Abstract
According to high incidence and prevalence of pressure ulcers worldwide, the purpose of this study is using of non-thermal atmospheric plasma as a novel therapy for pressure ulcers. Cold plasma was produced by applying a high-voltage (5 kV) and high-frequency (25 kHz), to helium gas. Under general anesthesia and sterile conditions, two circular magnets were used to create pressure ulcers on the dorsal skin of adult rats. The wounds were divided randomly into control and plasma-treated groups. Animals in the plasma-treated group received plasma radiation for 5 days, each day 3 times and every time 60 s. Mechanical assays were performed to determine plasma effects on the mechanical strength of the repaired tissue. The results showed that mechanical strength of repaired wound in the plasma-treated group was significantly higher than that in the control group (p < 0.05). In addition, evidence from histological studies indicates a significantly accelerated wound re-epithelialization in comparison with the control group; angiogenesis and fibrosis (collagen synthesis) were also significantly increased and the inflammation phase of wound healing was shorter in the plasma-treated group. The plasma treatment also resulted in significant wound contraction and acceleration of wound healing. The findings of present study indicate the effects of cold plasma on pressure ulcer treatment.
Similar content being viewed by others
Introduction
Pressure ulcer (also described as bed sore and decubitus ulcer) is a major health challenge worldwide that imposes a significant financial burden on health care systems and negatively affects quality of life, so quick and opportune curative actions are needed1. Definition of pressure ulcer provided by the National Pressure Ulcer Advisory Panel is “localized injury to the skin and/or underlying tissue usually over a bony prominence, as a result of pressure or pressure in combination with shear”2,3. The main groups of people at risk of developing pressure ulcers include elderly people over 70 years, patients with spinal cord injuries, hospitalized patients, especially those undergoing orthopedic surgeries and patients in especial care2,3,4,5,6. According to the epidemiological studies, approximately 40% of patients with spinal cord injury experience a pressure ulcer7,8.
The current care strategies of pressure ulcers comprise surgical debridement of all necrotic tissue, negative pressure wound therapy, maintenance of a favorable moist wound bed by employing suitable dressings, relief of pressure and verifying issues such as nutritional, metabolic and circulatory status9,10. The consequences of pressure ulcers that afflict patients and health care systems, justify the requirement of extensive efforts to reduce this problem. Using of non-thermal atmospheric pressure plasma, as a possible safe new method in chronic wound therapy has been reported in many different studies11,12,13,14,15,16,17,18,19,20.
Plasma is defined as a partially or completely ionized medium that is composed of many elements such as charged particles (electrons, ions), neutral and excited atoms, UV photons and radicals21,22,23,24. In recent years the application of non-thermal plasma in biomedical researches such as wound healing, blood coagulation23, angiogenesis suppression25, cancer treatment26,27 and deactivation of microorganisms28,29 like MDR bacteria30 and even viruses31 is rapidly growing, primarily due to its bactericidal properties. A large volume of studies have identified the positive effects of non-thermal plasma on healing acne32 and eczema33, venous ulcers34,35,36, chronic leg ulcers17,37, burn wounds38,39, full-thickness cutaneous wounds in healthy and diabetic mice19,40, and skin rejuvenation41. Unfortunately, the main mechanism of the non-thermal plasma interaction with cells or living tissue still remains unknown23, but the effects of non-thermal plasma on the key wound-related cells have been reported in several studies. These studies have shown that plasma induces fibroblast migration and proliferation42, promotes the proliferation of endothelial43 and keratinocyte14 cells, as well as the growth of epithelial cells44; these effects are attributed to the production of intracellular reactive oxygen species (ROS)45. In addition, NO-containing plasma induces skin acidification (pH reduction) as a result of NOx interaction with water and increases dermal microcirculation46. These effects are similar to those found in the natural process of wound healing. Therefore, plasma therapy represents a promising new medication against chronic/infected wounds.
To our knowledge, this is the first study that reports the effects of cold plasma on the treatment of an animal model of pressure ulcer. In order to compare the wound healing process between plasma-treated and control specimens, mechanical and histological parameters were measured and wound contraction was evaluated during the healing period.
Results
Wound area reduction
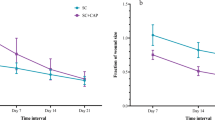
Wounds were observed and evaluated daily from day 0 to complete healing. As shown in Fig. 1 there were no particular differences in the appearance of the wound surfaces between two groups, except in the wound size in several days of the healing period. Wound size reduction was observed in both control and plasma-treated groups, but from Day 7 to Day 21 the wound size in the plasma-treated group was significantly smaller than that in the control group. Wound healing duration in the plasma-treated group was 17.83 ± 0.83 days whereas in the control group after Day 21 some wounds didn’t heal completely yet and wound healing duration was about 19.37 ± 0.62 days.
The wound area and the percentage decrease in wound size, in all animals were calculated and reported in Fig. 2(a,b), respectively. According to these Figures, there are no significant differences in wound area between plasma-treated and control groups on days 0 and 3 after wounding. On days 7–21, wound area surfaces in the plasma-treated group are significantly smaller than those in the control group and so the percentage decrease of wound area is significantly higher in the plasma-treated group (p < 0.05). F value for the wound area data on days 14 and 21 post wounding was obtained 10.16 and 13.09 respectively, and 13.82 for the percentage decrease in wound size on day 21.
Effect of non-thermal plasma on histological and mechanical parameters
Histological microscopic images from the tissue samples prepared on 3, 7, 14, and 21 days after wounding are presented in Fig. 3. It was observed that at day 3 after wounding: a new epidermal layer formed in plasma-treated animals which did not appear in control rats; production of scar tissue in control wounds was more, and less collagen generation happened in this group in comparison with the plasma-treated samples. Angiogenesis was not observed and infiltration of inflammatory cells was severe in both groups, indicating that immune defense increased in the early phases of wound healing.
At day 7: in the plasma-treated group, the epidermis began to regenerate and local presence of epidermis can be observed. In addition, unlike to the control group, production of scar tissue was stopped at this stage. Moreover, according to Masson’s trichrome staining, plasma caused stimulation of collagen synthesis in the dermis layer. The angiogenesis in the two groups increased from day 3 to 7, but it can be demonstrated that more angiogenesis was induced by plasma treatment.
At day 14: unlike to the control group, a thickened epidermis layer which covered the wound surface completely can be observed in the lesions treated with plasma. Moreover, angiogenesis was significantly increased because of plasma treatment. The inflammation decreased in both groups from day 7 to 14, but plasma caused a more rapidly reduction of inflammation in comparison with the control group.
At day 21: a new epidermis layer was covered the whole surface of all wounds, but in the plasma-treated wounds the epidermis was significantly thicker compared to the control group. The most important point obtained from the images is the formation of the new hair follicle and sebaceous glands in the epidermis and dermis layer of plasma-treated samples which did not appear in control rats. In addition, no inflammation was observed after plasma treatment while it cannot be ignored in control self-healed lesions.
The results of statistical analysis of histopathological studies are shown in Fig. 4(a–d). According to data, significant differences in epithelialization, inflammation, collagen synthesis and angiogenesis were observed between groups (p < 0.05). In the plasma-treated group, epithelialization and angiogenesis were significantly more than that of the control group on days 14 and 21. On the other hand, after plasma treatment, the inflammation was significantly less than that of the control group on day21. At the same time in the plasma-treated group, there was significantly more collagen synthesis in comparison with that of the control group (p < 0.05).
Maximum force and stress
As exhibited in Fig. 5(a,b), the maximum force (Fmax) and maximum stress (maximum force per mm2), on days 3 and 7 after wounding decreased significantly (decreasing of tissue tolerance versus rupture) in both plasma-treated and control groups compared to normal skin because of tissue injury and tensile strength reduction. On days 14 and 21, a significant increase in maximum force and maximum stress was observed in the plasma-treated group compared to the control group, which showed a higher tensile strength of plasma- treated wounds (p < 0.05). The amount of maximum force and maximum stress in plasma-treated and control groups were always lower than those in normal skin but on days 14 and 21, the means value in the plasma-treated group were not significantly different from normal skin(p > 0.05), that means cold plasma radiation increases the tolerance of the repaired tissue against rupture.
Work up to maximum force (W up to Fmax)
W up to Fmax is the area under the Stress-Strain curve and represents energy absorption by the tissue under the tensile force applying. As shown in Fig. 5(c), similarly to maximum force and maximum stress, W up to Fmax on days 3 and 7 decreased significantly in both plasma-treated and control groups compared to normal skin (p < 0.05). In the other hand on days 14 and 21, W in the plasma-treated group increased significantly compared to the control group but there was no significant difference between normal skin and plasma-treated wounds (p > 0.05). The increase of work up to maximum force in the plasma-treated group compared to the control group can be attributed to more regeneration of tissue in plasma-treated sores.
Elastic Stiffness (E-Modulus-N/mm2)
Elasticity module (E-Modulus) is the slope of the linear part of the Stress-Strain curve and represents the elastic stiffness of tissue that is a very important parameter in the evaluation of wound healing process. Due to wounding, the stiffness and elasticity of tissue were significantly decreased so the skin was loosed. As shown in Fig. 5(d), E-Modulus in both plasma-treated and control groups was significantly lower than that in normal skin in all days of healing period, but the means in plasma-treated specimens were more than control and finally on day 21, E-Modulus increasing in the plasma-treated group was significant compared to the control group (p < 0.05). The increasing of the elastic stiffness of the plasma-treated wounds indicated that those tissues gained more elasticity during the repairing period. According to this fact that collagen fibers are responsible for the stiffness and elasticity of the skin tissue, increased elastic stiffness in plasma-treated wounds can be due to increased collagen synthesis in those tissues, as confirmed by the results of the histological test.
Characterization of the He plasma jet
Optical emission spectroscopy
Our results of the optical emission spectroscopy of the helium plasma jet were in agreement with other findings according to the literature47. According to Fig. 6(a), OES measurement at the distance of 10 mm under the nozzle and pick-to-pick voltage of 5 kv, showed the emission of species such as OH radical (306 nm), N2 or NO lines (316, 337, 358, 427.5 nm), N2+ (391 nm), He (706 nm), O (777 nm), NO (297 nm) and N2 lines(351.8, 353.6, 357.6, 375.4, 380, 399.7, 406 nm).
(a) Optical emission spectra of He plasma jet and excited species produced by it (Gas flow rate 4 slpm, peak to peak voltage = 5 kv, d = 10 mm distance from the nozzle). (b) Reactive species concentration against distance from the nozzle at 5 kV. (c) Reactive species concentration against peak-to-peak voltage at 10 mm distance from the nozzle.
In this work, the effects of voltage and distance from the nozzle on the concentration of reactive species were studied. As shown in Fig. 6(b,c), by increasing the distance from the nozzle and the peak-to-peak voltage, the concentration of reactive species decreases and increases respectively.
Plasma temperature approximation
The rotational and vibrational temperatures can be approximated through comparing experimental and simulated spectra. As shown in Fig. 7, the best fitting was obtained at the rotational temperature of 300 k and the vibrational temperature of 3900 k.
Mouse skin temperature versus voltage
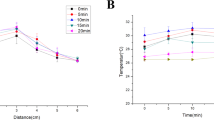
The results of temperature measurement in different voltages during 1 min plasma exposure were shown in Fig. 8(a). At the constant distance of 10 mm, by increasing the peak-to-peak voltage, the tissue temperature increases, it is because of increasing the electron energy and ionization rate in higher voltages. Plasma treatment with voltages of 4, 5 and 6 kv caused ΔT (elevated normal skin temperature) of about 1, 1.4 and 5 °C respectively. ΔT at 4 kv was lower than those at 5 and 6 kv, but according to the weak emission intensity of reactive species at 4 kv it’s not the optimum voltage and 5 kv was considered as the optimum. Figure 8(b) represents an infrared thermal image of normal and unwounded skin under plasma exposure at 60 s.
Discussion
The results of the present study showed that cold plasma as a safe treatment method can positively affect the healing process of pressure sores. In this study after plasma characterization to find the optimal power and temperature of plasma, by controlling the input voltage and the distance from the tip nozzle, we focused on the changes of mechanical and histological parameters of treated tissue.
The results showed that plasma treatment accelerated the wound healing process in contrast to self-healed wounds. In addition, histological analysis revealed plasma supported effective re-epithelialization, angiogenesis, the formation of a new hair follicle and collagen fibers, and controlling of inflammation. Moreover, the mechanical analysis demonstrated that plasma can improve the mechanical strength and tissue tolerance versus uniaxial tensile load.
It’s believed that reactive oxygen and nitrogen species (ROS and NOS) generates in plasma play an important role in the plasma and tissue interaction. When the discharge gas entered the ambient air, the collisions and interactions of energetic and ionized helium molecules with the surrounding air, led to the ionization of nitrogen and oxygen molecules; so, ROS and NOS can be observed in helium plasma spectrum. By increasing the distance from the nozzle, the electrical field decreases and both the electron density and energy are too low to happen ionization and excitation. Therefore, the concentration and intensity of reactive species in this region is lower. In the other hand, more ionization happens in higher voltages and it results in increasing reactive species production.
By analyzing the histological microscopic images, it can be obtained that plasma treatment is significantly effective for the acceleration of wound re-epithelialization and angiogenesis. In confirmation of our findings, similar results were reported in previous studies. Nasruddin et al.18, and M.-H. Ngo Thi et al.38, reported that cold plasma accelerated the re-epithelialization of full-thickness and burn wounds respectively. Furthermore, the influence of plasma on angiogenesis acceleration as an important physiological process in wound healing has been reported by Haertel et al.48. It was hypothesized that ROS and RNS produced in the plasma, induced the angiogenesis and epithelialization processes through enhancing cell proliferation and migration by ROS/RNS signals38,49. Furthermore, in several studies, it was reported that topical application of NO significantly accelerated wound healing, followed by less inflammation, promoted re-epithelialization, and more angiogenesis50,51. Therefore NO-containing plasma can be suggested as a promising strategy for controlling wound inflammation and so improve chronic wound care.
Based on our observation on day 3 post wounding, the inflammation score in plasma-treated and control groups was approximately the same. It showed that cold plasma treatment didn’t have any effects on the early stage of inflammation. On days 7, 14 and 21 after wound creation, the inflammation score in the plasma-treated was fewer than in the control. This observation suggested that cold plasma caused acceleration in the late phase of inflammation. Similar results have been reported previously by Tipa RS et al.52, and Nasruddin et al.18. These reports stated that this event may be related to the proliferative effect of cold plasma, and the early presence of myofibroblasts, respectively.
On the basis of mechanical data, we showed that cold plasma is efficacious for improving the mechanical strength of repaired tissue on days 14 and 21 after wounding. According to this fact that collagen fibers are responsible for the stiffness and elasticity of the skin tissue, increasing the tensile strength of the tissue can be due to plasma induced collagen synthesis in those tissues, as confirmed by the results of the microscopic evaluation. In addition, there is a strong correlation between increasing the mechanical strength with the wound closure and increasing of tissue tolerance versus rupture.
The present study indicates that wound healing phases were promoted by cold plasma treatment and the wound closure rate was significantly increased from day 7 to 21. Because an increase in wound closure rate was observed, it can be suggested that plasma exposer can inhibit the re-opening of sores, which, in turn, can have therapeutic value in clinical studies. In line with this results, Jacofsky et al. reported that helium cold plasma treatment accelerated the wound healing rate in a diabetic murine model19. In addition, it was recently demonstrated that cold plasma treatment had a positive effect and support the healing process of chronic wounds in clinical trials17,34,36. However, there were no mechanical data in this report. This is the first report describing such findings supported by the mechanical data.
In conclusion, this research demonstrates that cold plasma jet provided an improvement in terms of accelerating the wound healing through promoting of re-epithelialization, wound closure, the late phase of inflammation, and improving strength and rate of maturity in tissue repair. Our results suggest a possible treatment effect of cold plasma for wound healing in pressure ulcers in the future.
Materials and Methods
Ethics statement
In this study 45 adult male Wistar rats weighing (250 ± 50 gr) were caged individually under standard laboratory conditions (room temperature, atmospheric pressure, relative humidity: 30 ± 10%, 12 hour light-dark cycle) with free access to food and water. All of the animal experiments and procedures were approved by Biomedical Ethics Committee of Shahid Beheshti University of Medical Sciences (IR.SBMU.RETECH.REC, ethic no. 1396.28). All surgeries were done under deep anesthesia in accordance with the animal ethical guidelines of Shahid Beheshti University of medical science, and all efforts were made to minimize suffering.
Plasma jet system
The plasma jet system used in this study is shown schematically in Fig. 9, which is mainly composed of a copper tube as the central electrode, a copper ring as the ground electrode and a cylinder of Acrylonitrile Butadiene Styrene (ABS), as the dielectric between the electrodes. An AC high-voltage, with a pick-to-pick voltage of 5 kV and frequency of 25 kHz, was applied to the central electrode when helium gas (99.999% purity) at a flow rate set to 4 standard liters per minute (slpm) was injected from one end of the copper tube. The carrier gas flow was controlled using a mass flow controller (APEX, AX-MC-5SLPM-D). A high-voltage probe (Tekteronix, P6015A, 1:1000), a current probe (TCP-202), and also an oscilloscope (Tekteronix, DPO3012) were used to characterization and analysis the electrical behavior of plasma.
Wound model and treatment protocol
In this study, we used the pressure ulcer model reported by Stadler et al. (2004)53. After weighing the animals and general anesthesia by injection a mixture of xylazine hydrochloride (10 mg/kg) and ketamine hydrochloride (100 mg/kg), the hair on the dorsum was shaved and the area cleaned with 70% alcohol. As shown in Fig. 10(a), the skin was gently pulled up and placed between two circular permanent magnets that had 10 mm diameter and 5 mm thick, with 4000 G magnetic force. Magnets were remained on the skin for 8 hours and 72 hours after ending pressure, 2 circular pressure ulcers with nearly the same diameter and area, were made in each animal (Fig. 10(b)).
In this study, animals were studied in three groups: plasma-treated, control and normal. The wounded animals were randomly classified into plasma-treated and control groups (20 rats in each group), and were compared to the normal group (5 rats without skin wounds) in the mechanical parameters. Histological and mechanical parameters of tissue were assessed for wounded animals at 4 time points during healing period (totally 5 animals or 10 wounded tissue were allocated in each of control and plasma-treated groups at each time point).
In the plasma-treated group, wounds were treated 3 times daily for 5 days. Each time, plasma radiation lasted 60 s with 30 s interruptions. The position of the wound surface was about 10 mm under the nozzle tip, as shown in Fig. 10(c).
Macroscopic evaluation
The day when wounds were appeared (72 hours after ending pressure), was considered as Day 0, and the wound healing process was evaluated morphologically, from Day 0 to Day 21 after wounding. Wounds were photographed on Days 0, 3, 7, 14 and 21 after wounding by a digital camera, and wound surface area was calculated using ImageJ 1.49 v image analysis software. The wound closure rate was calculated by
Histological analysis
Following euthanasia with chloroform inhalation, on days 3, 7, 14 and 21 post-wounding the wounds and the surrounding normal skin, were excised and fixed in neutral buffered 10% formalin solution. 3 samples from each of control and plasma-treated groups were dehydrated in an alcohol series, cleaned in xylene and embedded in paraffin then 5-μm sections were prepared for general staining using hematoxylin-eosin (H&E) and specific staining using Masson’s trichrome staining. Stained tissues were evaluated under a light microscope (Euromex, FE.2025) with attached digital camera system (euromex, CMEXDC.5000) and scored using Ehrlich-Hunt numerical scale54. Inflammation, the intensity of fibrosis (collagen synthesis) and angiogenesis were graded as 0 for absence, 1 for minimal, 2 for mild, and 3 for severe. Epithelialization was also scored as 0 for absence, 1 for focal presence, 2 for thin-complete surface, and 3 for thick-complete surface. Finally, the average data was reported.
Mechanical assay
On Days 3, 7, 14 and 21 after wounding, strips of skin (60 mm × 20 mm) included the wounds and the surrounding normal skin, were excised from sacrificed animals (7 specimens from each of control and plasma-treated groups). The sample thickness was measured by a digital caliper. The specimens were kept moistened in 0.9% NaCl solution at −20°c until the test time55,56. In this test, skin strips were fixed between two clamps of a material testing machine (Zwick-Roll, Z25-ph1F, Germany) and stretched by uniaxial tensile test at a constant speed of 10 mm/min until the skin strips were ruptured. Stress-Strain curves were recorded and the related mechanical parameters [maximum force (Fmax-N), maximum stress (N/mm2), work-up to maximum force (W up to Fmax-Nmm) and elastic stiffness (E-Modulus-N/mm2)] were calculated by a computer connected to the material testing instrument. The mechanical results were analyzed to evaluate plasma effects on mechanical parameters of tissue.
The maximum force (N) was obtained directly from the curve and represents the maximum tolerance of the tissue against rupture or maximum tensile force applied to the specimen to rupture it. Maximum stress (N/mm2) was calculated by dividing the maximum force over the cross-sectional area of the specimen. W up to Fmax (Nmm) was measured by calculating the area under the curve and represents energy absorption by the tissue under the tensile force applying. E-Modulus (N/mm2) was obtained from the slope of the linear part of the Stress-Strain curve and represents the elastic stiffness of tissue that is a very important parameter in the evaluation of wound healing process57,58.
Optical emission spectroscopy
Optical emission spectroscopy (OES), is an appropriate noninvasive method to determine different reactive species produced in plasma. OES was done using an optical fiber connected to the spectrometer (AVANTES, AvaSpec 3648).
Thermal evaluation
The temperature of plasma is a very important parameter and its thermal effect on living tissue and safety must be evaluated. So prior to plasma application on the wound it was tested on normal skin of the anesthetized mouse. The temperature of the skin was measured with a non-contact infra-red digital camera (FLIR E4) in different voltages during 1 min plasma treatment (about 6 images were taken during 1 min).
The rotational and vibrational temperatures can be easily estimated by fitting the experimental and simulated spectra. Specair software and wavelengths 370–381 nm (nitrogen second positive system transition) were used to simulate and compare the spectra.
Statistical analysis
Statistical analysis was performed using SPSS 19.0. Normal distribution of data was analyzed using Shapiro-Wilk test (p > 0.05). Therefore repeated-measures analysis (to compare data in each group) and independent t-test (to compare data between two groups) were used for wound area decrease analysis. Mechanical parameters were compared among groups by ANOVA followed by the Tukey test. For comparison of the groups in terms of collagen synthesis, angiogenesis, inflammation, and epithelialization, Mann-Whitney-U test was used because variables were categorical data. Differences with p < 0.05 were considered statistically significant.
References
Moore, Z. E. H. & Cowman, S. Wound cleansing for pressure ulcers. Cochrane Libr. (2013).
Haesler, E. Prevention and treatment of pressure ulcers: clinical practice guideline. (Cambridge Media, 2014).
McNICHOL, L., FENCL, J., GUPTA, S. A. T. & HASEEB KAZI, M. D. Pressure Ulcers: Factors Contributing to Their Development in the OR. (2016).
White-Chu, E. F., Flock, P., Struck, B. & Aronson, L. Pressure ulcers in long-term care. Clin. Geriatr. Med. 27, 241–258 (2011).
Wyndaele, M. & Wyndaele, J.-J. Incidence, prevalence and epidemiology of spinal cord injury: what learns a worldwide literature survey? Spinal Cord 44, 523 (2006).
Strong, A. L. et al. Adipose Stromal Cells Repair Pressure Ulcers in Both Young and Elderly Mice: Potential Role of Adipogenesis in Skin Repair. Stem Cells Transl. Med. 4, 632 (2015).
Cao, Y., Krause, J. S. & DiPiro, N. Risk factors for mortality after spinal cord injury in the USA. Spinal Cord 51, 413 (2013).
Krause, J. S., Zhai, Y., Saunders, L. L. & Carter, R. E. Risk of mortality after spinal cord injury: an 8-year prospective study. Arch. Phys. Med. Rehabil. 90, 1708–1715 (2009).
Senn, N. Principles of Surgery. In The Journal of Nervous and Mental Disease 21, 575 (1896).
Smith, M. E. B. et al. Pressure Ulcer Treatment Strategies A Systematic Comparative Effectiveness Review. Ann. Intern. Med. 159, 39–50 (2013).
Nastuta, A. V., Topala, I., Grigoras, C., Pohoata, V. & Popa, G. Stimulation of wound healing by helium atmospheric pressure plasma treatment. J. Phys. D. Appl. Phys. 44, 105204 (2011).
Emmert, S. et al. Atmospheric pressure plasma in dermatology: Ulcus treatment and much more. Clin. Plasma Med. 1, 24–29 (2013).
García-Alcantara, E. et al. Accelerated mice skin acute wound healing in vivo by combined treatment of argon and helium plasma needle. Arch. Med. Res. 44, 169–177 (2013).
Haertel, B., von Woedtke, T., Weltmann, K.-D. & Lindequist, U. Non-thermal atmospheric-pressure plasma possible application in wound healing. Biomol. Ther. (Seoul). 22, 477 (2014).
Schmidt, A., Bekeschus, S., Wende, K., Vollmar, B. & von Woedtke, T. A cold plasma jet accelerates wound healing in a murine model of full-thickness skin wounds. Exp. Dermatol. 49 (2016).
Gweon, B., Kim, K., Choe, W. & Shin, J. H. Therapeutic uses of atmospheric pressure plasma: cancer and wound. In Biomedical Engineering: Frontier Research and Converging Technologies 357–385 (Springer, 2016).
Isbary, G. et al. Successful and safe use of 2 min cold atmospheric argon plasma in chronic wounds: Results of a randomized controlled trial. Br. J. Dermatol. 167, 404–410 (2012).
Nasruddin et al. Cold plasma on full-thickness cutaneous wound accelerates healing through promoting inflammation, re-epithelialization and wound contraction. Clin. Plasma Med. 2, 28–35 (2014).
Jacofsky, M. C. et al. Spatially resolved optical emission spectroscopy of a helium plasma jet and its effects on wound healing rate in a diabetic murine model. Plasma Med. 4, 177–191 (2014).
Nakajima, Y. et al. A simple technique to improve contractile effect of cold plasma jet on acute mouse wound by dropping water. Plasma Process. Polym. 12, 1128–1138 (2015).
Lieberman, M. A. & Lichtenberg, A. J. Principles of Plasma Discharges and Materials Processing: Second Edition. Principles of Plasma Discharges and Materials Processing: Second Edition. https://doi.org/10.1002/0471724254 (2005)
Fridman, G. et al. Applied plasma medicine. Plasma Process. Polym. 5, 503–533 (2008).
Fridman, A. & Friedman, G. Plasma Medicine. 18 (John Wiley & Sons, Inc., 2012).
Fridman, A. Plasma Chemistry. Vasa https://doi.org/10.1017/CBO9780511546075 (2008).
Gweon, B. et al. Suppression of angiogenesis by atmospheric pressure plasma in human aortic endothelial cells. Appl. Phys. Lett. 104, 133701 (2014).
Kang, S. U. et al. Nonthermal plasma induces head and neck cancer cell death: the potential involvement of mitogen-activated protein kinase-dependent mitochondrial reactive oxygen species. Cell Death Dis. 5, e1056 (2014).
Panngom, K. et al. Preferential killing of human lung cancer cell lines with mitochondrial dysfunction by nonthermal dielectric barrier discharge plasma. Cell Death Dis. 4, e642 (2013).
Matthes, R. et al. Antimicrobial efficacy of two surface barrier discharges with air plasma against in vitro biofilms. PLoS One 8, e70462 (2013).
Ehlbeck, J. et al. Low temperature atmospheric pressure plasma sources for microbial decontamination. J. Phys. D. Appl. Phys. 44, 13002 (2010).
Daeschlein, G. et al. Skin and wound decontamination of multidrug‐resistant bacteria by cold atmospheric plasma coagulation. JDDG J. der Dtsch. Dermatologischen Gesellschaft 13, 143–149 (2015).
Ahlfeld, B. et al. Inactivation of a foodborne norovirus outbreak strain with nonthermal atmospheric pressure plasma. MBio 6, e02300–14 (2015).
Chutsirimongkol, C., Boonyawan, D., Polnikorn, N., Techawatthanawisan, W. & Kundilokchai, T. Non-Thermal Plasma for Acne and Aesthetic Skin Improvement. Plasma Med. 4 (2014).
Tiede, R. et al. Plasma applications: a dermatological view. Contrib. to Plasma Phys. 54, 118–130 (2014).
Brehmer, F. et al. Alleviation of chronic venous leg ulcers with a hand‐held dielectric barrier discharge plasma generator (PlasmaDerm® VU‐2010): results of a monocentric, two‐armed, open, prospective, randomized and controlled trial (NCT01415622). J. Eur. Acad. Dermatology Venereol. 29, 148–155 (2015).
Emmert, S. et al. Treatment of Chronic Venous Leg Ulcers with a Hand-Held DBD Plasma Generator. Plasma Med. 2, (2012).
Brehmer, F. et al. Treatment of chronic venous leg ulcers with a hand-held DBD plasma generator (PlasmaDerm® VU-2010): results of a monocentric, two-armed, open, randomized, and controlled trial (NCT01415622). Exp. Dermatol. 22, e11 (2013).
Ulrich, C. et al. Clinical use of cold atmospheric pressure argon plasma in chronic leg ulcers: A pilot study. J. Wound Care 24, (2015).
Ngo Thi, M., Shao, P., Liao, J., Lin, C. K. & Yip, H. Enhancement of Angiogenesis and Epithelialization Processes in Mice with Burn Wounds through ROS/RNS Signals Generated by Non‐Thermal N2/Ar Micro‐Plasma. Plasma Process. Polym. 11, 1076–1088 (2014).
Lee, O. J. et al. An experimental burn wound‐healing study of non‐thermal atmospheric pressure microplasma jet arrays. J. Tissue Eng. Regen. Med. 10, 348–357 (2016).
Fathollah, S. et al. Investigation on the effects of the atmospheric pressure plasma on wound healing in diabetic rats. Sci. Rep. 6, 19144 (2016).
Kim, Y.-J. et al. Plasma apparatuses for biomedical applications. IEEE Trans. Plasma Sci. 43, 944–950 (2015).
Brun, P. et al. Helium generated cold plasma finely regulates activation of human fibroblast-like primary cells. PLoS One 9, e104397 (2014).
Kalghatgi, S., Friedman, G., Fridman, A. & Clyne, A. M. Endothelial cell proliferation is enhanced by low dose non-thermal plasma through fibroblast growth factor-2 release. Ann. Biomed. Eng. 38, 748–757 (2010).
Hoentsch, M., von Woedtke, T., Weltmann, K.-D. & Nebe, J. B. Time-dependent effects of low-temperature atmospheric-pressure argon plasma on epithelial cell attachment, viability and tight junction formation in vitro. J. Phys. D. Appl. Phys. 45, 25206 (2011).
Kalghatgi, S. et al. Effects of non-thermal plasma on mammalian cells. PLoS One 6, e16270 (2011).
Heuer, K. et al. The topical use of non-thermal dielectric barrier discharge (DBD): nitric oxide related effects on human skin. Nitric Oxide 44, 52–60 (2015).
Wang, M. et al. Cold atmospheric plasma for selectively ablating metastatic breast cancer cells. PLoS One 8, e73741 (2013).
Haertel, B., Eiden, K., Deuter, A., von Woedtke, T. & Lindequist, U. Differential effect of non-thermal atmospheric-pressure plasma on angiogenesis. Orléans-France 152 (2014).
Graves, D. B. The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. J. Phys. D. Appl. Phys. 45, 263001 (2012).
Blecher, K. et al. Nitric oxide-releasing nanoparticles accelerate wound healing in NOD-SCID mice. Nanomedicine Nanotechnology, Biol. Med. 8, 1364–1371 (2012).
Georgii, J. L., Amadeu, T. P., Seabra, A. B. & de Oliveira, M. G. & Monte‐Alto‐Costa, A. Topical S‐nitrosoglutathione‐releasing hydrogel improves healing of rat ischaemic wounds. J. Tissue Eng. Regen. Med. 5, 612–619 (2011).
Tipa, R. S. & Stoffels, E. Effects of plasma treatment on wounds. In 13th International Conference on Biomedical Engineering 1385–1388 (Springer, 2009).
Stadler, I., Zhang, R.-Y., Oskoui, P., Whittaker, M. B. S. S. & Lanzafame, R. J. Development of a simple, noninvasive, clinically relevant model of pressure ulcers in the mouse. J. Investig. Surg. 17, 221–227 (2004).
Ehrlich, H. P., Tarver, H. & Hunt, T. K. Effects of vitamin A and glucocorticoids upon inflammation and collagen synthesis. Ann. Surg. 177, 222 (1973).
Quirinia, A. & Viidik, A. Freezing for postmortal storage influences the biomechanical properties of linear skin wounds. J. Biomech. 24, 819–823 (1991).
Foutz, T. L., Stone, E. A. & Abrams, J. C. F. Effects of freezing on mechanical properties of rat skin. Am. J. Vet. Res. 53, 788–792 (1992).
Asadi, M. R., Torkaman, G., Hedayati, M. & Mofid, M. Role of sensory and motor intensity of electrical stimulation on fibroblastic growth factor-2 expression, inflammation, vascularization, and mechanical strength of full-thickness wounds. J. Rehabil. Res. Dev. 50, (2013).
Pouriran, R. et al. The effect of combined pulsed wave low-level laser therapy and human bone marrow mesenchymal stem cell-conditioned medium on open skin wound healing in diabetic rats. Photomed. Laser Surg. 34, 345–354 (2016).
Acknowledgements
We are grateful for the support provided by the Laser applications in medical sciences research center of the Shahid Beheshti University of Medical Sciences, Tehran.
Author information
Authors and Affiliations
Contributions
B.Sh., G.T., M.Kh. and M.Ch. initiated the research and designed the experiments. B.Sh., G.T. and M.Kh. conducted all part of the research. M.Ch. performed the experiments and analysed the data. G.T. and H.S. performed biological analysis. M.Ch. wrote manuscript. All authors discussed the result and revised the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chatraie, M., Torkaman, G., Khani, M. et al. In vivo study of non-invasive effects of non-thermal plasma in pressure ulcer treatment. Sci Rep 8, 5621 (2018). https://doi.org/10.1038/s41598-018-24049-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-24049-z
This article is cited by
-
Enhanced Fat Graft Viability and Remodeling Using a Helium-based Radiofrequency Device to Prepare the Recipient Site
Aesthetic Plastic Surgery (2024)
-
Applications of atmospheric cold plasma in agricultural, medical, and bioprocessing industries
Applied Microbiology and Biotechnology (2022)
-
Influences of cold atmospheric plasma on apoptosis related molecules in osteoblast-like cells in vitro
Head & Face Medicine (2021)
-
Antibacterial and safety tests of a flexible cold atmospheric plasma device for the stimulation of wound healing
Applied Microbiology and Biotechnology (2021)
-
Potential effect of non-thermal plasma for the inhibition of scar formation: a preliminary report
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.