–15503 (2011).
Mok, Y. G. et al. Domain structure of the DEMETER 5-methylcytosine DNA glycosylase. Proc. Natl. Acad. Sci. USA 107, 19225–19230 (2010).
Penterman, J. et al. DNA demethylation in the Arabidopsis genome. Proc. Natl. Acad. Sci. USA 104, 6752–6757 (2007).
Zemach, A. et al. Local DNA hypomethylation activates genes in rice endosperm. Proc. Natl. Acad. Sci. USA 107, 18729–18734 (2010).
Ibarra, C. A. et al. Active DNA demethylation in plant companion cells reinforces transposon methylation in gametes. Science 337, 1360–1364 (2012).
Yu, A. et al. Dynamics and biological relevance of DNA demethylation in Arabidopsis antibacterial defense. Proc. Natl. Acad. Sci. USA 110, 2389–2394 (2013).
Bharti, P., Mahajan, M., Vishwakarma, A. K., Bhardwaj, J. & Yadav, S. K. AtROS1 overexpression provides evidence for epigenetic regulation of genes encoding enzymes of flavonoid biosynthesis and antioxidant pathways during salt stress in transgenic tobacco. J. Exp. Bot. 66, 5959–5969 (2015).
Choi, Y. et al. DEMETER, a DNA Glycosylase domain protein, is required for endosperm gene imprinting and seed viability in Arabidopsis. Cell 110, 33–42 (2002).
Kinoshita, T. et al. One-way control of FWA imprinting in Arabidopsis endosperm by DNA methylation. Science 303, 521–523 (2004).
Gehring, M. et al. DEMETER DNA glycosylase establishes MEDEA polycomb gene self-imprinting by allele-specific demethylation. Cell 124, 495–506 (2006).
Ortega-Galisteo, A. P., Morales-Ruiz, T., Ariza, R. R. & Roldán-Arjona, T. Arabidopsis DEMETER-LIKE proteins DML2 and DML3 are required for appropriate distribution of DNA methylation marks. Plant Mol. Biol. 67, 671–681 (2008).
Gong, Z. et al. ROS1, a repressor of transcriptional gene silencing in Arabidopsis, encodes a DNA glycosylase/lyase. Cell 111, 803–814 (2002).
Ono, A. et al. A null mutation of ROS1a for DNA demethylation in rice is not transmittable to progeny. Plant J. 71, 564–574 (2012).
Burge, C. B. & Karlin, S. Finding the genes in genomic DNA. Curr. Opin. Struct. Biol. 8, 346–354 (1998).
Thibaudnissen, F., Ouyang, S. & Buell, C. R. Identification and characterization of pseudogenes in the rice gene complement. BMC Genomics 10, 317 (2009).
Agius, F., Kapoor, A. & Zhu, J. K. Role of the Arabidopsis DNA glycosylase/lyase ROS1 in active DNA demethylation. Proc. Natl. Acad. Sci. USA 103, 11796–11801 (2006).
Liu, R. et al. A DEMETER-like DNA demethylase governs tomato fruit ripening. Proc. Natl. Acad. Sci. USA 112, 10804–10809 (2015).
Prade, V. M. et al. The Pseudogenes of Barley. Plant J. 93, 502–514 (2018).
Trapnell, C. et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 7, 562–578 (2012).
Mittelsten Scheid, O., Williams, B. P., Pignatta, D., Henikoff, S. & Gehring, M. Methylation-sensitive expression of a DNA demethylase gene serves as an epigenetic rheostat. PLoS genetics 11, e1005142 (2015).
Sunkar, R. & Zhu, J. K. Novel and stress-regulated microRNAs and other small RNAs from Arabidopsis. Plant Cell 16, 2001–2019 (2004).
Kim, J. Y., Kwak, K. J., Jung, H. J., Lee, H. J. & Kang, H. MicroRNA402 affects seed germination of Arabidopsis thaliana under stress conditions via targeting DEMETER-LIKE protein3 mRNA. Plant Cell Physiol. 51, 1079–1083 (2010).
Baksa, I. et al. Identification of Nicotiana benthamiana microRNAs and their targets using high throughput sequencing and degradome analysis. BMC Genomics 16, 1–21 (2015).
Dai, X. & Zhao, P. X. psRNATarget: a plant small RNA target analysis server. Nucleic Acids Res. 39, W155–W159 (2011).
Xu, X. et al. Deep sequencing identifies tissue-specific microRNAs and their target genes involving in the biosynthesis of tanshinones in Salvia miltiorrhiza. PloS one 9, e111679 (2014).
Li, M. J. et al. Transcriptome/degradome-wide identification of R. glutinosa miRNAs and their targets: the role of miRNA activity in the replanting disease. PloS one 8, e68531 (2013).
Lu, S. et al. Novel and mechanical stress responsive microRNAs in Populus trichocarpa that are absent from Arabidopsis. Plant Cell 17, 2186–2203 (2005).
Rhoades, M. W. et al. Prediction of plant microRNA targets. Cell 110, 513–520 (2002).
Schwab, R. et al. Specific effects of microRNAs on the plant transcriptome. Dev. Cell 8, 517–527 (2005).
Wu, F. Y. et al. Transcriptome analysis explores genes related to shikonin biosynthesis in Lithospermeae plants and provides insights into Boraginales’ evolutionary history. Scientific Reports 7, 4477 (2017).
Zhou, L., Zuo, Z. & Chow, M. S. Danshen: an overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J. Clin. Pharmacol. 45, 1345–1359 (2005).
Yang, H., Han, L., Sheng, T., He, Q. & Liang, J. Effects of Replenishing Qi, promoting blood circulation and resolving phlegm on vascular endothelial function and blood coagulation system in senile patients with hyperlipemia. J. Tradit. Chin. Med. 26, 120–124 (2006).
Cui, G. et al. Functional divergence of diterpene syntheses in the medicinal plant Salvia miltiorrhiza. Plant Physiol. 169, 1607–1618 (2015).
Xu, Z. et al. Full-length transcriptome sequences and splice variants obtained by a combination of sequencing platforms applied to different root tissues of Salvia miltiorrhiza and tanshinone biosynthesis. Plant J. 82, 951–961 (2015).
Cao, D. et al. Genome-wide identification of cytosine-5 DNA methyltransferases and demethylases in Solanum lycopersicum. Gene 550, 230–237 (2014).
Xu, W., Yang, T., Dong, X., Li, D. Z. & Liu, A. Genomic DNA methylation analyses reveal the distinct profiles in castor bean seeds with persistent endosperms. Plant Physiol. 171, 1242–1258 (2016).
Jia, Y. et al. Loss of RNA–Dependent RNA Polymerase 2 (RDR2) function causes widespread and unexpected changes in the expression of transposons, genes, and 24-nt small RNAs. PLoS genetics 5, e1000737 (2009).
Madzima, T. F., Huang, J. & Mcginnis, K. M. Chromatin structure and gene expression changes associated with loss of MOP1 activity in Zea mays. Epigenetics 9, 1047–1059 (2014).
Cao, X. & Jacobsen, S. E. Role of the Arabidopsis DRM methyltransferases in de novo DNA methylation and gene silencing. Curr. Biol. 12, 1138–1144 (2002).
Lei, M. et al. Regulatory link between DNA methylation and active demethylation in. Arabidopsis. Proc. Natl. Acad. Sci. USA 112, 3553–3557 (2015).
Kidner, C. A. & Martienssen, R. A. The developmental role of microRNA in plants. Curr. Opin. Plant Biol. 8, 38–44 (2005).
Zhang, L. et al. Genome-wide analysis and molecular dissection of the SPL gene family in Salvia miltiorrhiza. J. Integr. Plant Biol. 56, 38–50 (2014).
Shao, F. & Lu, S. Genome-wide identification, molecular cloning, expression profiling and posttranscriptional regulation analysis of the Argonaute gene family in Salvia miltiorrhiza, an emerging model medicinal plant. BMC Genomics 14, 512 (2013).
Li, C. & Lu, S. Genome-wide characterization and comparative analysis of R2R3-MYB transcription factors shows the complexity of MYB-associated regulatory networks in Salvia miltiorrhiza. BMC Genomics 15, 1–12 (2014).
Zhang, H. et al. Identification and characterization of Salvia miltiorrhizain miRNAs in response to replanting disease. PloS One 11, e0159905 (2016).
Voinnet, O. Origin biogenesis and activity of plant microRNAs. Cell 136, 669–687 (2009).
Ha, M. & Kim, V. N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 15, 509–524 (2014).
Zhang, H., Xia, R., Meyers, B. C. & Walbot, V. Evolution, functions, and mysteries of plant ARGONAUTE proteins. Curr. Opin. Plant Biol. 27, 84–90 (2015).
Fahlgren, N. et al. High-throughput sequencing of Arabidopsis microRNAs: evidence for frequent birth and death of MIRNA genes. PloS One 2, e219 (2007).
Meyers, B. C. et al. Criteria for annotation of plant microRNAs. Plant Cell 20, 3186–3190 (2008).
Skoog, F. & Murashige, T. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiologia plantarum 15, 473–497 (1962).
Altschul, S. F. et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402 (1997).
Kumar, S., Stecher, G. & Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33, 1870–1874 (2016).
Rozas, J. & Rozas, R. DnaSP, DNA sequence polymorphism: an interactive program for estimating population genetics parameters from DNA sequence data. Comput. Appl. Biosci. 11, 621–625 (1995).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408 (2001).
Langmead, B., Trapnell, C., Pop, M. & Salzberg, S. L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25 (2009).
Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 31, 3406–3415 (2003).
Acknowledgements
This work was supported by the CAMS Innovation Fund for Medical Sciences (CIFMS) (2016-I2M-3-016), the Natural Science Foundation of China (31370327, 31570667 and 81773836), and the Beijing Natural Science Foundation (5152021).
Author information
Authors and Affiliations
Contributions
J.L. analyzed the data, performed qRT-PCR and RACE experiments and participated in writing the manuscript. C.L. contributed to data analysis and RNA extraction. S.L. designed the experiment, participant in bioinformatics analysis, and wrote the manuscript. All authors have read and approved the version of manuscript
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, J., Li, C. & Lu, S. Systematic analysis of DEMETER-like DNA glycosylase genes shows lineage-specific Smi-miR7972 involved in SmDML1 regulation in Salvia miltiorrhiza. Sci Rep 8, 7143 (2018). https://doi.org/10.1038/s41598-018-25315-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-25315-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.