Abstract
In this paper, efficient phosphorescent white organic light-emitting diodes (WOLEDs) were fabricated based on ultrathin doping-free emissive layers and mixed bipolar interlayers. The energy transfer processes were proved via the research of WOLEDs with different interlayer thicknesses and transient photoluminescence lifetime. WOLEDs with optimized thickness of doping-free emissive layers show maximum current efficiency of 47.8 cd/A and 44.9 cd/A for three-colors and four-colors WOLEDs, respectively. The Commission Internationale de L’Eclairage coordinates shows a very slight variation of ( ± 0.02, ± 0.02) from 5793 cd/m2 to 11370 cd/m2 for three-colors WOLEDs and from 3038 cd/m2 to 13720 cd/m2 for four-colors WOLEDs, respectively. The stability of the spectra is attributed to the stable and sequential energy transfer among the various dyes. The color temperature of four-colors WOLEDs can be obtained from 2659 to 6636 by adjusting the thickness of ultrathin emissive layer.
Similar content being viewed by others
Introduction
White organic light-emitting diodes (WOLEDs) have attracted much attention because of their advantages of low-cost, light weight, high-contrast, flexible and full-color1,2,3,4. Phosphorescent emitters can improve the efficiency of WOLEDs by harvesting both singlet and triplet energy for lighting, so it leads to a nearly 100% internal quantum efficiency (IQE) compared with fluorescent materials (25%)5,6,7.
To realize white-light emission covering the whole visible light, it is inevitable to utilize different dyes with diverse emissions. There are some general methods to fabricate WOLEDs, such as mixing multiple emitters into a single emission layer (EML)8,9,10, employing two or three EMLs structure with different emitters11,12,13,14,15, using stacking and tandem structure16,17. The traditional method to fabricate WOLEDs is co-evaporation the EMLs, which significantly improves electroluminescent (EL) efficiency18. However, it also brings some problems in entire fabrication process. It is difficult to precisely control the co-evaporation rate and dopant concentration, which leads to a bad reproducibility. Besides, the co-evaporation technology increases the cost, which is an adverse factor of commercialization. To solve these problems, there is a method to simplify the structures of devices by employing doping-free EMLs instead of the co-evaporation system. Since the first application of the doping-free WOLEDs (DF-WOLEDs) was demonstrated by Tsuji et al.19, a lot of attention have been paid20,21,22,23,24,25. Although the DF-WOLEDs have a great commercial potential because of the simple fabrication process, good reproducibility and low-cost, there are still some urgent problems need to be solved. For example, many studies were based on the research of devices using two kinds of emitters26,27,28. Lee et al.20 added yellow phosphorescent layer between the double blue phosphorescent emitters. Although the power efficiency is up to 35 lm/W at 1000 cd/m2, which is a comparable to that of the WOLEDs that are fabricated for achieving a yellow emission by using complicated host-guest doping system, two-colors WOLEDs are not suitable for lighting and display. On the other hand, Zhao et al.25 has fabricated three-colors and four-colors WOLEDs by using doping-free EMLs. They introduced ultrathin emission layers to the different position of hole transport layer (HTL) and electron transport layer (ETL) to realize the white emission. The power efficiencies were 29 lm/W and 19 lm/W at 1000 cd/m2 for the three-colors and four-colors WOLEDs, respectively. However, the power efficiency of WOLEDs based on the ultrathin doping-free EMLs is far from the requirement of practical lighting applications and lower than the traditional WOLEDs29,30, which is urgent to further improve. Besides, Liu et al.31,32 used blue fluorescent emission and phosphorescent emissions to fabricate hybrid WOLEDs which exhibit high-performance and long lifetime. Ma et al.33,34 doped phosphorescent materials into bipolar-transporting host to reduce the exciton quenching. However, there is little research on doping-free phosphorescent EMLs with bipolar interlayers.
In this paper, bipolar interlayers and ultrathin doping-free phosphorescent EMLs were adopted. The hole-transporting material 4,40,400-tri(N-carbazolyl) triphenylamine (TCTA) and electron-transporting materials 1,3,5-tri(m-pyrid-3-yl-phenyl) benzene (Tm3PyPB) were mixed as the bipolar interlayers. According to our previous study, the current density of hole only devices and electron only devices were close when the mass ratio of TCTA: Tm3PyPB was 3.5: 135. The thicknesses of bipolar interlayers were changed to adjust the energy transfer from short-wave emitters to long-wave emitters. Moreover, the working mechanism of the phosphorescent WOLEDs was discussed, and we concluded that the combined effects of stable energy transfer and even excitons distribution were the factor for achieving the relatively stable spectra. As a result, the high efficiency phosphorescent WOLEDs can be achieved by use of doping-free phosphorescent EMLs and mixed bipolar interlayers.
Methods
Materials
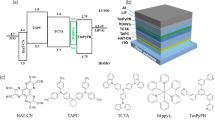
All compounds were purchased from commercial sources unless noted and used without further purification. Figure 1 shows the chemical structures and detailed energy level diagram of the materials proposed. The highest occupied molecular orbital (HOMO) level of MoO3 (HOMO = 5.2 eV) is between ITO (HOMO = 4.8 eV) and TCTA (HOMO = 5.7 eV), so MoO3 is beneficial to the hole injection36. Meanwhile, 1 nm LiF is used to enhance the electrons injection. Since TCTA has a relatively poor electron transport ability, which merely has an electron-mobility of 10−8 cm2/Vs37, and the electron-injection barrier between the lowest unoccupied molecular orbitals (LUMO) of Tm3PyPb (LUMO = 2.9 eV) and TCTA (LUMO = 2.40 eV) is merely 0.5 eV, it is difficult for electrons to be injected from Tm3PyPb into TCTA. Therefore, TCTA and Tm3PyPb can be a good confinement zone for excitons. Because the fact that the triplet energy levels of TCTA (2.86 eV) and Tm3PyPB (2.8 eV) are higher than that of all four phosphorescent dyes, the excitons generated between TCTA and Tm3PyPb can effectively transfer to dyes and then generate efficient emission.
Device fabrication
Various layers of WOLEDs were fabricated by a vacuum evaporation technique. The thickness value and deposition rates of the materials were monitored by an oscillating quartz thickness monitor. All layers were fabricated on a glass substrate with a patterned indium tin oxide (ITO), which is 150 nm thickness, ~10 Ω/sq. The ITO glasses were cleaned for 15 min in a sequential ultrasonic bath with detergent, de-ionized water, acetone and isopropanol, and then cleaned in a UV-ozone chamber for 15 min. The HTL, electron blocking layer (EBL), EML, ETL, electron injection layer (EIL) and the cathode (Al) were deposited by thermal evaporation at a high vacuum of ~10-4 Pa. The MoO3 doped TCTA as a p-type HTL was co-evaporated onto the ITO substrate at the speeds of 0.4 Å/s and 0.6 Å/s, respectively. Then TCTA was evaporated as EBL on the top of HTL layer. FIrpic, Ir(ppy)2acac, PO-01 and Ir(mphmq)2acac were evaporated as dyes at a speed of 0.1 Å/s. The mixed interlayers were evaporated among different dyes at a speed of 1 Å/s. Dyes and the mixed interlayers were combined as EML. A 40-nm-thick Tm3PyPB was deposited as ETL at a rate of 1 Å/s following the EML. Finally, a ~1-nm-thick LiF and ~120-nm-thick Al cathode were sequentially deposited. The deposition rates of LiF and Al were 0.1 and 5.0 Å/s, respectively. The active areas were 3 × 3 mm2 in all devices.
Measurements
The voltage-current density, luminance and EL spectra were measured with a programmable source meter (Keithley 2400), luminance meter (LS110, Konica Minolta) and a spectrophotometer (Spectrascan PR670, Photo Research). The photoluminescence (PL) spectra and transient PL lifetime were measured by FLSP 920 spectrometer series.
Result and Discussion
Two-colors OLEDs devices research
In order to verify the characteristic of energy transfer in the OLEDs device, the thickness of interlayer between blue and green dyes was investigated. Devices with four different thickness of interlayers were proposed. The structure of the devices was ITO/MoO3: TCTA (2:3, 35 nm)/TCTA (10 nm)/TCTA: Tm3PyPB (3.5:1, 20-X nm)/Ir(ppy)2acac (0.15 nm)/TCTA: Tm3PyPB (3.5:1, X nm)/FIrpic (0.4 nm)/Tm3PyPB (40 nm)/LiF (1 nm)/Al (120 nm), where X = 0,2,3,4.
Figure 2(a) shows the normalized EL spectra of the four devices at 6 V. When there is no interlayer between blue and green dyes, the device only shows a main green emission from Ir(ppy)2acac. With the increase of interlayer thickness, the blue emission appeared and gradually enhanced. So we can conclude that the energy transfer from blue to green dye can be controlled by the thickness of interlayer. The spectra of devices achieved an optimal balance when the thickness of interlayer is 3 nm. We can see that the spectra show a good stability due to the stable and sequential energy transfer from blue dye to green dye. The Commission Internationale de L’Eclairage (CIE) coordinates of the device with 3 nm interlayer are (0.209, 0.460), (0.209, 0.459), (0.207, 0.458) and (0.206, 0.455) at the operating voltage of 4 V, 5 V, 6 V and 7 V (which correspond to the luminance of 548 cd/m2, 2446 cd/m2, 5438 cd/m2 and 7996 cd/m2), respectively. The EL performances of devices with different thickness of interlayer between blue and green dyes are shown in Fig. S1, Supporting Information. Considering the triplet energy lever of blue dye is higher than green dye, energy transfer between blue and green dye is reasonable. It has been reported that the exciton lifetime will change with the advent of energy transfer38,39. If the energy transfer from the donor to the acceptor happens, the lifetime of the donor will decrease. The mixed bipolar material (TCTA: Tm3PyPB = 3.5: 1) was utilized as co-host, and then emitting dyes were doped into the co-host. Finally, the energy transfer can be confirmed by measuring the transient PL lifetime of the emitting dyes. Blue dye was separately doped into co-host, as depicted in Fig. 2(b), and the lifetime of blue emission was fitted to be about 1.21 μs (τ1) at 470 nm. When blue and green dye were doped into co-host together, the lifetime of blue emission dropped to 0.98 μs (τ2). According to the equation40
where ηET is the energy transfer ratio from the donor to the acceptor, ηET is 19.0%. It proves that there indeed exists an energy transfer process from FIrpic to Ir(ppy)2acac.
Three-colors WOLEDs devices research
On the basis of two-colors devices, orange dye was added to achieve three-colors warm WOLEDs. The structure of the three-colors devices is ITO/MoO3: TCTA (2:3, 35 nm)/TCTA (30-Y nm)/PO-01 (0.4 nm)/TCTA: Tm3PyPB (3.5:1, Y nm)/Ir(ppy)2acac (0.15 nm)/TCTA: Tm3PyPB (3.5:1, 3 nm)/FIrpic (0.1 nm)/Tm3PyPB (40 nm)/LiF (1 nm)/Al (120 nm). Here, Y is 3,4 and 5 corresponding to devices T1, T2 and T3.
The EL spectra of devices T1, T2 and T3 are normalized to the orange emission peak and shown in Fig. 3(a). The orange emission peak is much higher than green emission peak in the device T1 with 3 nm interlayer between PO-01 and Ir(ppy)2(acac). It illustrates that the energy transfer from Ir(ppy)2(acac) to PO-01 is too strange. So the thickness of interlayer between PO-01 and Ir(ppy)2(acac) was added. A better spectrum appeared When the interlayer reached 5 nm, the orange emission peak was almost equal with green emission peak. Although the current efficiency is dropped with the weakening of the orange emission, the efficiencies of device T3 is still comparable with device T1 and T2. As shown in Fig. 3(b), the maximum current efficiencies of device T1, T2 and T3 are 49.5 cd/A, 49.6 cd/A and 47.8 cd/A, respectively. The maximum power efficiencies and external quantum efficiencies (EQE) of the three devices are 50.5 lm/W (16.0%), 50.7 lm/W (16.1%) and 48.8 lm/W (15.4%) accordingly as shown in Fig. S2(a) and S2(b), Supporting Information. All the devices show the turn-on voltages is about 2.73 V ( ± 0.1 V), which is as low as the triplet energy gap (triplet energy~2.7 eV) of phosphorescent dye, FIrpic. The detailed EL datum of devices T1~T3 is listed in the Table 1. We can see the spectra just show a little change in Fig. 3(c). The CIE coordinates of the device T3 are (0.329, 0.479), (0.336, 0.479) and (0.343, 0.482) at the operating voltage of 5 V, 6 V and 7 V (which correspond to the luminance of 5793 cd/m2, 10240 cd/m2 and 11370 cd/m2), respectively. The color-rendering index (CRI) of device T3 is 54.2 at 7 V. Green dye was separately doped into co-host, as depicted in Fig. 3(d), and the lifetime of green emission was fitted to be about 0.95 μs at 520 nm. The lifetime of green emission dropped to 0.82 μs while green and orange dyes were doped into co-host together, and the energy transfer ratio is ηET = 1 – 0.82/0.95 = 13.9%. The stable and sequential energy transfer also exists between Ir(ppy)2(acac) and PO-01. Meanwhile, since interlayer was bipolar materials, both holes and electrons can flexibly transport across it, resulting in the relatively even excitons distribution. As shown in Fig. S2(c), the spectrum exhibits three main emission peaks from the PO-01, Ir(ppy)2(acac) and FIrpic at low operating voltage of 3.5 V (which correspond to the luminance of 365 cd/m2). A relatively weak orange emission can be ascribed to the fact that more triplet excitons created near cathode side (blue dye side) at low voltages since the doped MoO3 enhanced the hole injection/transport36. With the increase of voltage, more excitons distributed near orange dye side (anode side), which leaded to the enhancement of orange emission. Under the combined effect of stable energy transfer and even excitons distribution at high voltage (above 5 V), relatively stable spectra were obtained from 5793 cd/m2 to 11370 cd/m2.
(a) EL spectra of devices (T1, T2 and T3) with different thickness of interlayers that between green and orange dyes at 6 V. (b) Current efficiencies versus current density of devices T1, T2 and T3. Inset: Current density-Voltage-Luminescence J-V-L characteristic of devices T1, T2 and T3. (c) The EL spectra of the device T3 at different operating voltages. (d) Transient PL lifetime of green film and green-orange film.
Besides, the devices that used TCTA or Tm3PyPB as interlayers were fabricated. However, the TCTA and Tm3PyPB cannot play a good role in energy transfer alone and only one or two main emission can be saw.
Four-colors WOLEDs devices research
To achieve four-colors WOLEDs, red dye was added on the basis of three colors devices. The structure of the four-colors devices is ITO/MoO3: TCTA (2:3, 35 nm)/TCTA (27-Z nm)/Ir(mphmq)2acac (0.07 nm)/TCTA: Tm3PyPB (3.5:1, Z nm)/PO-01 (0.2 nm)/TCTA: Tm3PyPB (3.5:1, 3 nm)/Ir(ppy)2acac (0.15 nm)/TCTA: Tm3PyPB (3.5:1, 3 nm)/FIrpic (0.1 nm)/Tm3PyPB (40 nm)/LiF (1 nm)/Al (120 nm). Here, Z is 4, 5 and 6 corresponding to devices F1, F2 and F3. When the four-colors device F1 was fabricated, we find that the intensity of Ir(mphmq)2acac was too strong. So the thickness of interlayer between Ir(mphmq)2(acac) and PO-01 was added. As shown in Fig. 4(a), the thicker the interlayer is, the weaker the red emission is. As we can see, a good white spectrum was obtained when thickness of interlayer between Ir(mphmq)2acac and PO-01 was 6 nm. Figure 4(b) shows the current efficiencies of devices F1, F2 and F3. With the reduction of red emission, the current efficiency improved. The maximum current efficiencies of device F1, F2 and F3 are 41.2 cd/A, 41.9 cd/A and 44.9 cd/A, respectively. As shown in Fig. 4(b) inset, the turn-on voltages of device F1, F2 and F3 are 2.77 V, 2.81 V and 2.80 V, respectively. The maximum power efficiencies and EQE of the three devices are 40.3 lm/W (16.9%), 39.3 lm/W (16.0%) and 42.5 lm/W (16.1%) accordingly as shown in Fig. S3(a) and S3(b), Supporting Information. The spectrum of the device F3 is shown in Fig. 4(c) and CIE coordinates of the device F3 are (0.368, 0.460), (0.378, 0.455) and (0.384, 0.449) at the operating voltage of 5 V, 6 V and 7 V (which correspond to the luminance of 3038 cd/m2, 8314 cd/m2 and 13720 cd/m2), respectively. The CRI of device F3 is 77.4 at 7 V. As depicted in Fig. 4(d), the lifetime of orange emission was fitted to be about 1.80 μs at 550 nm. The lifetime of green emission dropped to 1.38 μs When orange and red dyes were doped into co-host together. The energy transfer ratio from PO-01 to Ir(mphmq)2acac is ηET = 1 – 1.38/1.80 = 23.3%. It illustrates that there is energy transfer between PO-01 and Ir(mphmq)2acac. Meanwhile, as shown in Fig. S3(c), there were more triplet excitons near cathode side (blue dye side) due to the adoption of MoO3, which lead to the red emission (anode side) was relatively weak at the low operating voltage of 4 V (which correspond to the low luminance of 424 cd/m2). With the increase of voltage, the enhancement of red emission can be ascribed to the fact that more excitons distributed near red dye side (anode side). Under the combined effect of stable energy transfer and even excitons distribution at high voltage (above 5 V), relatively stable spectra were obtained from 3038 cd/m2 to 13720 cd/m2.
(a) EL spectra of devices (F1, F2 and F3) with different thickness of interlayers that between orange and red dyes at 6 V. (b) Current efficiencies versus current density of devices F1, F2 and F3. Inset: Current density-Voltage-Luminescence J-V-L characteristic of devices F1, F2 and F3. (c) The EL spectra of the device F3 at different operating voltages. (d) Transient PL lifetime of orange film and orange-red film.
Since there is energy transfer between yellow and red dyes, changing the thickness of PO-01 can affect not only the intensity of orange light, but also the intensity of red light. The thickness of PO-01 in device F3 (the thickness of PO-01 was 0.2 nm) was changed, and devices F4 (the thickness of PO-01 was 0.1 nm) and F5 (the thickness of PO-01 was 0.3 nm) were fabricated.
Figure 5(a) depicts the EL spectra of devices F3, F4 and F5 at 6 V. We can see that the thicker the PO-01 layer is, the stronger the red emission is. In other word, thickening the PO-01 layer can increase the orange-to-red energy transfer. The color correlated temperature (CCT) of devices F4, F3 and F5 are 6636, 4565 and 2659 at 5000 cd/m2, respectively. Since the CCT can be adjusted by changing the thickness of PO-01, we can get a sun-like emission covering the entire daylight at different times (i. e., 2500, 3500 and 5000 K at sunset, sunrise and noon, respectively). Figure 5(c) and (d) shows the efficiencies of devices F3, F4 and F5. The maximum efficiencies of device F4 and F5 are 44.9 cd/A (44.5 lm/W) and 37.7 cd/A (35.1 lm/W), respectively. As shown in Fig. S3(d), the maximum EQE of the device F4 and F5 are15.3% and 16.4%, respectively. As shown in Fig. 5(c) inset, the turn-on voltages of the device F4 and F5 are 2.75 V and 2.85 V, respectively. The detailed EL datum of devices F1~F5 are listed in the Table 2.
EL performances of the devices with different thickness of orange dyes. (a) EL spectra of devices F3, F4 and F5 at 6 V. (b) CCT of devices F3, F4 and F5 at 6 V. (c) Current efficiencies versus current density of devices F3, F4 and F5. Inset: Current density-Voltage-Luminescence J-V-L characteristic of devices F3, F4 and F5. (d) Power efficiencies versus current density of devices F3, F4 and F5.
Conclusions
In summary, we reported an efficient WOLEDs based on ultrathin doping-free EMLs and mixed bipolar interlayers, and verified the existence of energy transfer among various dyes. The three-colors and four-colors WOLEDs have maximum efficiencies of 47.8 cd/A (48.8 lm/W) and 44.9 cd/A (42.5 lm/W), respectively, while the maximum EQE of the two devices are15.4% and 16.1% accordingly. The CIE coordinates of the three-colors and four-colors WOLEDs show a very slight variation. A sun-like emission covering the CCT of entire daylight at different times has been obtained by changing the thickness of PO-01. Besides, phosphorescent OLEDs usually show poor lifetime, which has been attributed to the traps caused by bimolecular triplet-polaron annihilation (TPA)41. In our devices, the adoption of ultrathin phosphorescent EML (<1 nm) and bipolar interlayers can broaden the exciton distribution zone and decrease the exciton density in EMLs. Therefore, the TPA will be reduced41, and it is hopeful to improve the OLEDs lifetime.
References
Wu, Z. B. et al. High-performance hybrid white organic light-emitting diodes with superior efficiency/color rendering index/color stability and low efficiency roll-off based on a blue thermally activated delayed fluorescent emitter. Adv. Funct. Mater. 26, 3306–3313 (2016).
Higuchi, T., Nakanotani, H. & Adachi, C. High-efficiency white organic light-emitting diodes based on a blue thermally activated delayed fluorescent emitter combined with green and red fluorescent emitters. Adv. Mater. 27, 2019–2023 (2015).
Yan, F. et al. Efficient three-color white organic light-emitting diodes with a spaced multilayer emitting structure. Appl. Phys. Lett. 106, 023302 (2015).
Oh, J. Y. et al. Down-conversion light outcoupling films using imprinted microlens arrays for white organic light-emitting diodes. Dyes and pigments. 136, 92–96 (2017).
Wang, H. et al. Highly efficient orange and red phosphorescent organic light-emitting diodes with low roll-off of efficiency using a novel thermally activated delayed fluorescence material as host. Adv. Mater. 27, 4041–4047 (2015).
Yook, K. S. & Lee, J. Y. Organic materials for deep blue phosphorescent organic light-emitting diodes. Adv. Mater. 24, 3169–3190 (2012).
Adachi, C., Baldo, M. A., Thompson, M. E. & Forrest, S. R. Nearly 100% internal phosphorescence efficiency in an organic light-emitting device. J. Appl. Phys. 90, 5048 (2001).
Shih, P. I., Shu, C. F., Tung, Y. L. & Chi, Y. Efficient white-light-emitting diodes based on poly(N-vinylcarbazole) doped with blue fluorescent and orange phosphorescent materials. Appl. Phys. Lett. 88, 251110 (2006).
Liu, B. Q. et al. Harnessing charge and exciton distribution towards extremely high performance: the critical role of guests in single-emitting-layer white OLEDs. Mater. Horiz. 2, 536–544 (2015).
Liu, B. Q. et al. Manipulation of exciton distribution for high-performance fluorescent/phosphorescent hybrid white organic light-emitting diodes. J. Mater. Chem. C. 5, 7668–7683 (2017).
Sun, Y. R. et al. Management of singlet and triplet excitons for efficient white organic light-emitting devices. Nature 440, 908–912 (2006).
Tokito, S., Iijima, T., Tsuzuki, T. & Sato, F. High-efficiency white phosphorescent organic light-emitting devices with greenishblue and red-emitting layers. Appl. Phys. Lett. 83, 2459 (2003).
Liu, B. Q. et al. Extremely high-efficiency and ultrasimplified hybrid white organic light-emitting diodes exploiting double multifunctional blue emitting layers. Light: Science & Applications 5, e16137 (2016).
Liu, B. Q. et al. Manipulation of charge and exciton distribution based on blue aggregation-induced emission fluorophors: a novel concept to achieve high-performance hybrid white organic light-emitting diodes. Adv. Funct. Mater. 26, 776–783 (2016).
Liu, B. Q. et al. Efficient hybrid white organic light-emitting diodes with extremely long lifetime: the effect of n-type interlayer. Sci. Rep. 4, 7198 (2014).
Ding, L. et al. A novel intermediate connector with improved charge generation and separation for large-area tandem white organic lighting devices. J. Mater. Chem. C. 2, 10403–10408 (2014).
Liu, B. Q. et al. Doping-free tandem white organic light-emitting diodes. Science Bulletin 62, 1193–1200 (2017).
Shi, J. & Tang, C. W. Doped organic electroluminescent devices with improved stability. Appl. Phys. Lett. 70, 1665 (1997).
Tsuji, T., Naka, S., Okada, H. & Onnagawa, H. Nondoped-type white organic electroluminescent devices utilizing complementary color and exciton diffusion. Appl. Phys. Lett. 81, 3329 (2002).
Lee, M. T., Chu, M. T., Lin, J. S. & Tseng, M. Host-free, yellow phosphorescent material in white organic light-emitting diodes. J. Phys. D: Appl. Phys. 43, 442003 (2010).
Yin, Y. M. et al. Efficient non-doped phosphorescent orange, blue and white organic light-emitting devices. Sci. Rep. 4, 6754 (2014).
Zhao, Y. B., Chen, J. S. & Ma, D. G. Ultrathin nondoped emissive layers for efficient and simple monochrome and white organic light-emitting diodes. ACS Appl. Mater. Interfaces 5, 965–971 (2013).
Luo, D. X. et al. Doping-free white organic light-emitting diodes without blue molecular emitter: An unexplored approach to achieve high performance via exciplex emission. Appl. Phys. Lett. 110, 061105 (2017).
Luo, D. X., Li, X. L., Zhao, Y., Gao, Y. & Liu, B. Q. High-performance blue molecular emitter-free and doping-free hybrid white organic light-emitting diodes: an alternative concept to manipulate charges and excitons based on exciplex and electroplex emission. ACS Photonics. 4, 1566–1575 (2017).
Xue, K. W. et al. Efficient non-doped monochrome and white phosphorescent organic light-emitting diodes based on ultrathin emissive layers. Org. Electron. 26, 451–457 (2015).
Liu, B. Q. et al. A hostguest system comprising high guest concentration to achieve simplified and high-performance hybrid white organic light-emitting diodes. J. Mater. Chem. C. 3, 6359–6366 (2015).
Xue, K. W. et al. Highly efficient orange and white phosphorescent organic light-emitting devices with simplified structure. Org. Electron. 26, 225–229 (2015).
Li, X. L. et al. Highly efficient blue and warm white organic light-emitting diodes with a simplified structure. Nanotechnology. 27, 124001 (2016).
Chang, Y. L. et al. Highly efficient warm white organic light-emitting diodes by triplet exciton conversion. Adv. Funct. Mater. 23, 705–712 (2013).
Su, S. J., Gonmori, E., Sasabe, H. & Kido, J. Highly efficient organic blue-and white-light-emitting devices having a carrier- and exciton-confining structure for reduced efficiency roll-off. Adv. Mater. 20, 4189–4194 (2008).
Liu, B. Q. et al. High-performance doping-free hybrid white OLEDs based on blue aggregation-induced emission luminogens. ACS Appl. Mater. Interfaces. 9, 34162–34171 (2017).
Liu, B. Q. et al. High-performance doping-free hybrid white organic light-emitting diodes: The exploitation of ultrathin emitting nanolayers (1 nm). Nano Energy 26, 26–36 (2016).
Sun, N. et al. High-performance hybrid white organic light-emitting devices without interlayer between fluorescent and phosphorescent emissive regions. Adv. Mater. 26, 1617–1621 (2014).
Sun, N. et al. A hybrid white organic light-emitting diode with above 20% external quantum efficiency and extremely low efficiency roll-off. J. Mater. Chem. C. 2, 7494–7504 (2014).
Wei, X., Yu, H. J., Cao, J., Jhun, C. & Chen, C. P. Efficient blue and white phosphorescent organic light-emitting diodes with a mixed host in emission layer. Molecular Crystals And Liquid Crystals 651, 108–117 (2017).
Lee, H. et al. The origin of the hole injection improvements at indium tin oxide/molybdenum trioxide/N, N′-bis (1-naphthyl)-N, N′-diphenyl-1,1′-biphenyl-4, 4′-diamine interfaces. Appl. Phys. Lett. 93, 043308 (2008).
Kang, J. W. et al. Low roll-off of efficiency at high current density in phosphorescent organic light emitting diodes. Appl. Phys. Lett. 90, 223508 (2007).
Zhao, Y. B., Zhu, L. P., Chen, J. S. & Ma, D. G. Improving color stability of blue/orange complementary white OLEDs by using single-host double-emissive layer structure: Comprehensive experimental investigation into the device working mechanism. Org. Electron. 13, 1340–1348 (2012).
D’Andrade, B. W., Holmes, R. J. & Forrest, S. R. Efficient organic electrophosphorescent white-light-emitting device with a triple doped emissive layer. Adv. Mater. 16, 624–628 (2004).
Zhu, L. P., Zhao, Y. B., Zhang, H. M., Chen, J. S. & Ma, D. G. Using an ultra-thin non-doped orange emission layer to realize high efficiency white organic light-emitting diodes with low efficiency roll-off. J. Appl. Phys. 115, 244512 (2014).
Zhang, Y. F., Lee, J. & Forrest, S. R. Tenfold increase in the lifetime of blue phosphorescent organic light-emitting diodes. Nature Communications 5, 5008 (2014).
Acknowledgements
Acknowledge to National Key Research and Development Program of China, No. 2017YFB0404503, Key Laboratory of Advanced Display and System Applications Ministry of Education Shanghai University.
Author information
Authors and Affiliations
Contributions
J.C. conceived the idea and designed the experiments. H.Y., X. W. and C.J. participated in structure designing. H.Y. and X.D. fabricated the devices and measured the device performance. J.C. and H.Y. analyzed most of the data and revised the manuscript. F.Y. and C.J. assisted experiments and participated discussion. All authors reviewed the manuscript and contributed to this work.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yu, H., Dai, X., Yao, F. et al. Efficient white phosphorescent organic light-emitting diodes using ultrathin emissive layers (<1 nm). Sci Rep 8, 6068 (2018). https://doi.org/10.1038/s41598-018-24434-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-24434-8
This article is cited by
-
Full-phosphorescence white organic light-emitting diode with high CRI: the exploitation of positive and reverse emitter sequences
Optical and Quantum Electronics (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.