Abstract
To investigate the effect of maternal MitoQ treatment on renal disorders caused by maternal cigarette smoke exposure (SE). We have demonstrated that maternal SE during pregnancy increases the risk of developing chronic kidney disease (CKD) in adult offspring. Mitochondrial oxidative damage contributes to the adverse effects of maternal smoking on renal disorders. MitoQ is a mitochondria-targeted antioxidant that has been shown to protect against oxidative damage-related pathologies in many diseases. Female Balb/c mice (8 weeks) were divided into Sham (exposed to air), SE (exposed to cigarette smoke) and SEMQ (exposed to cigarette smoke with MitoQ supplemented from mating) groups. Kidneys from the mothers were collected when the pups weaned and those from the offspring were collected at 13 weeks. Maternal MitoQ supplementation during gestation and lactation significantly reversed the adverse impact of maternal SE on offspring’s body weight, kidney mass and renal pathology. MitoQ administration also significantly reversed the impact of SE on the renal cellular mitochondrial density and renal total reactive oxygen species in both the mothers and their offspring in adulthood. Our results suggested that MitoQ supplementation can mitigate the adverse impact of maternal SE on offspring’s renal pathology, renal oxidative stress and mitochondrial density in mice offspring.
Similar content being viewed by others
Introduction
It has been increasingly recognised that maternal programming during fetal development predisposes the offspring to future disease. Maternal smoking imposes a significant adverse impact on fetal renal development that determines the future risk of chronic kidney disease (CKD) in adulthood1. Human studies have shown that intrauterine exposure to cigarette smoke (SE) is closely linked to impaired fetal renal growth2. Maternal smoking is associated with a 1.24-times increased risk of child proteinuria compared with offspring of non-smoking mothers3. These phenomena have also been confirmed in our mouse model of maternal smoking, which demonstrated that maternal SE leads to renal underdevelopment in offspring at birth and renal dysfunction in adulthood4.
Mitochondria are intracellular organelles that generate the energy required for cellular functions through oxidative phosphorylation, which involves a series of oxidation-reduction reactions. During this process, reactive oxygen species (ROS) are released as a by-product. Thus, mitochondria are the major source of ROS during energy synthesis5, which is subsequently cleared by the endogenous antioxidants, such as manganese superoxide dismutase (MnSOD). Mitochondrial abnormalities, such as the accumulation of mitochondrial DNA mutations and damaged mitochondria structure due to metabolic stress, can overconsume the antioxidant enzymes or impair the production of antioxidants leading to oxidative stress which in turn triggers pro-inflammatory response6,7. Renal tubular cells contain abundant mitochondria, therefore mitochondrial density plays a fundamental role in the pathogenesis of kidney diseases. Growing evidence suggests that mitochondrial damage is implicated in the pathophysiology of renal diseases8,9. It has been reported that nicotine can accumulate in the kidney10. Several studies indicated that maternal smoking is closely related to increased levels of oxidative stress in the mothers, infants and newborns11,12, and reduced levels of the antioxidant enzymes superoxide dismutase (SOD) in the arteries of offspring from nicotine treated rats13. Moreover, we also demonstrated that oxidative stress and mitochondrial dysfunction are closely associated with the adverse effects of maternal smoking on the kidney pathology in the male offspring14,15.
Coenzyme Q10 (CoQ10) is a mitochondrial endogenous antioxidant. It has been shown that CoQ10 supplementation in mice can lower hepatic oxidative stress and inflammation associated with diet-induced obesity in mice16. Amniotic fluid CoQ10 levels are significantly lower among women delivering preterm babies, a risk which is increased by maternal smoking17,18. In addition, plasma CoQ10 levels are reduced in smokers19. However CoQ10 is not a viable treatment option due to poor bioavailability and delayed mitochondrial uptake20. Mitoquinone mesylate, also known as MitoQ, is a mitochondria-targeted antioxidant. It consists of a ubiquinone moiety, the same structure to the ubiquinone found in CoQ10, which allows its rapid uptake and accumulation in the mitochondria to restore the antioxidant efficacy of the mitochondrial respiratory complex21. As such, it has been reported that MitoQ has a protective role against oxidative damage-related pathologies in metabolic22 and neurodegenerative diseases23. Moreover, our previous study demonstrated that maternal MitoQ supplementation during pregnancy and lactation is beneficial in reducing lung inflammatory and oxidative stress responses caused by maternal SE in the adult offspring24. Therefore, this study aimed to investigate whether maternal MitoQ supplementation can also mitigate the adverse impact on renal disorders caused by SE and whether the benefits of MitoQ administered to the SE mother are transmitted to the fetus and result in reduced future risk of CKD.
Materials and Methods
Animal experiments
The animal experiments were approved by the Animal Care and Ethics Committee at the University of Technology Sydney (ACEC#2014-638 and #2016-419). All protocols were performed according to the Australian National Health & Medical Research Council Guide for the Care and Use of Laboratory Animals. Female Balb/c mice (8 weeks) were housed at 20 ± 2 °C and maintained on a 12:12 hour light/dark cycle with ad libitum access to standard laboratory chow and water. After the acclimatisation period, mice were divided into three groups: SHAM (exposed to air), SE (exposed to cigarette smoke from 2 cigarettes twice daily, 6 weeks before mating and throughout gestation and lactation, as previously described4), and SEMQ (SE mothers supplied with MitoQ (500 µM in drinking water25,26,27) during gestation and lactation). Male breeders and suckling pups stayed in the home cage when the mothers were exposed to normal air or cigarette smoke. Pups were weaned at postnatal day 20 and maintained without additional intervention.
Since we have previously demonstrated that maternal SE have a greater impact on the male offspring28, only male offspring were assessed in this study. One cohort of pups were randomly selected at postnatal day 1 from each litter to prevent selection bias29. The rest of the pups were kept to week 13. The birthweight of the latter group was not measured to avoid disturbance to the new born litter and mothers and problems with attachment which may influence later results30. Briefly, male offspring were euthanized (4% isoflurane, 1% O2, Veterinary companies of Australia, Kings Park, NSW) at adulthood (13 weeks). A terminal urine collection was undertaken via direct bladder puncture and the blood was collected via cardiac puncture after mice were anesthetized. The kidney tissues were collected and stored at −80 °C for later analysis.
Albumin and creatinine assays
The levels of urinary albumin and creatinine were measured using Murine Microalbuminuria ELISA kit (Albuwell M) and Creatinine Companion kit, respectively (Exocell Inc, PA, USA) following the manufacturer’s instructions.
Kidney histology
Kidney structure was examined in the male offspring at 13 weeks as previously described. Briefly, fixed kidney samples were embedded in paraffin and sectioned. Kidney sections were stained with hematoxylin and eosin (H&E) and periodic acid-Schiff (PAS). Glomerular number and size were assessed as we have previously described4 and quantitated using Image J software (National Institute of Health, Bethesda, Maryland, USA).
Confocal Microscopy Imaging
Confocal laser scanning microscopy images of frozen kidney sections were acquired using Leica SP2 confocal laser scanning microscope (Leica, Wetzlar, Germany). Data was generated from 5–6 animals/group. Four to 6 Images were collected from each kidney and averaged before the analysis. All imaging parameters including laser intensities, Photomultiplier tubes voltage and pinholes were kept constant during imaging. For total reactive oxygen species (ROS) detection, CellROX Deep Red (Thermo Fisher Scientific, Australia) was used at 5 µM final concentration, images were acquired at 633 nm excitation wavelength and detected in the 640–680 nm emission range. MitoTracker Green (Thermo Fisher Scientific, Australia) was used for staining the mitochondria at 200 nM final concentration, Images were acquired at 488 nm excitation wavelength and detected in the 510–550 nm emission range.
Western blotting
Kidney tissues were homogenized in lysis buffer with phosphatase inhibitors (Thermo Fisher Scientific, CA, USA). Protein concentrations were measured using DC Protein assay (Bio-rad, Hercules, CA, USA). Equal amount of proteins (20 μg) were separated on 4–12% Criterion™ XT Bis-Tris Protein Gel (Bio-rad, Hercules, CA, USA) and transferred to PVDF membranes. The membranes were blocked with TBS-0.05% Tween 20 (TBS-T) containing 5% BSA or skim milk for 1 h, before incubation with primary antibodies against endogenous antioxidant Manganese superoxide dismutase (MnSOD, 1:2000, Millipore, Billerica, MA, USA), translocase of the outer membrane-20 (TOM-20, 1:2000, Santa Cruz Biotechnology), fibronectin (1:1000, Abcam, Cambridge, UK), phospho-extracellular signal-regulated kinase-1/2 (Erk1/2, 1:1000, Cell Signaling Technology Inc), Erk1/2 (1:1000, Cell Signaling Technology Inc), phospho-JNK (1:1000, Cell Signaling Technology Inc, MA, USA), c-JUN N-terminal kinase (JNK, 1:500, Cell Signaling Technology Inc), phospho-p38 Mitogen-activated protein kinase (MAPK, 1:1000, Cell Signaling Technology Inc), p38 MAPK (1:1000, Cell Signaling Technology Inc), transcription factor nuclear factor-κ-light-chain-enhancer of activated B cells (NFκB, 1:1000, Cell Signaling Technology Inc), phospho-NFκB (1:1000, Cell Signaling Technology Inc), F4/80 (1:1000, Sigma Aldrich, New South Wales, Australia), Collagen I (1:500, Santa Cruz Biotechnology, Texas, USA), Collagen III (1:500, Santa Cruz Biotechnology, Texas, USA) and Collagen IV (1:500, Santa Cruz Biotechnology, Texas, USA) overnight at 4 °C, then followed by secondary antibodies (peroxidase-conjugated goat anti-mouse or anti-rabbit IgG or rabbit anti-goat IgG,1:2000,Santa Cruz Biotechnology Inc). The blots were then incubated in Super Signal West Pico Chemiluminescent substrate (Thermo Fisher Scientific, CA, USA) and the membranes were then visualized by an Amersham Imager 600 (GE Healthcare, NSW, Australia). Protein band density determined using ImageJ software (National Institute of Health, Maryland, USA) was used for densitometry, and β-actin (1:5000, Santa Cruz Biotechnology, Texas, USA) was used as the control.
Quantitative real-time PCR
Total mRNA was isolated from kidney tissues using TRIzol Reagent (LifeTechnologies, CA, USA). First strand cDNA was generated using M-MLV Reverse Transcriptase, RNase H, Point Mutant Kit (Promega, Madison, WI, USA). Genes of interest were measured using pre-optimized SYBR green primers (Sigma-Aldrich) and RT-PCR master mix (LifeTechnologies, CA, USA). The primers used in real-time RT-PCR experiments were as follows: macrophage chemoattractant protein (MCP)-1 forward primer: 5′-GTTGTTCACAGTTGCTGCCT-3′, and reverse primer: 5′-CTCTGTCATACTGGTCACTTCTAC-3′. Interleukin (IL)-1α, IL-6 and cluster of differentiation (CD) 68 mRNA expressions were measured using Taqman probe (IL-1α: ACCTGCAACAGGAAGTAAAATTTGA, NCBI gene references: NM_010554.4, mCT192405.0, BC003727.1, ID: Mm00439620_m1; IL-6: ATGAGAAAAGAGTTGTGCAATGGCA, NCBI gene references: NM_031168.1, X06203.1, X54542.1, ID: Mm00446190_m1; CD68: CACTTCGGGCCATGTTTCTCTTGCA, NCBI gene references: NM_001291058.1, ID: Mm03047343_m1). The average expression of the control group was assigned as the calibrator against which all other samples were expressed as fold difference. The 18S rRNA was used as the house-keeping gene for all gene of interest.
Mitochondrial DNA copy number
Genomic DNA was extracted from renal tissue using the DNeasy blood and tissue kit (Qiagen). The content of mtDNA was calculated using real-time quantitative PCR by measuring the threshold cycle ratio (∆Ct) of the mitochondrial-encoded gene cytochrome c oxidase subunit 1 (COX1) (forward primers 5′-ACTATACTACTACTAA-CAGACCG-3′, reverse primers 5′-GGTTCTTTTTTTCCGGAGTA-3′) vs. the nuclear-encoded gene cyclophilin A (forward primers 5′-ACACGCCATAATGGCACTGG-3′, reverse primers 5′-CAGTCTTGGCAGTGCAGAT-3′ as we have previously shown15.
ATP assay
ATP determination kit (Thermo Fisher Scientific, CA, USA) was used to extract ATP according to manufacturer instructions. In brief, kidney tissues (15–20 mg) were homogenized in 0.5 ml ice-cold Phenol-TE (Sigma-Aldrich, New South Wales, Australia). Chloroform (200 μl) and de-ionised water (200 μl) were added and followed by twenty seconds shaking. Aqueous phase was extracted and ATP was determined with luciferin-luciferase assay.
Statistical analysis
Results are presented as the mean ± S.E.M. The differences between the groups were analysed by one-way ANOVA followed by post hoc Bonferroni test (Prism 7, Graphpad CA, USA). The differences were considered statistically significant at P < 0.05.
Results
Effects of cigarette smoke exposure on the mothers
Results in Table 1 show that body weight was not different between the SE mothers and control mothers. Kidney mass was marginally reduced without statistical significance in the SE mothers. Mitochondrial density, total ROS levels, and mitochondrial copy number were significantly increased in the kidney’s from the SE mothers (P < 0.01 vs SHAM, Fig. 1).
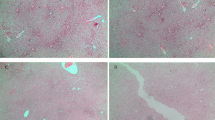
Renal mitochondrial density (a), mitochondrial DNA copy number (b) and total ROS level (c) in the mothers. Results are expressed as mean ± SE.**P < 0.01 SE vs Sham, ##P < 0.01 SEMQ vs SE. SE: cigarette smoke exposure; SEMQ: cigarette smoke exposure with MitoQ supplementation. Representative confocal images of (a and c) showing Mitotracker and Cell Rox staining in the SHAM, SE and SEMQ groups respectively (d).
MitoQ supplementation during gestation and lactation significantly reversed the impact of SE on mitochondrial density (P < 0.01 vs SE, Fig. 1a). In addition, renal DNA copy number in the SEMQ mothers was similar as the SHAM mothers (Fig. 1b). Maternal MitoQ administration also significantly ameliorated ROS level in the kidneys (P < 0.01 SEMQ vs SE, Fig. 1c).
Effect of maternal cigarette smoke exposure on the growth of the male offspring
At postnatal day 1, body weight and kidney mass were significantly reduced in the male offspring from the SE mothers (P < 0.01 and P < 0.05 vs SHAM, respectively; Table 1). A lower body weight was maintained until 13 weeks of age (P < 0.01, Table 1) but kidney mass was only marginally reduced without statistical significance in offspring of SE mothers. This is consistent with our previous study using the same model4.
Maternal MitoQ supplementation significantly enhanced body weight at P1 and normalised the body weight at week13 of the SEMQ offspring (P < 0.01, Table 1). Moreover, kidney mass was significantly normalised in the SEMQ offspring at P1 (P < 0.05 vs SE, Table 1). Interestingly, there were fewer male offspring in the SEMQ group in comparison to both SE and control groups.
Effect of maternal cigarette smoke exposure on kidney development
At 13 weeks, the average number of glomeruli was significantly decreased in the SE offspring compared to the SHAM offspring (P < 0.01, Fig. 2a). The mature glomerular size in the SE offspring was also significantly larger than those of the offspring from the SHAM mothers (P < 0.05, Fig. 2b). Maternal MitoQ supplementation normalised glomerular number (P < 0.01 vs SE) and size in the SEMQ offspring (Fig. 2a,b).
Effect on renal inflammatory and fibrotic markers and kidney function
Renal mRNA expression of the pro-inflammatory markers MCP-1 and CD68, in addition to the protein levels of F4/80, mice macrophage marker were significantly increased in the offspring kidneys due to maternal SE (P < 0.05 vs SHAM offspring, Fig. 3a,d,e). The levels of the pro-fibrotic marker fibronectin and collagen IV protein were also significantly increased in the offspring kidneys due to maternal SE (P < 0.05 vs SHAM offspring, Fig. 4a,d). IL-1α and IL-6 expression, as well as Collagen I,III protein levels were not changed by maternal SE (Figs 3b,c, 4b,c). Maternal MitoQ administration ameliorated MCP-1 expression although this did not reach statistical significance (Fig. 3a); whereas CD68 expression and F4/80 protein level were normalised in the SEMQ offspring (P < 0.01 vs SE, Fig. 3d; P < 0.05 vs SE, Fig. 3e).
Renal MCP-1 mRNA expression (a), IL-1α mRNA expression (b), IL-6 mRNA expression (c), CD68 mRNA expression (d), F4/80 protein levels (e) in the offspring at 13 weeks. Whole gel images (e) in Supplementary Fig. 3. Results are expressed as mean ± SE. *P < 0.05, SE vs Sham. ##P < 0.01; #P < 0.05, SEMQ vs SE. SE: cigarette smoke exposure; SEMQ: cigarette smoke exposure with MitoQ supplementation.
Renal fibronectin protein levels (a), collagen I protein levels (b), collagen III protein levels (c), collagen IV protein levels (d), and Urinary Albumin to creatinine ratio (ACR) in the offspring (e) at 13 weeks. Whole gel images (a–d) in Supplementary Fig. 4, (a,b). Results are expressed as mean ± SE. *P < 0.05, SE vs Sham. SE: cigarette smoke exposure; SEMQ: cigarette smoke exposure with MitoQ supplementation.
The urinary albumin-to-creatinine ratio as a marker of renal damage was significantly higher in the SE group (P < 0.05, Fig. 4e). Maternal MitoQ supplementation showed a trend to normalization of urinary albumin-to-creatinine ratio. However, this was not significant (Fig. 4e).
Effect on renal mitochondrial and stress markers in the offspring
Altered mitochondrial number and DNA content have been proposed as a surrogate of mitochondrial function. Here, mitochondrial density and DNA copy number were significantly increased in the offspring from the SE mothers (P < 0.01 and P < 0.05 vs SHAM offspring, respectively; Fig. 5a,b). As such, total ROS level was significantly increased in SE offspring’s kidneys (P < 0.01 vs SE offspring, Fig. 5c), with reduced endogenous antioxidant MnSOD level (P < 0.01 vs SHAM offspring, Fig. 5e). We also investigated TOM-20, a mitochondrial outer membrane receptor for the translocation of cytosolically synthesized mitochondrial pre-proteins. Renal TOM-20 protein level was significantly reduced in the SE offspring (P < 0.05 vs SHAM offspring, Fig. 5f).
Renal cellular mitochondrial density (a), mitochondrial DNA copy number (b), total ROS (c), Representative confocal images of (a and c) showing Mitotracker and Cell Rox staining in the SHAM, SE and SEMQ groups respectively (d), mitochondrial MnSOD (e), and TOM-20 (f), and ATP (g) in the male offspring at 13 weeks. Whole gel images of (e,f) in Supplementary Fig. 5. Results are expressed as mean ± SE. *P < 0.05, **P < 0.01 SE vs Sham, #P < 0.05, ##P < 0.01 SEMQ vs SE. SE: cigarette smoke exposure; SEMQ: cigarette smoke exposure with MitoQ supplementation.
Cellular oxidative stress level was significantly reduced by maternal MitoQ treatment. Maternal MitoQ treatment normalised cellular mitochondrial density (P < 0.01 vs SE offspring) and total ROS level (P < 0.05 vs SE offspring, Fig. 5a,c) although no change in copy number was seen. It also marginally improved TOM-20 level (Fig. 5f). There was a trend of increased ATP levels in the SE offspring’s kidneys (P = 0.27 vs SHAM), which was significantly reduced by maternal MitoQ supplementation (P < 0.05 SEMQ vs SE, Fig. 5g).
Effect on Receptors for Advanced Glycation End-products (RAGE) pathway
RAGE is a multi-ligand receptor of the immunoglobulin superfamily, which plays a role in cigarette smoke-related disease through the AGEs-RAGE axis31,32. Our data demonstrated that maternal SE has no effect on RAGE, p38 MAPK, ERK1/2, JNK, and NFκB in the offspring’s kidneys at week 13. MitoQ administration also has no effect on these markers (Supplementary Fig. 1).
Discussion
Maternal smoking during pregnancy affects fetal renal development which is linked to an increased risk of CKD in the offspring in the adulthood. We have previously demonstrated that maternal SE significantly reduced renal development in the male offspring and induced renal pathology in adulthood associated with increased oxidative stress and mitochondrial dysregulation4,14. Such effects were male specific28. However, it is not clear whether this is due to the direct effect of cigarette smoke on maternal mitochondrial DNA, which can be transmitted to the offspring.
In this study, we demonstrated that SE increased mitochondrial density and maternal renal DNA copy number and as a consequence increased total ROS levels in the mothers’ kidneys. We additionally demonstrated that the administration of the mitochondrial-targeted antioxidant MitoQ during gestation and lactation can significantly reverse the impact of SE on the abovementioned renal changes. Furthermore, we demonstrated that maternal SE induced renal underdevelopment and renal dysfunction in the male offspring at adulthood associated with increased renal inflammatory markers, mitochondrial alteration and oxidative stress, which were also ameliorated by maternal MitoQ supplementation. Interestingly, mitochondrial DNA copy number and density were increased in both SE mothers and their offspring suggesting that smoking during pregnancy can alter mitochondrial DNA predisposing the offspring to future kidney disease through foetal programing.
Maternal MitoQ administration reversed the effect of maternal SE on the offspring body weight, kidney size at birth, renal development, as well as renal function in the adult offspring. Interestingly, although maternal MitoQ administration was able to reverse the effect of maternal SE on renal mitochondrial density and total ROS levels in the offspring, it had no effect on mitochondrial DNA copy number. This finding suggests that the effect of SE on mitochondrial DNA copy number in the mothers may be transmitted to the offspring as mitochondrial DNA is inherited from the maternal lineage. Such change can’t be reversed by gestational MitoQ supplementation. However, whether such effect occurs prior to gestation requires further validation by examining the females before mating, which is beyond the scope of this study. It is important to note that there were less male offspring in the SEMQ group in comparison to both SE and control groups. The reason for that is to date unclear and is worth further investigation.
Several studies have indicated that maternal cigarette smoking during pregnancy was the most common cause of fetal growth restriction and reduced size of the fetal organs33,34. We have previously shown, using the same animal model, that maternal SE is linked to smaller glomerular size and delayed kidney development in the male offspring4,14. In addition, oxidative stress and mitochondrial dysfunction are closely associated with the adverse effects of maternal smoking on offspring’s kidney pathology14,15. Such phenotype has also been presented in the SE offspring in this study, reflected by smaller glomerular number with adaptive enlargement of glomerular size and impaired renal function. Mitochondrial DNA can only be inherited from the mothers, not the fathers35. Indeed in this study, the changes in renal mitochondrial DNA copy number and density in the SE offspring mirrored that in the SE mothers. While mitochondrial number and DNA copy number were deregulated by maternal SE, renal total ROS were increased in such offspring in line with increased mitochondrial activity of ATP production, suggesting oxidative stress, which is consistent with our previous studies14,15. Correlatively, the level of mitochondrial endogenous antioxidant MnSOD was reduced in the offspring’s kidney in response to increased oxidative stress, with lower expression of the mitochondrial import receptor subunit (TOM-20) which may be induced by increased work load for ATP synthesis.
Oxidative stress is often linked to inflammatory responses and fibrotic changes, which were also observed in the SE offspring with increased levels of inflammatory (MCP-1, CD68 and F4/80) and fibrotic markers (fibronectin and collagen IV). The AGEs-RAGE interaction has also been shown to associate with enhanced production of intracellular ROS, which can mediate further inflammatory response36,37. Several studies have suggested that RAGE can also influence the pathogenesis of renal disorders38,39. Our previous study demonstrated that maternal SE can increase RAGE and its signalling elements, as well as promoting oxidative stress and inflammatory responses in offspring’s lung24. However in this study, none of the RAGE signaling elements including RAGE, p38 MAPK, ERK1/2, JNK, and NFκB, were changed in the SE offspring’s kidney. These findings suggest that the RAGE pathway does not seem to be involved in maternal SE induced inflammatory response in the offspring’s kidney. There is also evidence suggesting that inflammatory cell infiltration correlates with both the extent of renal fibrosis and the severity of renal damage in CKD40. Irreversible renal fibrosis is a common consequence after renal injury and leads to a gradual loss of kidney function, which is a hallmark of CKD. In this study, there was a significant increase in fibronectin level in the SE offspring’s kidney. We have previously demonstrated that maternal SE induced subtle pathological changes in the offspring’s kidneys at 13 weeks. Increased risk of CKD may prevail if the offspring are exposed to additional insult after weaning, such as obesity or diabetes. Such hypothesis requires validation in future studies.
Mitochondrial dysfunction occurs in several human disorders, which is considered as the major driver for cellular and organ failure. Adverse effects of cigarette smoke have been attributed to increased oxidative stress together with mitochondrial dysregulation, which play a key role in the progression of renal injury and development of CKD41,42,43,44. Therefore, therapeutic application of mitochondrial-targeted therapies may offer potential alternatives for the prevention and treatment of such conditions, instead of the generic antioxidants which are normally poorly taken up by the mitochondria. The most widely investigated mitochondria-specific antioxidant to date is MitoQ21,45. The beneficial effects of MitoQ have been reported in various disorders, such as metabolic disease22, neurodegenerative diseases23, kidney damage related to diabetes46, Parkinson’s disease47, and liver inflammation in hepatitis C virus infection48. Importantly, our previous study using the same cohort of mice demonstrated that maternal MitoQ supplementation during pregnancy and lactation is beneficial in reducing lung inflammatory and oxidative stress responses in the adult offspring caused by maternal SE24. As we have demonstrated that oxidative stress plays an important role in maternal SE related renal disorders in the male offspring4,14,15, this study extended the investigation of the impact of maternal MitoQ supplementation on renal disorders.
In the current study, our results showed that MitoQ supplementation during pregnancy can significantly mitigate small body weight due to in-utero SE. Moreover, we demonstrated that MitoQ treatment can restore smaller kidney size and glomerular numbers with nearly normalised renal function in adult offspring from the SE mother. These results suggested that MitoQ exert beneficial effects on offspring’s health, despite continuing maternal SE during gestation and lactation. Our results are consistent with earlier reports that showed that MitoQ treatment prevented renal disorders in a mouse model of type 1 diabetes46. Mukhopadhyay and colleagues also found that MitoQ treatment prevented renal dysfunction caused by cisplatin nephrotoxicity49. Such improvement in the offspring is closely related to reduced renal ROS level and normalised mitochondrial density in both mothers and offspring. Interestingly, renal MnSOD level was not increased in the offspring as a consequence of maternal administration of MitoQ. This was different to that observed in the lungs24, suggesting that ROS was suppressed by other antioxidative mechanisms or due to reduced mitochondrial ATP production. The impact of maternal SE on TOM-20, the mitochondrial outer membrane receptor for the translocation of cytosolically synthesized mitochondrial preproteins, was partially reversed in SE offspring, suggesting some improvement in mitochondrial function. However, mitochondrial DNA copy number was not reversed in the SEMQ offspring compared with the SEMQ mothers, suggesting oxidative stress may not be the only factor to damage mitochondrial DNA in the offspring. Foetal kidneys are likely to be more vulnerable to the damage from the chemicals in the cigarette smoke, since nicotine level is 15% higher in the foetal blood than the maternal blood50. This may also affect the fibronectin level in the offspring’s kidney51, which was also unaffected by maternal MitoQ supplementation although the inflammatory markers MCP-1, CD68, F4/80 and ROS level were reduced. As the aim of the study was to determine whether MitoQ protects the offspring from maternal smoking we did not include a sham group treated with MitoQ. Hence we are unable to be definitive about whether MitoQ may cause changes in the parameters studied in “normal’ animals. However, Rodriguez-Cuenca et al., have previously examined the long-term consequences of MitoQ on wild-type mice in the absence of injury and demonstrated that MitoQ has no effect on mitochondrial function, mitochondrial DNA, food consumption or whole body metabolism25.
In summary, our study demonstrates the beneficial effects of maternal MitoQ supplementation during gestation and lactation on renal under development and pathology by maternal SE. It also reduced renal ROS accumulation and mitochondrial density in both mothers’ and offspring’s kidneys. Although MitoQ was unable to reverse the increase in fibrotic markers, it may still protect the offspring against maternal SE induced renal pathology and potentially future CKD through reduction of inflammation and oxidative stress. This is yet to be confirmed in future human clinical trials.
References
Taal, H. R. et al. Maternal smoking during pregnancy and kidney volume in the offspring: the Generation R Study. Pediatr Nephrol 26, 1275–1283, https://doi.org/10.1007/s00467-011-1848-3 (2011).
Anblagan, D. et al. Maternal smoking during pregnancy and fetal organ growth: a magnetic resonance imaging study. PloS one 8, e67223, https://doi.org/10.1371/journal.pone.0067223 (2013).
Shinzawa, M. et al. Maternal Smoking during Pregnancy, Household Smoking after the Child’s Birth, and Childhood Proteinuria at Age 3 Years. Clinical journal of the American Society of Nephrology: CJASN 12, 253–260, https://doi.org/10.2215/cjn.05980616 (2017).
Al-Odat, I. et al. The impact of maternal cigarette smoke exposure in a rodent model on renal development in the offspring. PLoS One 9, e103443, https://doi.org/10.1371/journal.pone.0103443 (2014).
Arany, I. et al. Chronic nicotine exposure exacerbates acute renal ischemic injury. American journal of physiology. Renal physiology 301, F125–133, https://doi.org/10.1152/ajprenal.00041.2011 (2011).
Pieczenik, S. R. & Neustadt, J. Mitochondrial dysfunction and molecular pathways of disease. Experimental and molecular pathology 83, 84–92, https://doi.org/10.1016/j.yexmp.2006.09.008 (2007).
Bulua, A. C. et al. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS). The Journal of experimental medicine 208, 519–533, https://doi.org/10.1084/jem.20102049 (2011).
Che, R., Yuan, Y., Huang, S. & Zhang, A. Mitochondrial dysfunction in the pathophysiology of renal diseases. Am J Physiol Renal Physiol 306, F367–378, https://doi.org/10.1152/ajprenal.00571.2013 (2014).
Granata, S. et al. Mitochondrial dysregulation and oxidative stress in patients with chronic kidney disease. BMC Genomics 10, 388, https://doi.org/10.1186/1471-2164-10-388 (2009).
Liu, J. P., Baker, J., Perkins, A. S., Robertson, E. J. & Efstratiadis, A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGFreceptor (Igf1r). Cell 75, 59–72, https://doi.org/10.1016/S0092-8674(05)80084-4 (1993).
Ermis, B. et al. Influence of Smoking on Maternal and Neonatal Serum Malondialdehyde, Superoxide Dismutase, and Glutathione Peroxidase Levels. Annals of Clinical & Laboratory Science 34, 405–409 (2004).
Noakes, P. S. et al. Association of maternal smoking with increased infant oxidative stress at 3 months of age. Thorax 62, 714–717, https://doi.org/10.1136/thx.2006.061630 (2007).
Xiao, D., Huang, X., Yang, S. & Zhang, L. Antenatal nicotine induces heightened oxidative stress and vascular dysfunction in rat offspring. Br J Pharmacol 164, 1400–1409, https://doi.org/10.1111/j.1476-5381.2011.01437.x (2011).
Nguyen, L. T. et al. l-Carnitine reverses maternal cigarette smoke exposure-induced renal oxidative stress and mitochondrial dysfunction in mouse offspring. Am J Physiol Renal Physiol 308, F689–696, https://doi.org/10.1152/ajprenal.00417.2014 (2015).
Stangenberg, S. et al. Oxidative stress, mitochondrial perturbations and fetal programming of renal disease induced by maternal smoking. Int J Biochem Cell Biol 64, 81–90, https://doi.org/10.1016/j.biocel.2015.03.017 (2015).
Sohet, F. M. et al. Coenzyme Q10 supplementation lowers hepatic oxidative stress and inflammation associated with diet-induced obesity in mice. Biochem Pharmacol 78, 1391–1400, https://doi.org/10.1016/j.bcp.2009.07.008 (2009).
Teran, E. et al. Maternal plasma and amniotic fluid coenzyme Q10 levels in preterm and term gestations: a pilot study. Archives of gynecology and obstetrics 283(Suppl 1), 67–71, https://doi.org/10.1007/s00404-011-1894-x (2011).
Kyrklund-Blomberg, N. B., Granath, F. & Cnattingius, S. Maternal smoking and causes of very preterm birth. Acta obstetricia et gynecologica Scandinavica 84, 572–577, https://doi.org/10.1111/j.0001-6349.2005.00848.x (2005).
Al-Bazi, M. M., Elshal, M. F. & Khoja, S. M. Reduced coenzyme Q(10) in female smokers and its association with lipid profile in a young healthy adult population. Archives of medical science: AMS 7, 948–954, https://doi.org/10.5114/aoms.2011.26605 (2011).
Hirano, M., Garone, C. & Quinzii, C. M. CoQ(10) deficiencies and MNGIE: two treatable mitochondrial disorders. Biochimica et biophysica acta 1820, 625–631, https://doi.org/10.1016/j.bbagen.2012.01.006 (2012).
Kelso, G. F. et al. Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. J Biol Chem 276, 4588–4596, https://doi.org/10.1074/jbc.M009093200 (2001).
Mercer, J. R. et al. The mitochondria-targeted antioxidant MitoQ decreases features of the metabolic syndrome in ATM+/−/ApoE−/− mice. Free radical biology & medicine 52, 841–849, https://doi.org/10.1016/j.freeradbiomed.2011.11.026 (2012).
Manczak, M. et al. Mitochondria-targeted antioxidants protect against amyloid-beta toxicity in Alzheimer’s disease neurons. J Alzheimers Dis 20(Suppl 2), S609–631, https://doi.org/10.3233/jad-2010-100564 (2010).
Sukjamnong, S. et al. The effect of long-term maternal smoking on the offspring’s lung health. American journal of physiology. Lung cellular and molecular physiology, ajplung.00134.02017, https://doi.org/10.1152/ajplung.00134.2017 (2017).
Rodriguez-Cuenca, S. et al. Consequences of long-term oral administration of the mitochondria-targeted antioxidant MitoQ to wild-type mice. Free Radical Biology and Medicine 48, 161–172, https://doi.org/10.1016/j.freeradbiomed.2009.10.039 (2010).
Mercer, J. R. et al. The mitochondria-targeted antioxidant MitoQ decreases features of the metabolic syndrome in ATM+/−/ApoE−/− mice. Free Radical Biology and Medicine 52, 841–849, https://doi.org/10.1016/j.freeradbiomed.2011.11.026 (2012).
Sukjamnong, S. et al. Effect of long-term maternal smoking on the offspring’s lung health. American Journal of Physiology - Lung Cellular and Molecular Physiology 313, L416–L423, https://doi.org/10.1152/ajplung.00134.2017 (2017).
Chan, Y. L. et al. Impact of maternal cigarette smoke exposure on brain and kidney health outcomes in female offspring. Clinical and experimental pharmacology & physiology 43, 1168–1176, https://doi.org/10.1111/1440-1681.12659 (2016).
Suresh, K. P. An overview of randomization techniques: An unbiased assessment of outcome in clinical research. Journal of Human Reproductive Sciences 4, 8–11, https://doi.org/10.4103/0974-1208.82352 (2011).
Lambert, M. Breeding strategies for maintaining colonies of laboratory mice. TJ Laboratory (2009).
Cerami, C. et al. Tobacco smoke is a source of toxic reactive glycation products. Proceedings of the National Academy of Sciences of the United States of America 94, 13915–13920 (1997).
Prasad, K., Dhar, I. & Caspar-Bell, G. Role of Advanced Glycation End Products and Its Receptors in the Pathogenesis of Cigarette Smoke-Induced Cardiovascular Disease. The International journal of angiology: official publication of the International College of Angiology, Inc 24, 75–80, https://doi.org/10.1055/s-0034-1396413 (2015).
Reeves, S. & Bernstein, I. Effects of maternal tobacco-smoke exposure on fetal growth and neonatal size. Expert review of obstetrics & gynecology 3, 719–730, https://doi.org/10.1586/17474108.3.6.719 (2008).
Esposito, E. R., Horn, K. H., Greene, R. M. & Pisano, M. M. An animal model of cigarette smoke-induced in utero growth retardation. Toxicology 246, 193–202, https://doi.org/10.1016/j.tox.2008.01.014 (2008).
Luo, S. M. et al. Unique insights into maternal mitochondrial inheritance in mice. Proceedings of the National Academy of Sciences of the United States of America 110, 13038–13043, https://doi.org/10.1073/pnas.1303231110 (2013).
Jiang, J. M., Wang, Z. & Li, D. D. Effects of AGEs on oxidation stress and antioxidation abilities in cultured astrocytes. Biomedical and environmental sciences: BES 17, 79–86 (2004).
Lin, L., Park, S. & Lakatta, E. G. RAGE signaling in inflammation and arterial aging. Frontiers in bioscience (Landmark edition) 14, 1403–1413 (2009).
Koyama, H. & Nishizawa, Y. AGEs/RAGE in CKD: irreversible metabolic memory road toward CVD? Eur J Clin Invest 40, 623–635, https://doi.org/10.1111/j.1365-2362.2010.02298.x (2010).
Tomino, Y., Hagiwara, S. & Gohda, T. AGE-RAGE interaction and oxidative stress in obesity-related renal dysfunction. Kidney Int 80, 133–135, https://doi.org/10.1038/ki.2011.86 (2011).
Eardley, K. S. et al. The role of capillary density, macrophage infiltration and interstitial scarring in the pathogenesis of human chronic kidney disease. Kidney Int 74, 495–504, https://doi.org/10.1038/ki.2008.183 (2008).
Orth, S. R., Schroeder, T., Ritz, E. & Ferrari, P. Effects of smoking on renal function in patients with type 1 and type 2 diabetes mellitus. Nephrol Dial Transplant 20, 2414–2419, https://doi.org/10.1093/ndt/gfi022 (2005).
Stengel, B., Couchoud, C., Cenee, S. & Hemon, D. Age, blood pressure and smoking effects on chronic renal failure in primary glomerular nephropathies. Kidney Int 57, 2519–2526, https://doi.org/10.1046/j.1523-1755.2000.00111.x (2000).
Cigremis, Y., Turkoz, Y., Akgoz, M. & Sozmen, M. The effects of chronic exposure to ethanol and cigarette smoke on the level of reduced glutathione and malondialdehyde in rat kidney. Urological research 32, 213–218, https://doi.org/10.1007/s00240-004-0406-x (2004).
Miro, O. et al. Smoking disturbs mitochondrial respiratory chain function and enhances lipid peroxidation on human circulating lymphocytes. Carcinogenesis 20, 1331–1336 (1999).
Smith, R. A., Hartley, R. C., Cocheme, H. M. & Murphy, M. P. Mitochondrial pharmacology. Trends Pharmacol Sci 33, 341–352, https://doi.org/10.1016/j.tips.2012.03.010 (2012).
Chacko, B. K. et al. Prevention of diabetic nephropathy in Ins2(+/)(−)(AkitaJ) mice by the mitochondria-targeted therapy MitoQ. The Biochemical journal 432, 9–19, https://doi.org/10.1042/bj20100308 (2010).
Snow, B. J. et al. A double-blind, placebo-controlled study to assess the mitochondria-targeted antioxidant MitoQ as a disease-modifying therapy in Parkinson’s disease. Mov Disord 25, 1670–1674, https://doi.org/10.1002/mds.23148 (2010).
Gane, E. J. et al. The mitochondria-targeted anti-oxidant mitoquinone decreases liver damage in a phase II study of hepatitis C patients. Liver Int 30, 1019–1026, https://doi.org/10.1111/j.1478-3231.2010.02250.x (2010).
Mukhopadhyay, P. et al. Mitochondrial-targeted antioxidants represent a promising approach for prevention of cisplatin-induced nephropathy. Free radical biology & medicine 52, 497–506, https://doi.org/10.1016/j.freeradbiomed.2011.11.001 (2012).
Chen, H. & Morris, M. J. Maternal smoking—A contributor to the obesity epidemic? Obesity research & clinical practice 1, 155–163 (2007).
Jensen, K. et al. General mechanisms of nicotine-induced fibrogenesis. FASEB journal: official publication of the Federation of American Societies for Experimental Biology 26, 4778–4787, https://doi.org/10.1096/fj.12-206458 (2012).
Acknowledgements
This study was supported by a postgraduate research support by Faculty of Science, University of Technology Sydney, and a Research grant awarded by Faculty of Allied Health Sciences, Chulalongkorn University support to A/Prof Rachana Santiyanont. Ms Razia Zakarya is supported by an Australia Postgraduate Award. Ms Suporn Sukjamnong is supported by Overseas Research Experience Scholarship for Graduate Students by Graduate School, Chulalongkorn University and the 90th Anniversary of Chulalongkorn University Fund and Grant for Joint Funding (Ratchadaphiseksomphot Endowment Fund). The MitoQ was provided by MitoQ Limited, New Zealand. A/Prof Oliver is supported by NH&MRC fellowship APP1110368. This work was partially supported by the Australian Research Council (CE140100003).
Author information
Authors and Affiliations
Contributions
H.C. and S. Saad designed the study. S. Sukjamnong Y.L.C., H.C., S. Saad A.A.Z., A.G.A. performed all the experiments. All authors contributed to the writing of the main manuscript text, and S. Sukjamnong, Y.L.C., S. Saad and H.C. prepared the figures and table. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sukjamnong, S., Chan, Y.L., Zakarya, R. et al. MitoQ supplementation prevent long-term impact of maternal smoking on renal development, oxidative stress and mitochondrial density in male mice offspring. Sci Rep 8, 6631 (2018). https://doi.org/10.1038/s41598-018-24949-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-24949-0
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.