Abstract
The anti-fluorite type Li5FeO4 has attracted significant interest as a potential cathode material for Li ion batteries due to its high Li content and electrochemical performance. Atomic scale simulation techniques have been employed to study the defects and Li ion migration in Li5FeO4. The calculations suggest that the most favorable intrinsic defect type is calculated to be the cation anti-site defect, in which Li+ and Fe3+ ions exchange positions. Li Frenkel is also found to be lower in this material (0.85 eV/defect). Long range lithium diffusion paths were constructed in Li5FeO4 and it is confirmed that the lower migration paths are three dimensional with the lowest activation energy of migration at 0.45 eV. Here we show that doping by Si on the Fe site is energetically favourable and an efficient way to introduce a high concentration of lithium vacancies. The introduction of Si increases the migration energy barrier of Li in the vicinity of the dopant to 0.59 eV. Nevertheless, the introduction of Si is positive for the diffusivity as the migration energy barrier increase is lower less than that of the lithium Frenkel process, therefore the activation energy of Li diffusion.
Similar content being viewed by others
Introduction
The ever increasing requirements for better capacity, safety, cycle performance, and durability led to solid-state lithium batteries with the research focusing mainly on the electrolyte and cathode materials1,2,3,4,5,6,7,8,9,10,11,12. In that respect, considerable effort has been devoted to identify alternative cathode materials for rechargeable lithium ion batteries in order to provide high energy density for large scale applications particularly in electric vehicles and to replace conventional positive electrode material LiCoO2 due to its issues associated with cost and safety13. Promising positive electrode materials require meeting various essential conditions such as safety requirements, a relatively low cost, and large density of Li+ ions leading to a higher energy density.
Polyanion based olivine-structured orthophosphate LiFePO4 has attracted considerable attention as viable alternative to LiCoO2 due to its high electrochemical stability and iron, phosphorous are relatively safe, abundant and low-cost14,15. Though LiFePO4 is now in commercial use, several other promising cathode materials identified in recent years include Li2FeSiO416,17,18, Li2MnSiO419,20, LiFeBO321, LiFeSO4F22, Li2Fe(SO4)223, Li2FePO4F24, Li2FeP2O725, Li2MnO326, and Li7Mn(BO3)327. Among these, “Li rich” Li7Mn(BO3)3 displayed an extremely large theoretical capacity (≈288 mAhg−1) upon extraction of three lithium ions per formula unit. Another “Li-rich” antifluorite Li5FeO4 has been reported as a promising cathode material for lithium ion batteries as it provides a high concentration of Li+ ions with a theoretical capacity of 867 mAhg−1 28,29,30,31,32. Four Li+ ions have been extracted per formula unit, electrochemically between 3.5 and 4.5 V, with the evidence of partial oxidation of Fe3+ to Fe4+ in the X-ray absorption spectroscopy during the initial charge30. There is no evidence of Fe3+ to Fe4+ oxidation in the delithiated samples but a change in coordination of the Fe3+ ion from tetrahedral to octahedral coordination has been observed. This suggested that lithium extraction is predominantly assisted by the release of oxygen with the net loss of Li2O leaving Fe2O3 rich residual product30.
Atomic scale modeling techniques are powerful tools to provide detailed information about the defect chemistry and Li ion migration pathways together with the activation barrier providing complementary information to experiment. In the literature, no attempts have been made to study the defect process and Li ion diffusion in Li5FeO4 theoretically. The present study uses well-established atomistic modeling techniques to carry out a detailed survey of the relative energetics of the formation of intrinsic defects and the possible pathways for lithium ion conduction in Li5FeO4.
Results and Discussion
Structure and intrinsic defects
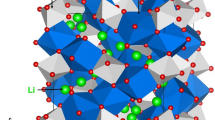
Crystal structure of Li5FeO4 exhibits a defect antifluorite structure with orthorhombic symmetry (space group Pbca). Experimentally determined lattice parameters are: a = 9.218, b = 9.213 and c = 9.153 Å33. Figure 1 shows the structure and the chemical environments of Li (forming a tetrahedron with four O atoms) and iron (forming a tetrahedron with four O atoms). Energy minimization calculations were performed on antifluorite bulk structure of Li5FeO4 to obtain the equilibrium lattice constants, thereby enabling an assessment (through comparison with experiment) of the quality of the pair potentials used in this study. The calculated equilibrium lattice constants (tabulated in Table S1) are in good agreement with experiment within a margin of 1% error.
A series of isolated point defect (vacancy and interstitial) energies were calculated, which were combined to determine the formation energies for Frenkel and Schottky-type defects in Li5FeO4. The equations represent the reactions involving these defects as written using Kröger-Vink notation and corresponding reaction energies are tabulated in Table 1. The most favorable intrinsic disorder is found to be the Li-Fe anti-site defect (equation 7 of Table 1). The exact concentration is dependent on the temperature and synthetic routes. The formation of Li Frenkel is the second most favorable process in this material. Fe Frenkel, O Frenkel and Schottky defects are highly unfavorable and thus unlikely to occur in any significant concentration in Li5FeO4.
Lithium ion diffusion
The lithium ion mobility in the Li5FeO4 material is of crucial importance when assessing its use as a possible high-rate cathode material in lithium batteries. Also it is important to observe the Li ion migration energies with paths in this material. However, the diffusion paths in the Li5FeO4 structures have not been established experimentally.
Atomistic simulation techniques enable the examination of various possible diffusion paths responsible for lithium ion conduction. We have identified two main long range diffusion channels connecting local Li hops, illustrated as X and Y in Fig. 2. In both channels Li ions can also diffuse in opposite directions as well. The lowest overall activation energy is calculated at 0.45 eV for the X channel. The second lowest activation energy channel, Y, has an overall activation barrier of 0.56 eV. Individual Li-Li separations and corresponding activation energy barriers are tabulated in Table 2. We have considered a range of other paths with longer Li-Li migration distances of >2.70 Å, but these revealed high migration barriers (>0.85 eV). Potential energy profile diagrams showing the activation energies are shown in Fig. 3.
Nine different energy profiles [as shown in Fig. 2] of Li vacancy hopping between two adjacent Li sites in Li5FeO4.
Tetravalent doping
There is a low migration activation energy for the migration of lithium via the vacancy mechanism (0.45 eV), however, the Li Frenkel energy (0.85 eV/defect) will limit the concentration of V′ Li . The latter are important as they effectively act as the vehicles mediating Li self-diffusion. A way to increase the concentration of V′ Li is by the introduction of tetravalent dopant atoms via the solution of other oxides in Li5FeO4. This processes can be described as (in the Kröger-Vink notation):
Analogous defect engineering strategies have been introduced in order to enhance the concentration of vacancy defects in oxides34. We considered the solution RO2 oxides (R = Ce, Zr, Ti, Si and Ge), aiming to find an oxide with a low solution enthalpy. As it can be observed in Fig. 4 the solution energy of SiO2 is the lowest one (−0.16 eV/defect) and interestingly it is negative. This in turn implies that the solution of SiO2 in Li5FeO4 is energetically favourable leading to the formation of a non-equilibrium concentration of V′ Li . Additionally, the solution of GeO2, although positive (0.46 eV/defect), is lower in energy and thus more energetically favourable as compared to the Li Frenkel. In essence doping with Si or Ge will result in the formation of V′ Li without the higher energies required by the Li Frenkel reaction. Importantly, these vacancies will be vehicles for Li self-diffusion, increasing the Li diffusivity. Given that the solution enthalpies for SiO2 and GeO2 will be lower than the enthalpies for Schottky and Frenkel disorder, the V′ Li will be prevalent due to this extrinsic processes.
In the Li5FeO4 crystal structure Fe forms a tetrahedral coordination. Fig. 5 shows the local coordination of dopants together with the bond lengths and bond angles in the relaxed structures. For comparison, bond lengths and bond angles of the FeO4 unit in the relaxed structure of Li5FeO4 are also given. In most silicates, a SiO4 structure is formed with four O atoms in tetrahedral coordination around the Si atom. A similar feature is observed in some solids containing Ge. This reflects in the solution energies. Lower solution energies were calculated for Si and Ge. Ti and Zr also form tetrahedral coordination but their solution energies are high. This can be due to their unusual tetrahedral coordination as Zr and Ti normally form octahedral six-coordinate complexes in their crystal structures. Ce clearly forms a distorted octahedral coordination revealing a high positive solution enthalpy.
Introducing Si or Ge dopants in Li5FeO4 will have an effect on the migration energies of lithium. Fig. 6 represents the impact of Si or Ge dopants on the migration barrier of Li. The presence of the Si substitutional will increase the migration energy barrier of Li by 0.14 eV (i.e. to 0.59 eV). Interestingly, the Ge substitutional will lead to the reduction of the migration energy barrier to 0.29 eV. Considering the activation energy of the diffusion process (formation energy + migration energy) it can be concluded that in undoped Li5FeO4 the activation energy is 1.30 eV, in GeO2 doped Li5FeO4 it is 0.75 eV and finally, in SiO2 doped Li5FeO4 only 0.59 eV (as the solution energy is negative we assume that there will be an non-equilibrium concentration of V′ Li so their formation energy will be 0).
Summary
Atomistic simulation techniques have been employed to provide detailed insights into intrinsic defects and lithium ion mobility in Li5FeO4. Our simulations reproduce the experimentally observed crystal structure of Li5FeO4. The most favorable intrinsic disorder type is the Li-Fe anti-site defect. This suggests that there will be a population of Li ion on Fe sites and Fe on Li sites. Frenkel and Schottky type disorders (except Li Frenkel) are highly unfavorable. The lowest activation energy for Li migration in Li5FeO4 is 0.45 eV. Considering the formation energy of vacancies via the Li Frenkel energy the activation energy of the process becomes 1.3 eV. The solution of SiO2 effectively forms V′ Li but can increase the migration energy barrier to 0.59 eV. There is therefore an activation energy reduction as compared to the undoped case of 0.71 eV. Finally, through the solution of GeO2 only 0.46 are required for the formation of every V′ Li , whereas there is a reduced migration energy barrier (0.29 eV) for Li in the vicinity of the Ge substitutionals. The activation energy of diffusion is 0.75 eV that is 0.55 eV lower as compared to the undoped case and only 0.16 eV higher as compared to Si-doped Li5FeO4. We propose experimental investigations and diffusion studies in Li5FeO4 doped with Si and or Ge. A key objective of the present investigation is to motivate experimental and theoretical studies35,36,37 to determine the defect processes of anti-fluorite materials such as Li5FeO4 and their potential application as energy materials.
Methods
In order to calculate the energetics for the formation of intrinsic defects and possible Li ion diffusion pathways, the classical pair potential method as implemented in the GULP package was employed38. This method is based on the classical Born model description of an ionic crystal lattice. All systems were treated as crystalline solids with interactions between ions consisting of the long-range attractions and short-range repulsive forces representing electron-electron repulsion and van der Waals interactions. The short range interactions were modelled using Buckingham potentials (refer to Table S2). Simulation boxes and the corresponding atom positions were relaxed using the Broyden-Fletcher-Goldfarb-Shanno (BFGS) algorithm39. The Mott-Littleton method40 was used to investigate the lattice relaxation about point defects and the migrating ions. It divides the crystal lattice into two concentric spherical regions, where the ions within the inner spherical region (on the order of >700 ions) immediately surrounding the defect relaxed explicitly. All defect calculations were performed using a perfect 2 × 2 × 2 supercell containing 640 atoms. Li ion diffusion was calculated considering two adjacent vacancy sites as initial and final configurations. Seven interstitial Li ions were considered in a direct linear route and they were fixed while all other ions were free to relax. The local maximum energy along this diffusion path is calculated and reported as activation energy. As the present model assumes a full charge ionic model with the calculations corresponding to the dilute limit the defect enthalpies will be overestimated, however, relative energies and trends will be consistent.
References
Tarascon, J.-M. & Armand, M. Issues and changes facing rechargeable lithium batteries. Nature 414, 359–367 (2001).
Armand, M. & Tarascon, J.-M. Building better batteries. Nature 451, 652–657 (2001).
Kamaya, N. et al. A lithium superionic conductor. Nat. Mater. 10, 682–686 (2011).
Zhao, Y. & Daemen, L. L. Superionic conductivity in lithium-rich anti-perovskites. J. Am. Chem. Soc. 134, 15042–15047 (2012).
Bruce, P. G., Freunberger, S. A., Hardwick, L. J. & Tarascon, J.-M. Li-O2 and Li-S batteries with high energy storage. Nat. Mater. 11, 19–29 (2012).
Jay, E. E., Rushton, M. J. D., Chroneos, A., Grimes, R. W. & Kilner, J. A. Genetics of superionic conductivity in lithium lanthanum titanates. Phys. Chem. Chem. Phys. 17, 178–183 (2015).
Shin, D. O. et al. Synergistic multi-doping effects on the Li7La3Zr2O12 solid electrolyte for fast lithium ion conduction. Sci. Rep. 5, 18053 (2015).
Klenk, M. & Lai, W. Local structure and dynamics of lithium garnet ionic conductors: tetragonal and cubic Li7La3Zr2O12. Phys. Chem. Chem. Phys. 17, 8758–8768 (2015).
Fisher, C. A. J., Kuganathan, N. & Islam, M. S. Defect chemistry and lithium-ion migration in polymorphs of the cathode material Li2MnSiO4. J. Mater. Chem. A 1, 4207–4214 (2013).
Kato, Y. et al. High-power all-solid-state batteries using sulfide superionic conductors. Nat. Energy 1, 16030 (2016).
Chen, C., Lu, Z. & Ciucci, F. Data mining of molecular dynamics data reveals Li diffusion characteristics in garnet Li7La3Zr2O12. Sci. Rep. 7, 40769 (2017).
He, X., Zhu, Y. & Mo, Y. Origin of fast ion diffusion in super-ionic conductors. Nat. Commun. 8, 15893 (2017).
Mizushima, K., Jones, P. C., Wiseman, P. J. & Goodenough, J. B. LixCoO2 (0 < x < −1): A new cathode material for batteries of high energy density. Mater. Res. Bull. 15, 783–789 (1980).
Padhi, A. K., Nanjundaswamy, K. S. & Goodenough, J. B. Phospho‐olivines as positive‐electrode materials for rechargeable lithium Batteries. J. Electrochem. Soc. 144, 1188–1194 (1997).
Kang, B. & Ceder, G. Battery materials for ultrafast charging and discharging. Nature 458, 190 (2009).
Nytén, A., Abouimrane, A., Armand, M., Gustafsson, T. & Thomas, J. O. Electrochemical performance of Li2FeSiO4 as a new Li-battery cathode material. Electrochem. Commun. 7, 156–160 (2005).
Nishimura, S.-I. et al. Structure of Li2FeSiO4. J. Am. Chem. Soc. 130, 13212–13213 (2008).
Armstrong, A. R., Kuganathan, N., Islam, M. S. & Bruce, P. G. Structure and lithium transport pathways in Li2FeSiO4 Cathodes for Lithium Batteries. J. Am. Chem. Soc. 133, 13031–13035 (2011).
Politaev, V. V., Petrenko, A. A., Nalbandyan, V. B., Medvedev, B. S. & Shvetsova, E. S. Crystal structure, phase relations and electrochemical properties of monoclinic Li2MnSiO4. J. Solid State Chem. 180, 1045–1050 (2007).
Dominko, R. et al. Structure and electrochemical performance of Li2MnSiO4 and Li2FeSiO4 as potential Li-battery cathode materials. Electrochem. Commun. 8, 217–222 (2006).
Yamada, A. et al. Lithium iron borates as high-capacity battery electrodes. Adv. Mater. 22, 3583–3587 (2010).
Recham, N. et al. A 3.6 V lithium-based fluorosulphate insertion positive electrode for lithium-ion batteries. Nat. Mater. 9, 68 (2009).
Reynaud, M. et al. Li2Fe(SO4)2 as a 3.83V positive electrode material. Electrochem. Commun. 21, 77–80 (2012).
Ellis, B. L., Makahnouk, W. R. M., Makimura, Y., Toghill, K. & Nazar, L. F. A multifunctional 3.5 V iron-based phosphate cathode for rechargeable batteries. Nat. Mater. 6, 749 (2007).
Nishimura, S.-I., Nakamura, M., Natsui, R. & Yamada, A. New lithium iron pyrophosphate as 3.5 V class cathode material for lithium ion battery. J. Am. Chem. Soc. 132, 13596–13597 (2010).
Thackeray, M. M. et al. Li2MnO3-stabilized LiMO2 (M=Mn, Ni, Co) electrodes for lithium-ion batteries. J. Mater. Chem. 17, 3112–3125 (2007).
Afyon, S., Wörle, M. & Nesper, R. A. Lithium-rich compound Li7Mn(BO3)3 containing Mn2+ in tetrahedral coordination: A cathode candidate for lithium-ion batteries. Angew. Chemie Inter. Ed 52, 12541–12544 (2013).
Hirano, A. et al. Electrochemical properties and Mössbauer effect of anti-fluorite type compound, Li5FeO4. Solid State Ionics 176, 2777–2782 (2005).
Narukawa, S. et al. Anti-fluorite type Li6CoO4, Li5FeO4, and Li6MnO4 as the cathode for lithium secondary batteries. Solid State Ionics 122, 59–64 (1999).
Johnson, C. S. et al. Li2O Removal from Li5FeO4: A cathode precursor for lithium-ion batteries. Chem. Mater. 22, 1263–1270 (2010).
Okumura, T., Shikano, M. & Kobayashi, H. Effect of bulk and surface structural changes in Li5FeO4 positive electrodes during first charging on subsequent lithium-ion battery performance. J. Mater. Chem. A 2, 11847–11856 (2014).
Park, M.-S. et al. Scalable integration of Li5FeO4 towards robust, high-performance lithium-ion hybrid capacitors. ChemSusChem 7, 3138–3144 (2014).
Luge, R. & Hoppe, R. Neues über Oxoferrate(III). I. Zur Kenntnis von Li5FeO4 [1] Mit einer Notiz über Mischkristalle Na5Fe1−xGaxO4. Z. Anorg. Allgem. Chemie 513, 141–150 (1984).
Rushton, M. J. D. & Chroneos, A. Impact of uniaxial strain and doping on oxygen diffusion in CeO2. Sci. Rep. 4, 6068 (2014).
Alexopoulos, K., Lazaridou, M. & Varotsos, P. Activation volumes in lead halides and other solids. Phys. Rev. B 33, 2838–2841 (1986).
Varotsos, P. A. Calculation of point defect parameters in diamond. Phys. Rev. B 75, 172107 (2007).
Saltas, V., Chroneos, A. & Vallianatos, F. Composition and temperature dependence of self-diffusion in Si1−xGex alloys. Sci. Rep. 7, 1374 (2017).
Gale, J. D. & Rohl, A. L. The General Utility Lattice Program (GULP). Molec. Simul. 29, 291–341 (2003).
Gale, J. D. G. U. L. P. A computer program for the symmetry-adapted simulation of solids. J. Chem. Soc. Faraday Trans. 93, 629–637 (1997).
Mott, N. F. & Littleton, M. J. Conduction in polar crystals. I. Electrolytic conduction in solid salts. Trans. Faraday Soc 34, 485–499 (1938).
Acknowledgements
Computational facilities and support were provided by High Performance Computing Centre at Imperial College London.
Author information
Authors and Affiliations
Contributions
N.K. performed the calculations. All the authors analyzed and discussed the results and contributed to the writing of the paper.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kuganathan, N., Iyngaran, P. & Chroneos, A. Lithium diffusion in Li5FeO4. Sci Rep 8, 5832 (2018). https://doi.org/10.1038/s41598-018-24168-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-24168-7
This article is cited by
-
Recent progress of in-situ characterization of LiNi1−x−yCoxMnyO2 cathodes for lithium metal batteries: A mini review
Nano Research (2024)
-
Breaking the hard-sphere model with fluorite and antifluorite solid solutions
Scientific Reports (2023)
-
Defect Chemistry and Li-ion Diffusion in Li2RuO3
Scientific Reports (2019)
-
Defects and dopant properties of Li3V2(PO4)3
Scientific Reports (2019)
-
Defects, dopants and Mg diffusion in MgTiO3
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.